Abstract
Background
Pharmacological therapy has been used as an alternative or complementary approach to surgery in central giant cell granuloma (CGCG) of the jaws. This systematic review examined the effectiveness of pharmacological therapy for CGCG of the jaws, focusing on clinical outcomes.
Material and Methods
Electronic searches were performed in six databases. Case reports and/or cases series were included. The Kaplan-Meier survival analysis method was used to evaluate outcomes related to clinical resolution and recurrence. The risk of bias was assessed using the Joanna Briggs Institute tool.
Results
A total of 74 studies comprising 205 cases of CGCG were included. About 65.4% of cases occurred in individuals under 20 years of age. Most of the treated patients were women (61%) and the mandible (72.2%) was the most reported site. Curettage and enucleation before or after pharmacological therapy were reported in 28.3% and 19% of cases, respectively. The main pharmacological agent used was triamcinolone (37.5%). Complete resolution of CGCG was reported at a rate of 77.1%, while side effects were experienced by 9.8% of individuals. The recurrence rate was 6.8%.
Conclusions
Pharmacological therapy may be an effective and safe option for managing CGCG, especially in the young population. Although the overall success rate in achieving complete resolution is encouraging, further controlled studies are needed to refine drug selection and protocols.
Key words:Calcitonin, Central giant cell lesion, Denosumab, Interferon, Pharmacological therapy, Triamcinolone.
Introduction
Central giant cell granuloma (CGCG) is a localized and benign but occasionally aggressive osteolytic lesion of the jaws (1,2). First described by Jaffe in 1953 (3), this lesion remains uncertain in terms of origin and etiology. Factors such as a reactive origin to a local irritant, developmental anomaly, and neoplasia have been proposed (4,5). Histopathologically, CGCG is characterized by the proliferation of osteoclast-like giant cells and a mononuclear cell population composed of macrophage/monocytic cells and spindle-shaped mesenchymal cells (2,6). CGCG has a striking predilection for females and the mandible, and about 50% of affected subjects are children and adolescents (7).
CGCG are usually painless, slow-growing lesions that do not affect vital structures, i.e., so-called non-aggressive lesions (7). Aggressive CGCG is characterized by pain, rapid growth, root resorption, cortical perforation, with an average diameter of 5.8 cm, and a high recurrence rate (1). Recent literature has reported challenges in treating aggressive lesions compared to non-aggressive ones (5,7). Conservative surgical treatment (e.g., enucleation and curettage) has been the mainstay approach to CGCG (7). Nevertheless, its limitations concern the significant probability of recurrence and esthetic and functional impairment, particularly in children, regarding maxillofacial development and growth (8,9).
Pharmacological therapy has been used as an alternative or complementary approach to surgery in CGCG, with the purpose of promoting healing and reducing size, thus minimizing damage caused by extensive surgical procedures and the risk of recurrence (7,10). Different drugs (e.g., triamcinolone, interferon, calcitonin, and denosumab) with multiple protocols have been described elsewhere (5,7,10,11). Two previous systematic review have been published on the topic. The first was in 2009 (12), while a recent analysis synthesized data from 15 studies on non-surgical treatments for CGCG (10). However, isolated case reports were not incorporated in the latter review (10). In particular, it is known that publishing case reports has become increasingly difficult; thus, the motivation to revisit existing literature without restrictions of study designs was based on the premise that unusual diseases treated with alternative approaches along with long-term follow-up, when well documented, can make an important contribution to decision making (14).
The aim of the present systematic review was to evaluate the pharmacological therapy used in CGCG of the jaws. Our focus was to examine information on complete or partial clinical resolution, side effects, recurrence rate, and need for additional interventions.
Material and Methods
-Eligibility criteria
The answer to the following question: “what are the pharmacological therapies that have been indicated for the treatment of CGCG of the jaws?”, was investigated based on the PICO framework: P (population): patients with CGCG of the jaws; I (intervention): pharmacological therapy; C (comparison): not applicable; and O (outcome): total or partial clinical resolution of the lesion, side effects, recurrence, and need for another intervention.
Studies published in English were included. Articles such as case reports or case series with sufficient data about the cytopathological and/or histopathological diagnosis of CGCG of the jaws treated with pharmacological agents were included. Exclusion criteria were articles whose data could not be extracted, experimental studies, letters to the editor, and expert opinions/comments, unless any of these types of articles provided sufficient and detailed data of interest. Disorders related to CGCG of the jaws, such as brown tumors of hyperparathyroidism, cherubism, and syndromes such as Noonan and LEOPARD, or neurofibromatosis type 1 were not considered.
-Search scheme
PubMed, Web of Science, Ovid, Embase, Cochrane Library, and Scopus were consulted without time constraints in July 2022. Search updates were made in October 2023 according to the strategy used in the databases with Boolean operators linking terms and keywords. Adjustments were applied to the search scheme according to the characteristics of each database ( Table 1). Hand searches were also undertaken by cross-checking the reference lists of the included articles. Duplicate references encountered in different databases were removed using the EndNote program (EndNote®, Clarivate Analytics, Toronto, Canada).
Table 1.
Search strategy employed to identify articles in electronic databases.
| PubMed and Web of Science | giant cell granuloma OR Central giant cell granuloma OR giant cell reparative granuloma OR central giant cell lesion OR CGCL OR CGCG AND calcitonin OR denosumab OR Triamcinolone acetonide OR triamcinolone hexacetonide OR Prolia OR "Xgeva" OR AMG 162 OR "Calcitrin" OR Thyrocalcitonin OR Ciba OR "Cinonide" OR "Tricort" OR Azmacort OR Kenacort OR Kenalog OR "Volon" OR Interferon OR Pegasys OR Imatinib mesylate OR Dasatinib OR nilotinib OR Bosutinib OR glivec OR gleevec OR STI571 OR CGP 57148 |
|---|---|
| Embase and Scopus | "giant cell granuloma" OR "central giant cell granuloma" OR "giant cell reparative granuloma" OR "central giant cell lesion" OR CGCL OR CGCG AND calcitonin OR denosumab OR ‘triamcinolone acetonide" OR "triamcinolone hexacetonide" OR prolia OR xgeva OR "AMG 162" OR calcitrin OR thyrocalcitonin OR ciba OR cinonide OR tricort OR azmacort OR kenacort OR kenalog OR volon OR interferon OR pegasys OR "imatinib mesylate" OR dasatinib OR nilotinib OR bosutinib OR glivec OR gleevec OR STI571 OR "CGP 57148" |
| Cochrane Library and Ovid | giant cell granuloma OR central giant cell granuloma OR giant cell reparative granuloma OR central giant cell lesion OR CGCL OR CGCG AND calcitonin OR denosumab OR triamcinolone acetonide OR triamcinolone hexacetonide OR prolia OR xgeva OR AMG 162 OR calcitrin OR thyrocalcitonin OR ciba OR cinonide OR tricort OR azmacort OR kenacort OR kenalog OR volon OR interferon OR pegasys OR imatinib mesylate OR dasatinib OR nilotinib OR bosutinib OR glivec OR gleevec OR STI571 OR CGP 57148 |
-Study selection
The studies were selected by two independent authors (F.A.C. and V.Z.D.) in two phases. In the first phase, the two authors evaluated the studies based on their titles and abstracts, and those that fulfilled the eligibility criteria were included. The full text of the articles without sufficient information in the titles/abstracts was acquired in order to permit the authors to decide whether to include or exclude. In the second phase, the authors evaluated the complete texts and included those that met the eligibility criteria. Disagreements were resolved by a discussion with other authors (E.R.C.R., R.A.M., and A.E.).
