Abstract
Background
The interplay between different uses of woody plants remains underexplored, obscuring our understanding of how a plant’s value for one purpose might shield it from other, more harmful uses. This study examines the protection hypothesis by determining if food uses can protect woody plants (trees and shrubs) from wood uses. We approached the hypothesis from two distinct possibilities: (1) the protective effect is proportional to the intensity of a species’ use for food purposes, and (2) the protective effect only targets key species for food purposes.
Methods
The research was conducted in a rural community within “Restinga” vegetation in Northeast Brazil. To identify important food species for both consumption and income (key species) and the collection areas where they naturally occur, we conducted participatory workshops. We then carried out a floristic survey in these areas to identify woody species that coexist with the key species. Voucher specimens were used to create a field herbarium, which, along with photographs served as visual stimuli during the checklist interviews. The interviewees used a five-point Likert scale to evaluate the species in terms of perceived wood quality, perceived availability, and use for food and wood purposes. To test our hypothesis, we used Cumulative Link Mixed Models (CLMMs), with the wood use as the response variable, food use, perceived availability and perceived quality as the explanatory variables and the interviewee as a random effect. We performed the same model replacing food use for key species food use (a binary variable that had value 1 when the information concerned a key species with actual food use, and value 0 when the information did not concern a key species or concerned a key species that was not used for food purposes).
Results
Consistent with our hypothesis, we identified a protective effect of food use on wood use. However, this effect is not directly proportional to the species’ food use, but is confined to plants with considerable domestic food importance. Perceived availability and quality emerged as notable predictors for wood uses.
Conclusion
We advocate for biocultural conservation strategies that enhance the food value of plants for their safeguarding, coupled with measures for non-edible woody species under higher use-pressure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13002-024-00719-3.
Keywords: Conservation through use, Ethnobiology, Traditional management, Wild edible plants, Wood uses
Background
A growing body of research points to the potential effects of chronic anthropogenic disturbances leading to the gradual extinction of local species and alterations in vegetation structure [1, 2]. Among these disturbances, the impact of forest product utilization has been highlighted, demonstrating that while wood use is crucial for local communities, especially in developing countries, it often results in more pronounced impacts on plant populations [3, 4].
While wood uses exerts considerable pressure on plant resources, in some socio-ecological contexts, the extraction of non-timber forest products (NTFP) can also be harmful to plant populations [5, 6]. For example, the intensive harvesting of foliage and bark from tree species [6]. Nevertheless, the extraction of NTFPs, particularly the harvesting of wild fruits, generally has a lesser impact on forest structure and ecosystem functions than other uses [7]. Moreover, the consumption of NTFPs fulfills multiple roles, frequently underpinning rural livelihoods and local economies, aiding food security, fostering trade, and preserving cultural traditions and knowledge [8]. These species are also excellent sources of micro and macronutrients [9, 10].
Hence, some researchers argue that discouraging the commercialization of wild food plants may adversely affect the subsistence and income of local populations, potentially leading to greater reliance on other forest resources with more harmful consequences than food collection itself [11]. One of these harmful consequences is deforestation, caused using wood for firewood and the construction of fences and houses, which require substantial amounts of green wood. Conversely, the commercial value of NTFPs, coupled with the opportunity for income generation, may incentivize conservation efforts among local communities for the forests that supply these resources [12].
Since the 1990s, investigations into the sustainability of food plant use have sought to determine the impact on species populations without conclusively addressing whether such use confers protective benefits. In contrast, research on plant domestication supports the notion that significant food value may lead to conservation practices, such as tolerance, protection, and promotion [13]. Plants with desirable traits may be maintained during deforestation or other disturbances, promoted through distribution and dispersal, and specifically safeguarded against competitors and herbivory [13].
However, the extent to which a plant’s significance for one use can shield it from more destructive applications,1 namely the interaction effects among different utilization types, remains underexamined. This gap hinders our comprehension of protective dynamics in socio-ecological systems and their economic benefits for humans. Such insights are vital for shaping biocultural conservation frameworks that recognize the multifaceted advantages of maintaining cultural practices intertwined with biodiversity.
