Abstract
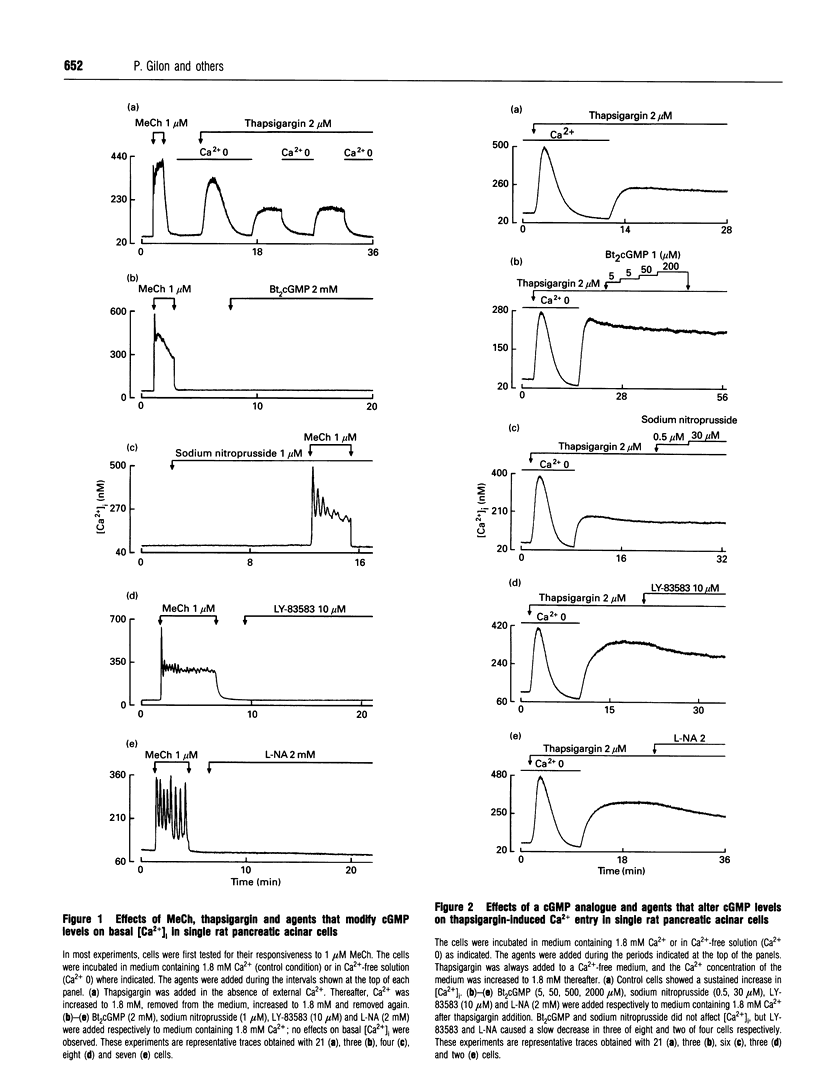
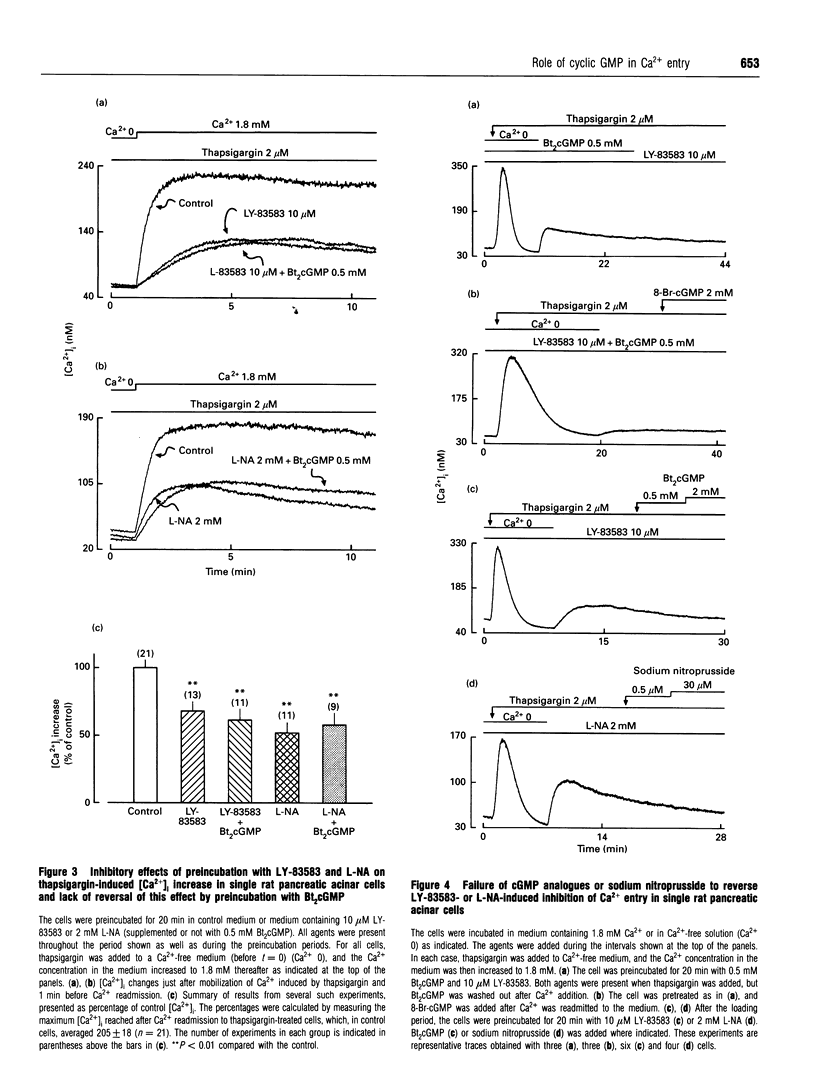
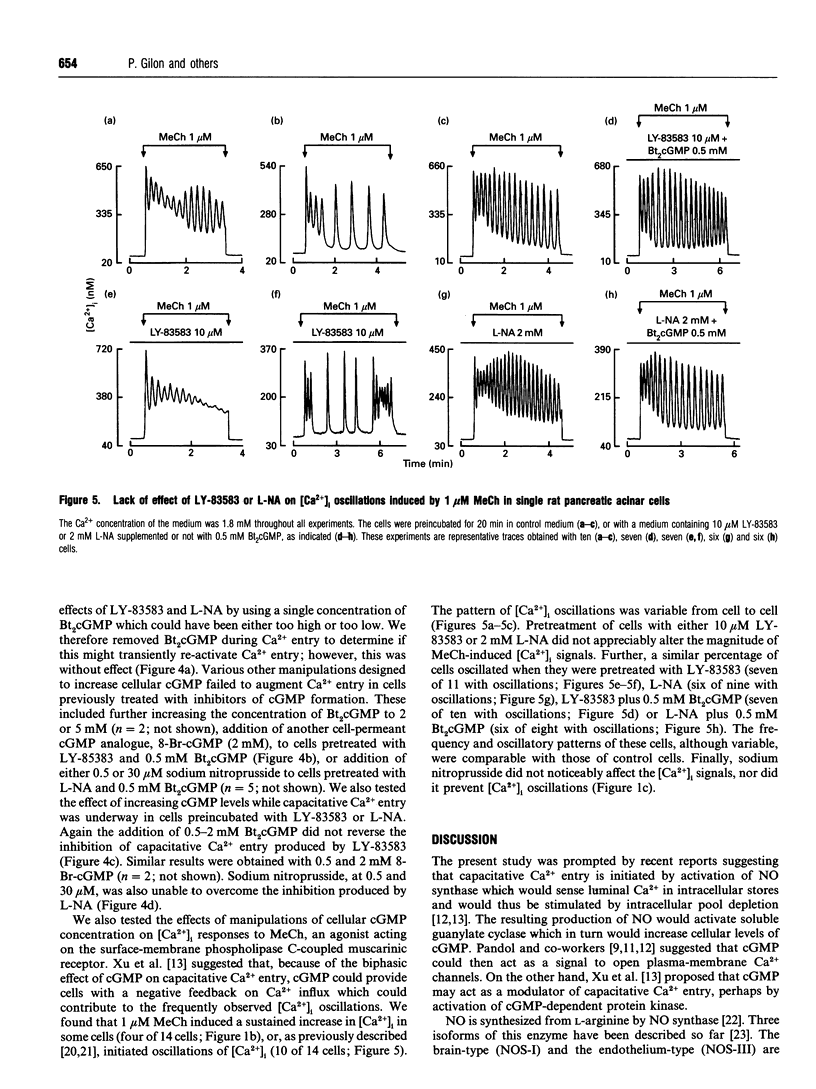
We have investigated the possible roles of cyclic GMP (cGMP) in initiating or regulating capacitiative Ca2+ entry in rat pancreatic acinar cells. In medium containing 1.8 mM external Ca2+, thapsigargin activated Ca2+ entry and slightly but significantly increased intracellular cGMP concentration. This rise in cGMP levels was prevented by pretreating the cells with the guanylate cyclase inhibitor, LY-83583, or by omitting Ca2+ during stimulation by thapsigargin or methacholine. LY-83583 and NG-nitro-L-arginine (L-NA, an inhibitor of NO synthase) both had a small inhibitory effect on Ca2+ entry when they were added after thapsigargin in Ca2(+)-containing medium, and they reduced by 32 and 48% respectively the thapsigargin-induced capacitative Ca2+ entry when added to the cells during a 20 min preincubation period. However, neither dibutyryl cGMP (Bt2cGMP) nor sodium nitroprusside, an NO mimic, affected either basal intracellular Ca2+ concentration [Ca2+]i or thapsigargin-induced capacitative Ca2+ entry. Further, the inhibitory effects observed after preincubation with LY-83583 or L-NA could not be prevented by preincubation with Bt2cGMP, nor could they be reversed by adding Bt2cGMP, 8-bromo-cGMP or sodium nitroprusside acutely after activation of capacitative Ca2+ entry by thapsigargin. Finally, pretreatment of cells with LY-83583 or L-NA did not affect Ca2+ signalling in response to 1 microM methacholine, including the pattern of [Ca2+]i oscillations. In conclusion, in pancreatic acinar cells, the rise in cellular cGMP levels appears to depend on, rather than cause, the increase in [Ca2+]i with agonist stimulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Hoth M., Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993 Oct 5;268(28):20737–20740. [PubMed] [Google Scholar]