-Data collection process and items
Data collection was performed independently by two reviewers and then cross-checked. For each study included, data referring to the surname of the first author, year of publication, study design, country where the study was performed, sample size, age and sex of the participants, anatomical location (maxilla or mandible), time of evolution, symptomatology, lesion size, imaging aspects, previous treatments (e.g., surgical and/or pharmacological interventions), current treatment (i.e., concentration, dose, route of administration, administration interval, time of use), additional treatment, clinical resolution, side effects, recurrence, and follow-up period.
-Classification of CGCG aggressiveness
The cases reported in the included studies were classified according to aggressiveness. For cases in which the authors did not classify the clinical behavior of aggressive and non-aggressive lesions, the criteria adopted by Chuong et al. (1) and Kaban et al. (14) were followed.
-Risk of bias assessment
The Joanna Briggs Institute (University of Adelaide) tools for case reports or case series were employed (15). The articles were appraised by two authors (F.A.C. and A.E.) according to the following parameters: demographic data of the patient’s characteristics, medical history and presentation as a timeline, current clinical condition of the patient, diagnostic tests and evaluation method, treatment provided, information about the post-intervention clinical picture, and identification or list of side effects. For each parameter, the risk of bias of each included article was defined as “yes” (low), “no” (high), or “not applicable”.
-Data analysis
Data were tabulated in Microsoft Office Excel 2019 (Microsoft® software, Redmond, WA, USA) and analyzed descriptively. The MedCalc software (MedCalc software bvba, Ostend, Flander, Belgium) was used to construct the Kaplan-Meier survival curves regarding total clinical resolution and recurrences.
-Protocol and registration
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (16). A protocol was drafted and registered with the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/prospero/) under registration number CRD42021266588.
Results
-Study selection
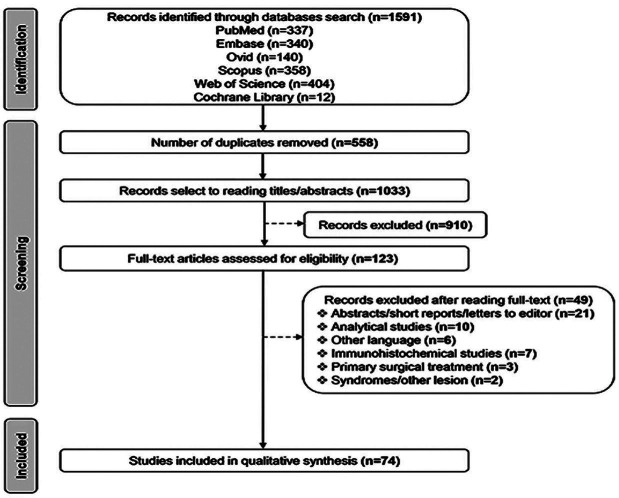
The electronic searches yielded 1591 articles. After removing duplicates, 1033 articles remained. After a comprehensive evaluation of titles and abstracts, 143 studies were eligible, 69 of which were excluded after reading the full text. Subsequently, 74 studies with a total sample of 205 cases of CGCG of the jaws were included for qualitative analysis. A flowchart depicting the search of articles and the selection process is shown in Figure 1.
Figure 1.
Flowchart showcasing the screening procedure.
-Study characteristics
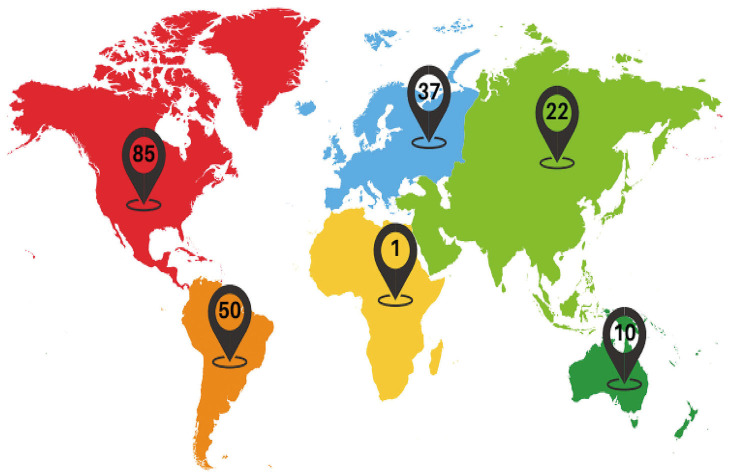
Of the 74 studies included, 57 were case reports (17-73) and 17 were case series (74-90). Case reports were published between 1998 and 2023, while case series were published between 1993 and 2023. The smallest case series comprised three individuals and the largest 45 individuals. North America was the continent with the highest number of cases published in the literature (Fig. 2).
Figure 2.
Global distribution of central giant cell granulomas of the jaws managed with pharmacological therapy.
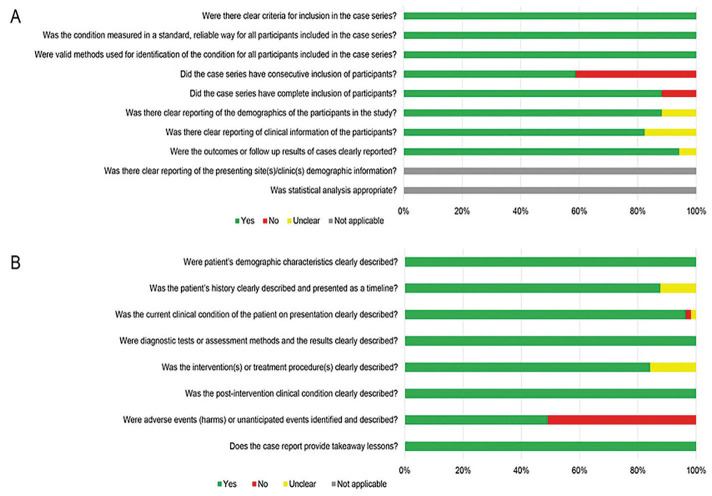
-Risk of bias of studies
The studies were classified as having low or high risk of bias. A high risk of bias was detected in one of the 17 case series ( Table 2) and in 11 of the 57 case reports ( Table 3). Figure 3 showcases a graphical representation of the results from the risk of bias assessment.
Table 2.
Joanna Briggs Institute (JBI) critical appraisal checklist for case series.
| Study | Items | Risk of bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | ||
| Allon et al. (74) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Low |
| Borges et al. (75) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NA | NA | Low |
| Bredell et al. (76) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NA | NA | Low |
| Carlos & Sedano (77) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Low |
| Chandna et al. (78) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Low |
| Choe et al. (79) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Low |
| de Lange et al. (80) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NA | NA | Low |
| Dolanmaz et al. (81) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NA | NA | Low |
| Harris (82) | Yes | Yes | Yes | No | No | Yes | Yes | Yes | NA | NA | High |
| Kim et al. (83) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NA | NA | Low |
| Nogueira et al. (56) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | NA | NA | Low |
| Niedzielska et al. (85) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Low |
| Nogueira et al. (86) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | NA | NA | Low |
| Pogrel (87) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NA | NA | Low |
| Rhou et al. (88) | Yes | Yes | Yes | Yes | No | Unclear | Yes | Unclear | NA | NA | High |
| Schreuder et al. (89) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | Low |
| Vanderniet et al. (90) | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | NA | Yes | Low |
Note: NA, not applicable. Q1: Were there clear criteria for inclusion in the case series? Q2: Was the condition measured in a standard, reliable way for all participants included in the case series? Q3: Were valid methods used for identification of the condition for all participants included in the case series? Q4: Did the case series have consecutive inclusion of participants? Q5: Did the case series have complete inclusion of participants? Q6: Was there clear reporting of the demographics of the participants in the study? Q7: Was there clear reporting of clinical information of the participants? Q8: Were the outcomes or follow up results of cases clearly reported? Q9: Was there clear reporting of the presenting site(s)/clinic(s) demographic information? Q10: Was statistical analysis appropriate?