It is conceivable that certain NTFP uses, including for food, may exert a protective effect against more damaging activities such as wood uses. Although overharvesting of fruits has been shown to affect the regeneration of wild fruit trees adversely [14, 15], food use is typically seen as specialized, with minimal impact on plant populations, whereas wood uses is often deemed generalist, posing broader threats [3].
The classification of plant uses as specialized or generalist may vary depending on the social-ecological context. In the context of several South American communities, specialized uses are defined by a narrower range of suitable plants meeting specific requirements, with the specialization premise reinforced by observations that plant availability exerts little to no influence on such uses [16, 17]. In contrast, generalist uses accommodate a broader spectrum of species, with the most utilized often being the most accessible, as with many wood uses practices [18]. For example, the use of wood for firewood (fuel category) is often classified as generalist because, although factors like durability and high calorific leads to a species’ preference, potentially all woody species would be useful for this purpose [19, 20]. As fuelwood use often requires large amounts of wood and/or frequent collection, it commonly targets the most abundant species [21]. Despite the fact that the uses in the construction (e.g., house and fence construction) and technology (e.g., tools, kitchen utensils) categories often require quality wood and are considered less generalist uses compared to the fuel category [3], they are still more generalist than the food use.
For woody species with edible fruits, some requirements such as nutritional value and flavor [22, 23] are needed, and a much smaller proportion of species meet these requirements. Thus, in general, regardless of how generalist the use of wood is, any tree can meet some wood application (fuel, construction, technology), but not every tree has edible fruits. Although quality may also be an important predictor of plant importance for generalist uses [20], the generalist nature of wood use is supported by studies investigating the apparency (availability) hypothesis, which posits a correlation between environmental availability and species utilization [18, 24, 25]. Therefore, for generalist applications, alternatives may spare certain species for specialized uses, such as food, where fewer species can act as substitutes.
Silva et al. [26] were the first to test the protection hypothesis. They analyzed woody plants from the Caatinga used domestically for medicinal and wood purposes to evaluate whether the importance of medicinal use (specialized use) had an impact on wood use (generalist use). Their findings revealed a modest yet significant medicinal use effect on wood uses, providing supportive evidence for the hypothesis as plants of greater medicinal value saw less wood utilization. Moreover, Silva et al suggested that the protective effect could be more pronounced in species with high medicinal importance.
The use of plants for food is also considered a likely candidate for conferring protective effects against wood uses. Wild food plants are often crucial for providing essential nutrients or for supplementing diets, playing a vital role in ensuring food security and offering economic benefits through the trade of these resources. Given food use’s specialized nature, dietary importance, and economic potential, there is a presumption that communities may prefer to preserve these plants from irreversible harm, such as wood uses. For the latter, alternative species are available due to the generalist nature of wood use.
In this context, we investigate the protection hypothesis from two distinct possibilities. We hypothesize that food uses (specialized) protect plants from wood uses (generalist). We examined: (1) whether the protective effect is proportional to the intensity of a species’ use for food purposes, and (2) if a protective effect only targets key species for food purposes. Here, ‘key species’ refer to wild food plants of high regional importance, which are well-established within the local community for both consumption and income generation.
This study is the inaugural inquiry into the protection hypothesis concerning the protective effect stemming from food use. Moreover, unlike Silva et al. [26], our study incorporates the commercial relevance of woody plants (trees and shrubs), providing income for the local population. Methodologically, we refine hypothesis testing by employing the checklist-interview technique [27] to boost respondent recall, ensuring all associated uses (food and wood) are considered.