- Fleisch J. H., Haisch K. D., Spaethe S. M., Rinkema L. E., Cullinan G. J., Schmidt M. J., Marshall W. S. Pharmacologic analysis of two novel inhibitors of leukotriene (slow reacting substance) release. J Pharmacol Exp Ther. 1984 Jun;229(3):681–689. [PubMed] [Google Scholar]
- Glennon M. C., Bird G. S., Takemura H., Thastrup O., Leslie B. A., Putney J. W., Jr In situ imaging of agonist-sensitive calcium pools in AR4-2J pancreatoma cells. Evidence for an agonist- and inositol 1,4,5-trisphosphate-sensitive calcium pool in or closely associated with the nuclear envelope. J Biol Chem. 1992 Dec 15;267(35):25568–25575. [PubMed] [Google Scholar]
- Gukovskaya A., Pandol S. Nitric oxide production regulates cGMP formation and calcium influx in pancreatic acinar cells. Am J Physiol. 1994 Mar;266(3 Pt 1):G350–G356. doi: 10.1152/ajpgi.1994.266.3.G350. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Jamieson J. D. Increased intracellular cyclic GMP does not correlate with protein discharge from pancreatic acinar cells. Nature. 1979 Jul 26;280(5720):318–320. doi: 10.1038/280318a0. [DOI] [PubMed] [Google Scholar]
- Heemskerk J. W., Vis P., Feijge M. A., Hoyland J., Mason W. T., Sage S. O. Roles of phospholipase C and Ca(2+)-ATPase in calcium responses of single, fibrinogen-bound platelets. J Biol Chem. 1993 Jan 5;268(1):356–363. [PubMed] [Google Scholar]
- Heisler S., Lambert M. Dissociation of cyclic GMP synthesis from cholinergic-stimulated secretion of protein from rat exocrine pancreas. Can J Physiol Pharmacol. 1978 Jun;56(3):395–399. doi: 10.1139/y78-059. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Isomers of inositol trisphosphate in exocrine pancreas. Biochem J. 1986 Sep 15;238(3):825–829. doi: 10.1042/bj2380825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morgan A. J., Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994 Jun 15;300(Pt 3):665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield-Payne M. S. Cyclic GMP mediates the agonist-stimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J Biol Chem. 1990 Aug 5;265(22):12846–12853. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield-Payne M. S. Cyclic GMP regulates free cytosolic calcium in the pancreatic acinar cell. Cell Calcium. 1990 Aug;11(7):477–486. doi: 10.1016/0143-4160(90)90080-e. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Bird G. S., Putney J. W., Jr Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through the actin microfilaments. Biochem J. 1991 Mar 15;274(Pt 3):643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt M. J., Sawyer B. D., Truex L. L., Marshall W. S., Fleisch J. H. LY83583: an agent that lowers intracellular levels of cyclic guanosine 3',5'-monophosphate. J Pharmacol Exp Ther. 1985 Mar;232(3):764–769. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. 1989. Agents Actions. 1994 Dec;43(3-4):187–193. doi: 10.1007/BF01986687. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y., Stuenkel E. L., Williams J. A. Oscillatory mode of calcium signaling in rat pancreatic acinar cells. Am J Physiol. 1990 Jan;258(1 Pt 1):C147–C155. doi: 10.1152/ajpcell.1990.258.1.C147. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Regulatory mechanisms in pancreas and salivary acini. Annu Rev Physiol. 1984;46:361–375. doi: 10.1146/annurev.ph.46.030184.002045. [DOI] [PubMed] [Google Scholar]
- Xu X., Star R. A., Tortorici G., Muallem S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J Biol Chem. 1994 Apr 29;269(17):12645–12653. [PubMed] [Google Scholar]