Table 3.
Joanna Briggs Institute (JBI) critical appraisal checklist for case report.
| Study | Items | Risk of Bias | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | ||
| Adornato et al. (17) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Al-Ahmad et al. (18) | Yes | Yes | Yes | Yes | Unclear | Yes | No | Yes | High |
| Al-Jandan (19) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Al-Layla & Mahazta (20) | Yes | Yes | Yes | Yes | Unclear | Yes | No | Yes | High |
| Aoki et al. (21) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Aurora et al. (22) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Baker et al. (23) | Yes | Yes | Yes | Yes | Unclear | Yes | No | Yes | High |
| Bayar & Ak (24) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Cavalcante et al. (25) | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | High |
| Chien et al. (26) | Yes | Unclear | Yes | Yes | Unclear | Yes | Yes | Yes | Low |
| Choi & Kraut (27) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Comert et al. (28) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| da Rosa et al. (29) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| da Silva et al. (30) | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | High |
| da Silva Sampieri et al. (31) | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| de Arruda et al. (32) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| de Lange et al. (33) | Yes | Yes | Yes | Yes | Unclear | Yes | No | Yes | High |
| de Mendonça et al. (34) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| de Oliveira et al. (35) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| de Oliveira et al. (36) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| El Hadidi et al. (37) | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Low |
| Fernandes Gonçalves et al. (38) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Ferretti & Muthray (39) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Fonseca et al. (40) | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | High |
| Goldman et al. (41) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Goyal et al. (42) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Gupta et al. (43) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Jerkins et al. (44) | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Low |
| Joshi et al. (45) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Khafif et al. (46) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Kurtz et al. (47) | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | High |
| Lietman & Levine (48) | Yes | Yes | No | Yes | Unclear | Yes | No | Yes | High |
| Mariz et al. (49) | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Matos et al. (50) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Mohanty et al. (51) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Moura et al. (52) | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Low |
| Mukdad et al. (53) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Naidu et al. (54) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Nilesh et al. (55) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Maia Nogueira et al. (84) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| O'Connell et al. (58) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| O'Connell et al. (57) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| O'Regan et al. (59) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Pogrel et al. (60) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Rachmiel et al. (61) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Rajeevan et al. (62) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Romero et al. (63) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Schreuder et al. (64) | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Low |
| Schütz et al. (65) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Sezer et al. (66) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Shirani et al. (67) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Stagner et al. (68) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Tallent et al. (69) | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Low |
| Tarsitano et al. (70) | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | High |
| Toferer et al. (71) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Wendt et al. (72) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Yazici et al. (73) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
Note: Q1: Were patient’s demographic characteristics clearly described? Q2. Was the patient’s history clearly described and presented as a timeline? Q3. Was the current clinical condition of the patient on presentation clearly described? Q4. Were diagnostic tests or assessment methods and the results clearly described? Q5. Was the intervention(s) or treatment procedure(s) clearly described? Q6. Was the post-intervention clinical condition clearly described? Q7. Were adverse events (harms) or unanticipated events identified and described? Q8. Does the case report provide takeaway lessons?
Figure 3.
Graphical illustration of the risk of bias appraisal.
-Clinicodemographic data
The mean age of individuals affected by CGCG was 18.1 ± 12.3 years. Women (61.0%) were more affected than men (39.0%). Most cases occurred in the mandible (72.2%) and in the anterior portion (44.4%), with a 59.5% prevalence of lesions with aggressive behavior. Pain and swelling were reported in 7.8% and 36.1% of cases, respectively. Detailed information regarding clinicodemographic data is provided in Table 4.
Table 4.
Clinicopathological data of individuals with central giant cell granulomas of the jaws managed with pharmacological therapy retrieved in the present systematic review.
| Variables | n (%) |
|---|---|
| Age | |
| Mean ± SD (range) | 18.1 ± 12.3 (0–80) |
| 0-9 years | 49 (23.9) |
| 10-19 years | 85 (41.5) |
| ≥20 years | 71 (34.6) |
| Sex | |
| Female | 125 (61.0) |
| Male | 80 (39.0) |
| Swelling | |
| Reported | 74 (36.1) |
| Not reported | 131 (63.9) |
| Pain | |
| Reported | 16 (7.8) |
| Not reported | 189 (92.2) |
| Other signs/symptoms | |
| Tooth mobility | 10 (4.8) |
| Tooth displacement | 8 (3.9) |
| Paresthesia | 3 (1.5) |
| Cortical perforation | 3 (1.4) |
| Evolution time | |
| Reported | 62 (21.7) |
| Not reported | 143 (78.3) |
| Mean ± SD (median; range) | 7.5 ± 8.6 (5.0; 0.2–48.0) months |
| Lesion behavior | |
| Aggressive | 122 (59.5) |
| Non-aggressive | 83 (40.5) |
| Radiographic features | |
| Radiolucent | 96 (46.8) |
| Radiopaque | – |
| Mixed | 7 (3.4) |
| Not reported | 102 (49.8) |
| Anatomical location | |
| Mandible | 148 (72.2) |
| Maxilla | 57 (27.8) |
| Topography | |
| Anterior | 91 (44.4) |
| Posterior | 85 (41.5) |
| Anterior and posterior | 1 (0.5) |
| Not reported | 28 (13.6) |
| Previous surgical treatment | |
| None | 137 (66.8) |
| Curettage/enucleation | 58 (28.3) |
| Recontouring | 1 (0.5) |
| Resection | 2 (1.0) |
| Not reported | 7 (3.4) |
| Previous pharmacological therapy | |
| None | 196 (95.6) |
| Triamcinolone | 7 (3.4) |
| Calcitonin | 1 (0.5) |
| Not reported | 1 (0.5) |
| Current pharmacological therapy | |
| Triamcinolone | 77 (37.5) |
| Interferon | 50 (24.4) |
| Calcitonin | 30 (14.7) |
| Denosumab | 25 (12.2) |
| Combination of drugs | 15 (7.3) |
| Other | 8 (3.9) |
| Route of administration | |
| Subcutaneous | 93 (45.4) |
| Intralesional | 80 (39.0) |
| Nasal | 13 (6.3) |
| Intralesional + subcutaneous | 11 (5.4) |
| Intralesional + nasal | 5 (2.4) |
| Intralesional + nasal + oral | 1 (0.5) |
| Subcutaneous + nasal | 2 (1.0) |
| Adverse effects | |
| Yes | 20 (9.8) |
| No | 185 (90.2) |
| Surgical procedure after pharmacological therapy | |
| None | 157 (76.6) |
| Curettage/enucleation | 39 (19.0) |
| Resection | 7 (3.4) |
| Not reported | 2 (1.0) |
| Clinical/image resolution | |
| Total | 158 (77.1) |
| Partial | 47 (22.9) |
| Follow-up period - mean ± SD, (median; range) | 46.9 ± 34.8, (36.6; 3.6–201.6) months |
| Recurrence | |
| Yes | 14 (6.8) |
| No | 190 (92.7) |
| Not reported | 1 (0.5) |
| Follow-up period - mean ± SD, (median; range) | 45.3 ± 34.7, (36.0; 3.0–201.6) months |
| Osteoplasty | |
| Yes | 16 (7.8) |
| No | 189 (92.2) |
Note: SD, standard deviation.
-Treatment and pharmacological approach
Surgical treatments prior to current pharmacological therapy were performed in 29.8% of cases, with conservative surgery (curettage and/or enucleation) being the most common (28.3%). Pharmacological therapy was previously used in the current treatment in 3.9% of cases. Drug combinations were administered to 15 (7.3%) individuals. The most common route of administration was subcutaneous (45.4%), followed by the intralesional one (39.0%).
Triamcinolone (concentration range: 10–40 mg) was used exclusively in 77 (37.5%) cases. The total dose range administered was 40–3120 mg. The time of use varied from 0.03 to 20 months. Interferon (15 mcg concentration and 9 MIU/m²) was used exclusively in 50 (24.4%) cases. The total dose range administered was 7020 mcg and 1095 MIU/m². The time of use varied from one to 14 months. Calcitonin (concentration range: 50–200 IU) was used exclusively in 30 (14.7%) cases. The total dose range administered was 74200–602000 IU. The time of use varied from 1.3 to 60 months. Denosumab (concentration range: 60–120 mg) was used exclusively in 25 (12.2%) cases. The total dose range administered was 60–1800 mg. The time of use varied from 0.03 to 12 months. Other drugs such as prednisolone, zoledronic acid, hydrocortisone, alendronate, flucortolone, and solumedrol were also reported.
Drug combinations were reported in 15/205 (7.3%) cases, in which at least two drugs were administered. Of these, 7/29 (24.1%) cases were triamcinolone with denosumab, 4/29 (13.79%) were triamcinolone with calcitonin, and 4/29 (13.79%) were zoledronic acid with interferon. Of the current treatments using drug associations, 8/15 (53.3%) were for aggressive lesions. Adverse effects were reported in 20/205 (9.8%) individuals, the most common being hypercalcemia, nausea, and hypocalcemia.
In 46/205 cases (22.4%) additional surgery was performed after current pharmacological therapy. Conservative surgery (curettage and/or enucleation) was performed in 39/205 (19.0%) cases, followed by aggressive surgical procedures (resection) in 7/205 (3.4%) cases.
Immunohistochemical analysis complementary to pharmacological treatment was performed in 5/205 (2.4%) cases. The markers analyzed were calcitonin and glucocorticoid receptors, RANKL, and CD34.
-Outcomes
Regarding the clinical/image resolution outcome, data of 200 individuals with information about outcome and follow-up were available; 158 exhibited total clinical/imaging resolution and 42 exhibited partial clinical/imaging resolution during the follow-up period. Of a total of 47 lesions that had exhibited partial resolution with current pharmacological therapy, 36/47 (76.5%) were aggressive.
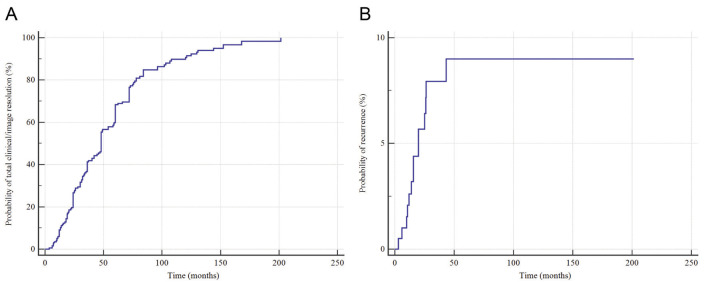
Mean follow-up time was 56.0 months (standard error = 3.2). The probability of total clinical/imaging resolution at 12 months of follow-up was 9.1%. Within the 144-month follow-up, the probability of total clinical/image resolution increased markedly to 95.0% (Fig. 4A).
Figure 4.
Outcomes of central giant cell granulomas of the jaws managed with pharmacological therapy. (A) Probability of lesion resolution and (B) probability of recurrence in relation to follow-up time.
Data from 199 individuals were pooled for the recurrence outcome. Mean follow-up time was 185.2 months (standard error = 4.24). The probability of recurrence at six months of follow-up was 1.0%. Within a 26.4-month follow-up, the probability of recurrence was 7.9% (Fig. 4B). Of the 14 cases with recurrence, six (42.9%) were treated only with interferon and 13 (92.9%) were aggressive.
Discussion
The objective of the present systematic review was to gather relevant data in order to assist clinicians and surgeons in choosing the best protocols for treating patients with CGCG of the jaws. In line with previous literature, CGCG occurred more frequently in young women and the mandible was reported as the most commonly affected site. Pain and swelling were the most frequently reported signs and symptoms associated with this condition (7,10). Most cases of CGCG examined in this systematic review were classified as having an aggressive behavior. This classification suggests that these cases may have been more destructive or fast-growing in nature, with consequent implications for treatment decisions (1,14). When considered aggressive, CGCG requires a more extensive surgical procedure and tends to have a greater chance of recurrence when compared to non-aggressive lesions (22.8% vs. 7.8%, respectively) (9). Multinucleated giant cells are a characteristic feature of CGCG and are believed to play a role in the pathogenesis and aggressiveness of the lesion, particularly with osteoclast-like activity (6). The specific mechanisms by which multinucleated giant cells contribute to CGCG aggressiveness are still unknown. The production of cytokines and growth factors related to inflammation and the recruitment of immune cells have been linked to the aggressive nature of these lesions (91).
Intralesional injections of triamcinolone acetonide were employed at varying concentrations (10, 20 and 40 mg) to manage CGCG cases. The concentration of 40 mg seems to have been the most effective in achieving a total resolution of the condition (92). Triamcinolone acetonide is a synthetic corticosteroid drug with anti-inflammatory and immunosuppressive properties. The impact of triamcinolone acetonide on bone varies depending on dosage, duration of use, and individual susceptibility (56,86). Notably, a recent study found no significant differences in glucocorticoid receptor immunoexpression in mononuclear stromal cells or multinucleated giant cells with respect to the aggressiveness of CGCG or its response to clinical treatment with triamcinolone (93). The advantages of this therapy are its less invasive nature, the likely lower cost for the patient, a lower risk, and the ability to treat the lesion surgically in the future, if necessary (56). Conversely, some authors have emphasized that high or prolonged doses of this corticosteroid have been associated with an increased risk of osteoporosis, fractures, and even avascular necrosis of long bones (5,56,86).
Interferon and calcitonin were introduced as alternative pharmacological therapies for the management of CGCG in the 1990s (11,14,33,80). Interferon therapy has been investigated as a potential inhibitor of giant cell growth and activity in CGCG due to its immunomodulatory effects, particularly in controlling lesion progression (14,23,33,41). Calcitonin is a hormone that regulates calcium and bone metabolism and has been used in the treatment of diseases involving bone resorption, such as osteoporosis (94). In the case of CGCG, calcitonin has been considered a treatment option because it might help inhibit bone resorption, which is a hallmark of CGCG (5). The present systematic review identified that the use of interferon varied greatly in terms of the concentration and total dose applied. Furthermore, most cases received interferon before lesion enucleation. Of the 50 cases exclusively treated with interferon, 8 (16.0%) showed partial resolution and required additional treatment (5,58,70). On the other hand, 83.3% of 30 cases treated with calcitonin exhibited complete resolution of the lesion. In this line, low recurrence rates were reported when compared to curettage (9.1% vs. 53.8%, respectively) in aggressive cases of CGCG (95). However, the disadvantage of calcitonin therapy is that it may have to be administered for more than two years and the route is usually nasal (5,95).
Recently, denosumab was approved for the treatment of common metabolic bone diseases and has been used off-label in rare metabolic bone diseases (96). Denosumab is a monoclonal antibody against the receptor activator of nuclear factor-κB (RANK) ligand (RANKL) which substantially suppresses osteoclast formation and activity (96). So far, about 25 patients with CGCG have been treated with denosumab. Studies have documented a reduction in the size of the lesion, decreased pain, and stabilization of the affected bone (90). The average complete treatment time was 10.5 months, a relatively short period when compared to other therapies that used just one drug. However, it is important to highlight that the choice of denosumab for CGCG depends particularly on the location and aggressiveness of the lesion, in addition to the healthcare provider’s experience (96). This is because denosumab-related adverse effects such as osteonecrosis, hypercalcemia, and increased serum urea and creatinine levels have been reported elsewhere. Another important factor to consider is the high cost of this drug (90,96).
The use of pharmacological therapy for the management of CGCG is a valuable strategy, both as an adjunct to surgical treatment and as the primary treatment in certain cases (5,7). The data provided indicated that overall recurrence after treatment occurred in almost 7% of cases. Recurrence is a significant concern in CGCG (9), and this relatively low recurrence rate suggests that a combination of surgical and pharmacological approaches has been effective in managing the condition. Nonetheless, a significant proportion of individuals (i.e., 53.3%) treated with drug association had lesions classified as aggressive. This suggests that, in aggressive cases, the combination of pharmacological therapy with surgery may be a preferred approach addressing the complexity of the condition and reducing the risk of recurrence. It is also necessary to consider possible side effects (e.g., hypercalcemia, hypocalcemia, nausea, and osteonecrosis), patient age, and treatment costs. Additionally, ossification caused by the use of corticosteroids, especially triamcinolone, can also be considered a side effect, potentially leading to the need for subsequent osteoplasty (56,86).
Since most of the studies available in the literature were cases reports, certain limitations and heterogeneity of the present review should be acknowledged such as the fact that different dosages and concentrations of medication were employed and that detailed information about the treatment period, image characteristics at follow-up, and the measurement of the size of the lesion were not standardized.
In summary, pharmacological therapy is considered to be a viable treatment option for CGCG of the jaws, with a relatively high rate of complete resolution. However, healthcare providers should closely monitor patients during treatment to ensure the best outcomes and to minimize any potential adverse effects. Further controlled studies are needed to refine drug selection and protocols considering the aggressiveness of the lesion.
Acknowledgement
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001), Brazil. L.G.A. (#305544/2022-5), T.A.S. (#305077/2021-0) and R.A.M. (#312830/2022-0; #407364/2021-8) are research fellows of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). J.A.A.A. is the recipient of a fellowship granted by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; E-26/200.331/2024), Brazil. Mrs. E. Greene provided English editing of the manuscript.
Institutional Review Board Statement
This survey did not require the approval of the research ethics committee.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author.
Author Contributions
FAC, ERC, RAM, and AE designed the study. FAC, VZD, and LGA participated in study design and collected the data. FAC and JAAA took the lead in writing the manuscript. IVBC, SBCT, and TAS participated in study design and critically edited the manuscript. FAC, JAAA, RAM, and AE revised critically the study for important intellectual content. All authors gave final approval of the version to be published.
Funding
All authors declare no funding.
Conflict of interest
The authors have no potential conflicts of interest to declare.
References
- 1.Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg. 1986;44:708–13. doi: 10.1016/0278-2391(86)90040-6. [DOI] [PubMed] [Google Scholar]
- 2.Nosé V, Lazar AJ. Update from the 5th edition of the World Health Organization classification of head and neck tumors: familial tumor syndromes. Head Neck Pathol. 2022;16:143–57. doi: 10.1007/s12105-022-01414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe HL. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol. 1953;6:159–75. doi: 10.1016/0030-4220(53)90151-0. [DOI] [PubMed] [Google Scholar]
- 4.Alsufyani NA, Aldosary RM, Alrasheed RS, Alsaif RF. A systematic review of the clinical and radiographic features of hybrid central giant cell granuloma lesions of the jaws. Acta Odontol Scand. 2021;79:124–31. doi: 10.1080/00016357.2020.1797160. [DOI] [PubMed] [Google Scholar]
- 5.Schreuder WH, van den Berg H, Westermann AM, Peacock ZS, de Lange J. Pharmacological and surgical therapy for the central giant cell granuloma: A long-term retrospective cohort study. J Craniomaxillofac Surg. 2017;45:232–43. doi: 10.1016/j.jcms.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Miguita L, de Souza JC, Bastos VC, Pereira NB, de Freitas RAB, Guimarães LM. Central giant cell granulomas of the jaws stromal cells harbour mutations and have osteogenic differentiation capacity, in vivo and in vitro. J Oral Pathol Med. 2022;51:206–16. doi: 10.1111/jop.13274. [DOI] [PubMed] [Google Scholar]
- 7.Chrcanovic BR, Gomes CC, Gomez RS. Central giant cell lesion of the jaws: an updated analysis of 2270 cases reported in the literature. J Oral Pathol Med. 2018;47:731–9. doi: 10.1111/jop.12730. [DOI] [PubMed] [Google Scholar]
- 8.Abdo EN, Alves LC, Rodrigues AS, Mesquita RA, Gomez RS. Treatment of a central giant cell granuloma with intralesional corticosteroid. Br J Oral Maxillofac Surg. 2005;43:74–6. doi: 10.1016/j.bjoms.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Chrcanovic BR, Gomes CC, Dos Santos TR, Abreu MHNG, Gomez RS. Clinical factors associated with the recurrence of central giant cell lesions. J Oral Pathol Med. 2019;48:799–802. doi: 10.1111/jop.12937. [DOI] [PubMed] [Google Scholar]
- 10.Camarini C, de Souza Tolentino E. Non-surgical treatment as an alternative for the management of central giant cell granuloma: a systematic review. Clin Oral Investig. 2022;26:2111–32. doi: 10.1007/s00784-021-04193-z. [DOI] [PubMed] [Google Scholar]
- 11.de Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:603–15. doi: 10.1016/j.tripleo.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Suárez-Roa Mde L, Reveiz L, Ruíz-Godoy Rivera LM, Asbun-Bojalil J, Dávila-Serapio JE, Menjívar-Rubio AH. Interventions for central giant cell granuloma (CGCG) of the jaws. Cochrane Database Syst Rev. 2009;2009:CD007404. doi: 10.1002/14651858.CD007404.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7:264. doi: 10.1186/1756-0500-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60:1103–11. doi: 10.1053/joms.2002.34975. [DOI] [PubMed] [Google Scholar]
- 15.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 17.Adornato MC, Paticoff KA. Intralesional corticosteroid injection for treatment of central giant-cell granuloma. J Am Dent Assoc. 2001;132:186–90. doi: 10.14219/jada.archive.2001.0153. [DOI] [PubMed] [Google Scholar]
- 18.Al-Ahmad HT, Anabtawi M, Salfiti F, Eid RA. Combination of surgery followed by intralesional steroids in treatment of aggressive mandibular giant cell granuloma: a case report. Jordan Med J. 2009;43:231–7. [Google Scholar]
- 19.Al-Jandan B. Combined management of large aggressive central giant cell granuloma of the mandible: a case report. Saudi J Dent Res. 2015;6:157–60. [Google Scholar]
- 20.Al-Layla AM, Mahazfa T. Giant cell granuloma of the maxillary sinus; a case report. Jordan Med J. 2013;47:260–5. [Google Scholar]
- 21.Aoki T, Karakida K, Sakamoto H, Yamazaki H, Otsuru M, Sasaki M. Successful treatment by intralesional steroid injection in management of central giant cell granuloma of the jaw-report of two cases. J Oral Maxillofac Surg Med Pathol. 2012;24:213–7. [Google Scholar]
- 22.Aurora JK, Chauhan H, Loomba K, Potlia I. Novel regimen of combined intralesional triamcinolone and salmon calcitonin nasal spray to treat a large central giant cell granuloma. Natl J Maxillofac Surg. 2022;13:S131–5. doi: 10.4103/njms.NJMS_185_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker SB, Parikh PM, Rhodes DN, Abu-Ghosh A, Shad AT. Aggressive central giant cell lesion of the maxilla: surgical management and the use of adjuvant interferon alfa-2a. Plast Reconstr Surg. 2008;122:77–9. doi: 10.1097/PRS.0b013e31817d5f3d. [DOI] [PubMed] [Google Scholar]
- 24.Bayar OF, Ak G. Treatment of giant cell granuloma with intralesional corticosteroid injections: a case report. J Istanb Univ Fac Dent. 2015;49:45–50. doi: 10.17096/jiufd.88120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalcante IL, Barros CCS, Osterne RLV, Cavalcante RB, Nogueira RLM, Medeiros RCT. Conservative therapy for central giant cell lesion: case report. J Bras Patol Med Lab. 2017;53:403–6. [Google Scholar]
- 26.Chien MC, Mascarenhas L, Hammoudeh JA, Venkatramani R. Zoledronic acid for the treatment of children with refractory central giant cell granuloma. J Pediatr Hematol Oncol. 2015;37:e399–401. doi: 10.1097/MPH.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Kraut RA. Management of central giant granuloma of mandible with intralesional triamcinolone injections: a case report. N Y State Dent J. 2013;79:34–6. [PubMed] [Google Scholar]
- 28.Comert E, Turanli M, Ulu S. Oral and intralesional steroid therapy in giant cell granuloma. Acta Otolaryngol. 2006;126:664–6. doi: 10.1080/00016480500468976. [DOI] [PubMed] [Google Scholar]
- 29.da Rosa MRP, de Sá JL, Martins VB, de Oliveira MV. Central giant cells lesion: report of a conservative management. Eur J Dent. 2018;12:305–10. doi: 10.4103/ejd.ejd_402_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva NG, Carreira AS, Pedreira EN, Tuji FM, Ortega KL, de Jesus Viana Pinheiro J. Treatment of central giant cell lesions using bisphosphonates with intralesional corticosteroid injections. Head Face Med. 2012;8:23. doi: 10.1186/1746-160X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva Sampieri MB, Yaedú RY, Santos PS, Gonçales ES, Santa'ana E, Consolaro A. Central giant cell granuloma: treatment with calcitonin, triamcinolone acetonide, and a cystic finding 3 years and 6 months after the primary treatment. Oral Maxillofac Surg. 2013;17:229–34. doi: 10.1007/s10006-012-0370-5. [DOI] [PubMed] [Google Scholar]
- 32.de Arruda JAA, Martins AFL, Abreu LG, Mesquita RA, von Zeidler SV, Estrela C. Central giant cell granuloma of the maxilla: long-term follow-up of a patient treated with an adjuvant corticosteroid. Spec Care Dentist. 2021;41:399–407. doi: 10.1111/scd.12569. [DOI] [PubMed] [Google Scholar]
- 33.de Lange J, van den Akker HP, van den Berg H, Richel DJ, Gortzak RA. Limited regression of central giant cell granuloma by interferon alpha after failed calcitonin therapy: a report of 2 cases. Int J Oral Maxillofac Surg. 2006;35:865–9. doi: 10.1016/j.ijom.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 34.de Mendonça RP, Mitre GP, Real FH, da Silva Kataoka MS, de Melo Alves Júnior S, Vianna P. Central giant cell granuloma treated with intralesional corticosteroid injections and bisphosphonates: a long-term follow-up case study. Head Neck Pathol. 2020;14:497–502. doi: 10.1007/s12105-019-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira JP, Olivete F, de Oliveira ND, Giovanini AF, Zielak JC, Klüppel L. Combination therapies for the treatment of recurrent central giant cell lesion in the maxilla: a case report. J Med Case Rep. 2017;11:74. doi: 10.1186/s13256-016-1173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira LJ, Lehman LFC, Gomez RS, Castro WH. Treatment of central giant cell lesions. Oral Surg. 2016;9:52–7. [Google Scholar]
- 37.El Hadidi YN, Ghanem AA, Helmy I. Injection of steroids intralesional in central giant cell granuloma cases (giant cell tumor): is it free of systemic complications or not? A case report. Int J Surg Case Rep. 2015;8C:166–70. doi: 10.1016/j.ijscr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandes Gonçalves IM, Barbosa Luna AH, de Melo DP, Weege Nonaka CF, Alves PM. Pharmacological therapy for treatment of recurrent central giant cell lesion in a child. J Dent Child (Chic) 2019;86:113–7. [PubMed] [Google Scholar]
- 39.Ferretti C, Muthray E. Management of central giant cell granuloma of mandible using intralesional corticosteroids: case report and review of literature. J Oral Maxillofac Surg. 2011;69:2824–9. doi: 10.1016/j.joms.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca FP, Ribeiro AC, Santos-Silva AR, Vargas PA, Lopes MA. Fine needle aspiration cytology and intralesional steroid injection in a central giant cell granuloma affecting the gingiva: a new clinical approach. Braz Dent J. 2013;24:420–7. doi: 10.1590/0103-6440201302196. [DOI] [PubMed] [Google Scholar]
- 41.Goldman KE, Marshall MK, Alessandrini E, Bernstein ML. Complications of alpha-interferon therapy for aggressive central giant cell lesion of the maxilla. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:285–91. doi: 10.1016/j.tripleo.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Goyal P, Narula R, Bansal S, Bansal S, Garg P. Conservative nonsurgical treatment of mandibular central giant cell granuloma in an adolescent: a case report. Pediatr Dent J. 2014;24:58–62. [Google Scholar]
- 43.Gupta B, Stanton N, Coleman H, White C, Singh J. A novel approach to the management of a central giant cell granuloma with denosumab: a case report and review of current treatments. J Craniomaxillofac Surg. 2015;43:1127–32. doi: 10.1016/j.jcms.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Jerkins D, Malotky M, Miremadi R, Dole M. Central giant cell granuloma of the mandible requiring multiple treatment modalities: a case report. J Oral Maxillofac Surg. 2016;74:1596–607. doi: 10.1016/j.joms.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Joshi S, Koranne V, Pawar S, Pawar P, Lakhani K, Salema H. Conservative management of central giant cell granuloma - A case report. J Indian Acad Oral Med Radiol. 2023;35:141–3. [Google Scholar]
- 46.Khafif A, Krempl G, Medina JE. Treatment of giant cell granuloma of the maxilla with intralesional injection of steroids. Head Neck. 2000;22:822–5. doi: 10.1002/1097-0347(200012)22:8<822::aid-hed12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Kurtz M, Mesa M, Alberto P. Treatment of a central giant cell lesion of the mandible with intralesional glucocorticosteroids. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:636–7. doi: 10.1067/moe.2001.115721. [DOI] [PubMed] [Google Scholar]
- 48.Lietman SA, Levine MA. Resolution of giant cell granuloma after treatment with calcitonin. Oral Oncology Extra. 2005;41:125–7. [Google Scholar]
- 49.Mariz BALA, Migliorati CA, Alves FA, Penteado FM, Carvalho NP Filho, Santos-Silva AR. Successful denosumab treatment for central giant cell granuloma in a 9-year-old child. Spec Care Dentist. 2021;41:519–25. doi: 10.1111/scd.12588. [DOI] [PubMed] [Google Scholar]
- 50.Matos FR, Sarmento DJS, Neto AC, Miguel MCC, da Silveira ÉJD. Central giant cell lesion in pediatric patient: case report and literature review of 33 cases. Int J Clin Dent. 2016;6:33–42. [Google Scholar]
- 51.Mohanty S, Jhamb A. Central giant cell lesion of mandible managed by intralesional triamcinolone injections. A report of two cases and literature review. Med Oral Patol Oral Cir Bucal. 2009;14:E98–102. [PubMed] [Google Scholar]
- 52.Moura LB, Tarquinio SBC, Gomes APN, Schinestsck AR, Torriani MA. Modified approach to central giant cell lesion. J Clin Pediatr Dent. 2018;42:292–4. doi: 10.17796/1053-4628-42.4.9. [DOI] [PubMed] [Google Scholar]
- 53.Mukdad M, Barut O, Sjöström M. Intralesional corticosteroid injections as first option for management of giant cell lesion of the lower jaw in a 56-year-old patient: a case report and brief literature review. Oral Maxillofac Surg Cases. 2022;8:100283. [Google Scholar]
- 54.Naidu A, Malmquist MP, Denham CA, Schow SR. Management of central giant cell granuloma with subcutaneous denosumab therapy. J Oral Maxillofac Surg. 2014;72:2469–84. doi: 10.1016/j.joms.2014.06.456. [DOI] [PubMed] [Google Scholar]
- 55.Nilesh K, Dadhich A, Patil R. Management of recurrent central giant cell granuloma of mandible using intralesional corticosteroid with long-term follow-up. BMJ Case Rep. 2020;13:e237200. doi: 10.1136/bcr-2020-237200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nogueira RL, Teixeira RC, Cavalcante RB, Ribeiro RA, Rabenhosrt SH. Intralesional injection of triamcinolone hexacetonide as an alternative treatment for central giant-cell granuloma in 21 cases. Int J Oral Maxillofac Surg. 2010;39:1204–10. doi: 10.1016/j.ijom.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 57.O'Connell JE, Bowe C, Murphy C, Toner M, Kearns GJ. Aggressive giant cell lesion of the jaws: a review of management options and report of a mandibular lesion treated with denosumab. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e191–8. doi: 10.1016/j.oooo.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 58.O'Connell JE, Kearns GJ. Aggressive giant cell granuloma of the jaws treated with interferon alpha: a report of two cases. Ir J Med Sci. 2013;182:163–70. doi: 10.1007/s11845-012-0858-x. [DOI] [PubMed] [Google Scholar]
- 59.O'Regan EM, Gibb DH, Odell EW. Rapid growth of giant cell granuloma in pregnancy treated with calcitonin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:532–8. doi: 10.1067/moe.2001.119246. [DOI] [PubMed] [Google Scholar]
- 60.Pogrel MA, Regezi JA, Harris ST, Goldring SR. Calcitonin treatment for central giant cell granulomas of the mandible: report of two cases. J Oral Maxillofac Surg. 1999;57:848–53. doi: 10.1016/s0278-2391(99)90828-5. [DOI] [PubMed] [Google Scholar]
- 61.Rachmiel A, Emodi O, Sabo E, Aizenbud D, Peled M. Combined treatment of aggressive central giant cell granuloma in the lower jaw. J Craniomaxillofac Surg. 2012;40:292–7. doi: 10.1016/j.jcms.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Rajeevan NS, Soumithran CS. Intralesional corticosteroid injection for central giant cell granuloma. A case report. Int J Oral Maxillofac Surg. 1998;27:303–4. doi: 10.1016/s0901-5027(05)80620-4. [DOI] [PubMed] [Google Scholar]
- 63.Romero M, Romance A, Garcia-Recuero JI, Fernández Á. Orthopedic and orthodontic treatment in central giant cell granuloma treated with calcitonin. Cleft Palate Craniofac J. 2011;48:519–25. doi: 10.1597/10-091. [DOI] [PubMed] [Google Scholar]
- 64.Schreuder WH, Coumou AW, Kessler PA, de Lange J. Alternative pharmacologic therapy for aggressive central giant cell granuloma: denosumab. J Oral Maxillofac Surg. 2014;72:1301–9. doi: 10.1016/j.joms.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 65.Schütz P, El-Bassuoni KH, Munish J, Hamed HH, Padwa BL. Aggressive central giant cell granuloma of the mandible. J Oral Maxillofac Surg. 2010;68:2537–44. doi: 10.1016/j.joms.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 66.Sezer B, Koyuncu B, Gomel M, Günbay T. Intralesional corticosteroid injection for central giant cell granuloma: a case report and review of the literature. Turk J Pediatr. 2005;47:75–81. [PubMed] [Google Scholar]
- 67.Shirani G, Abbasi AJ, Mohebbi SZ, Shirinbak I. Management of a locally invasive central giant cell granuloma (CGCG) of mandible: report of an extraordinary large case. J Craniomaxillofac Surg. 2011;39:530–3. doi: 10.1016/j.jcms.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 68.Stagner AM, Sajed DP, Nielsen GP, Ebb DH, Faquin WC, Chebib I. Giant cell lesions of the maxillofacial skeleton express RANKL by RNA in situ hybridization regardless of histologic pattern. Am J Surg Pathol. 2019;43:819–26. doi: 10.1097/PAS.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 69.Tallent B, Padilla RJ, McKay C, Foreman AKM, Fan Z, Blatt J. Response of central giant cell granuloma of the jaw to imatinib. J Pediatr Hematol Oncol. 2023;45:278–80. doi: 10.1097/MPH.0000000000002608. [DOI] [PubMed] [Google Scholar]
- 70.Tarsitano A, Del Corso G, Pizzigallo A, Marchetti C. Aggressive central giant cell granuloma of the mandible treated with conservative surgical enucleation and interferon-α-2a: complete remission with long-term follow-up. J Oral Maxillofac Surg. 2015;73:2149–54. doi: 10.1016/j.joms.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 71.Toferer A, Truschnegg A, Merl L, Liegl-Atzwanger B, Zemann W, Beham A. Dilemma in the treatment of a central giant cell granuloma. J Clin Pediatr Dent. 2021;45:337–40. doi: 10.17796/1053-4625-45.5.8. [DOI] [PubMed] [Google Scholar]
- 72.Wendt FP, Torriani MA, Gomes AP, de Araujo LM, Torriani DD. Intralesional corticosteroid injection for central giant cell granuloma: an alternative treatment for children. J Dent Child (Chic) 2009;76:229–32. [PubMed] [Google Scholar]
- 73.Yazici N, Yalçin B, Yilmaz T, Akyüz C, Oguz KK, Arzu Sungur. Surgery and calcitonin therapy in childhood central giant cell granuloma. Int J Pediatr Otorhinolaryngol. 2006;1:297–300. [Google Scholar]
- 74.Allon DM, Anavi Y, Calderon S. Central giant cell lesion of the jaw: nonsurgical treatment with calcitonin nasal spray. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:811–8. doi: 10.1016/j.tripleo.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Borges HO, Machado RA, Vidor MM, Beltrão RG, Heitz C, Filho MS. Calcitonin: a non-invasive giant cells therapy. Int J Pediatr Otorhinolaryngol. 2008;72:959–63. doi: 10.1016/j.ijporl.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 76.Bredell M, Rordorf T, Kroiss S, Rücker M, Zweifel DF, Rostetter C. Denosumab as a treatment alternative for central giant cell granuloma: a long-term retrospective cohort study. J Oral Maxillofac Surg. 2018;76:775–84. doi: 10.1016/j.joms.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Carlos R, Sedano HO. Intralesional corticosteroids as an alternative treatment for central giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:161–6. doi: 10.1067/moe.2002.119971. [DOI] [PubMed] [Google Scholar]
- 78.Chandna P, Srivastava N, Bansal V, Wadhwan V, Dubey P. Peripheral and central giant cell lesions in children: institutional experience at Subharti dental college and hospital. Indian J Med Paediatr Oncol. 2017;38:440–6. doi: 10.4103/ijmpo.ijmpo_17_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choe M, Smith V, Okcu MF, Wulff J, Gruner S, Huisman TAGM. Treatment of central giant cell granuloma in children with denosumab. Pediatr Blood Cancer. 2021;68:e28778. doi: 10.1002/pbc.28778. [DOI] [PubMed] [Google Scholar]
- 80.de Lange J, Rosenberg AJ, van den Akker HP, Koole R, Wirds JJ, van den Berg H. Treatment of central giant cell granuloma of the jaw with calcitonin. Int J Oral Maxillofac Surg. 1999;28:372–6. doi: 10.1034/j.1399-0020.1999.285280513.x. [DOI] [PubMed] [Google Scholar]
- 81.Dolanmaz D, Esen A, Mihmanlı A, Işık K. Management of central giant cell granuloma of the jaws with intralesional steroid injection and review of the literature. Oral Maxillofac Surg. 2016;20:203–9. doi: 10.1007/s10006-015-0530-5. [DOI] [PubMed] [Google Scholar]
- 82.Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg. 1993;31:89–94. doi: 10.1016/0266-4356(93)90168-v. [DOI] [PubMed] [Google Scholar]
- 83.Kim TS, Usera GL, Ruggiero SL, Weinerman SA. Improvement of giant cell lesions of the jaw treated with high and low doses of denosumab: a case series. JBMR Plus. 2017;1:101–6. doi: 10.1002/jbm4.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maia Nogueira RL, Osterne RL, Cavalcante RB, Abreu RT. Surgical treatment, oral rehabilitation, and orthognathic surgery after failure of pharmacologic treatment of central giant cell lesion: a case report. J Oral Maxillofac Surg. 2016;74:2567.e1–2567. doi: 10.1016/j.joms.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 85.Niedzielska I, Bielecki M, Bąk M, Dziuk B, Niedzielski D. Bony canal method of dexamethasone injections in aggressive form of central giant cell granuloma-case series. Medicina (Kaunas) 2023;59:250. doi: 10.3390/medicina59020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nogueira RLM, Osterne RLV, Lima Verde RMB, Azevedo NO, Teixeira RC, Cavalcante RB. Intralesional injection of triamcinolone hexacetonide as an alternative treatment for central giant cell lesions: a prospective study. Br J Oral Maxillofac Surg. 2020;58:e283–9. doi: 10.1016/j.bjoms.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 87.Pogrel MA. Calcitonin therapy for central giant cell granuloma. J Oral Maxillofac Surg. 2003;61:649–53. doi: 10.1053/joms.2003.50129. [DOI] [PubMed] [Google Scholar]
- 88.Rhou YJJ, Wang CJ, Nguyen M, Vanderniet JA, Munns CF, Coleman H. Clinical and radiologic response of central giant cell granuloma to denosumab: a 6-year prospective observational study. Calcif Tissue Int. 2022;110:464–74. doi: 10.1007/s00223-021-00935-z. [DOI] [PubMed] [Google Scholar]
- 89.Schreuder WH, Peacock ZS, Ebb D, Chuang SK, Kaban LB. Adjuvant antiangiogenic treatment for aggressive giant cell lesions of the jaw: a 20-year experience at Massachusetts general hospital. J Oral Maxillofac Surg. 2017;75:105–18. doi: 10.1016/j.joms.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 90.Vanderniet JA, Wall CL, Mullins A, London K, Lim L, Hibbert S. Denosumab for central giant cell granuloma in an Australian tertiary paediatric centre. Bone. 2022;159:116395. doi: 10.1016/j.bone.2022.116395. [DOI] [PubMed] [Google Scholar]
- 91.Elias LS, Costa RF, Carvalho MA, Batista AC, Silva TA, Leles CR. Markers of bone remodeling in neoplastic and bone-related lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:624–31. doi: 10.1016/j.tripleo.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 92.Osterne RL, Araújo PM, de Souza-Carvalho AC, Cavalcante RB, Sant'Ana E, Nongueira RL. Intralesional corticosteroid injections in the treatment of central giant cell lesions of the jaws: a meta-analytic study. Med Oral Patol Oral Cir Bucal. 2013;18:e226–32. doi: 10.4317/medoral.18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Batista Severo ML, Lopes MLDS, Miguel MCDC, Germano AR, Nogueira RLM, Turatti E. Immunoexpression of calcitonin and glucocorticoid receptors in central giant cell lesions of the jaws. J Oral Pathol Med. 2018;47:907–13. doi: 10.1111/jop.12766. [DOI] [PubMed] [Google Scholar]
- 94.Xie J, Guo J, Kanwal Z, Wu M, Lv X, Ibrahim NA. Calcitonin and bone physiology: in vitro, in vivo, and clinical investigations. Int J Endocrinol. 2020;2020:3236828. doi: 10.1155/2020/3236828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tabrizi R, Fardisi S, Zamiri B, Amanpour S, Karagah T. Can calcitonin nasal spray reduce the risk of recurrence of central giant cell granuloma of the jaws? A double-blind clinical trial. Int J Oral Maxillofac Surg. 2016;45:756–9. doi: 10.1016/j.ijom.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 96.Polyzos SA, Makras P, Tournis S, Anastasilakis AD. Off-label uses of denosumab in metabolic bone diseases. Bone. 2019;129:115048. doi: 10.1016/j.bone.2019.115048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author.