Materials and methods
Study area
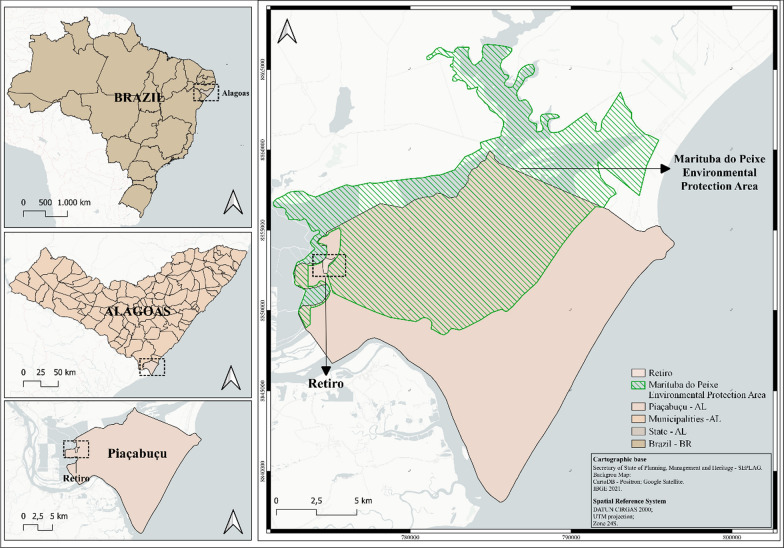
The research was carried out in a rural community within the coastal “Restinga” vegetation of Piaçabuçu, situated on the southern coast of Alagoas state. Piaçabuçu spans an area of 243.686 km2, housing a population of 15,908 individuals [28]. It features a tropical ‘As’ climate in the Köppen and Geiger classification, with an average annual temperature of 25.3 °C and an annual rainfall average of 1283 mm [29]. Notably, the municipality is designated with two sustainable use Conservation Units: the federally instituted Piaçabuçu Environmental Protection Area, established in 1983, and the state-sanctioned Marituba do Peixe Environmental Protection Area, created in 1988.
The Marituba do Peixe Environmental Protection Area spans 18,556 hectares and extends over portions of the Alagoan municipalities of Piaçabuçu (45%), Feliz Deserto (43%), and Penedo (6%) [30]. This area boasts diverse vegetation, including native “Restinga”, “Várzea”, and other forest formations [30]. Within the Indirect Influence Area of Marituba do Peixe Environmental Protection Area lies the village of Retiro (depicted in Fig. 1), which was the focal point for the ethnobiological segment of this study.
Fig. 1.
Geographic Location of the Retiro Community in the Municipality of Piaçabuçu-Alagoas, Brazil
The Retiro community is structured with a residents’ association and a family farmers’ association. It is equipped with a primary healthcare unit and a municipal elementary school. The predominant faith among residents is Christianity, represented by two Catholic and two evangelical churches. Currently, the community comprises approximately 288 families, a decrease of 81 families since before the COVID-19 pandemic, as reported by Gomes et al. [31]. This discrepancy may be partly due to some families not being documented, a requirement for health unit registration.
Retiro was selected for this study due to the local reliance on plant resources for both food and wood. The community’s economy is significantly driven by the extraction and commercialization of wild food plant fruits [31], along with shrimp and fish [32]. Wood resource extraction for personal use and commerce, particularly firewood, charcoal, and materials for fencing, is also prevalent. These resources are marketed through open markets or direct orders in Piaçabuçu and Penedo, whereas wood products are solely distributed by order.
Firewood is the primary cooking fuel in the community, though some households use both cooking gas and firewood. Meals are typically prepared on traditional clay or makeshift brick stoves. Firewood also serves in roasting shrimp and baking cakes from rice straw, a common bait for shrimp in local fishing gear known as “cóvu.”
Architecturally, many “taipa” houses (rammed earth) are present within the community, often serving as dwellings for individuals from other regions staying temporarily in the area.
Ethical and legal aspects of the research
This research project received approval from the Research Ethics Committee by Federal University of Alagoas (UFAL), No. 1998673, securing authorization for studies involving human participants as per the stipulations of National Health Council Resolution 466/2012. Additionally, scientific activities involving the collection and transport of botanical specimens within the Marituba do Peixe Environmental Protection Area were duly registered with Chico Mendes Institute for Biodiversity Conservation/Biodiversity Authorization and Information System (ICMBio/SISBIO), No. 87,112-1.
To ensure ethical compliance, all community members aged 18 or over—to whom the objectives of the research were explained—and who consented to participate, were asked to provide a signature or thumbprint on the Informed Consent Form (ICF), as well as on the image use authorization form.
Data collection
Data collection was carried out in three distinct phases: a participatory workshop, a forest inventory, and checklist-interviews.
1st data collection stage: participatory workshops
Participatory workshops with the residents of the Retiro community aimed to identify significant wild food plant species for consumption and commercial use, as well as their harvesting locations. These workshops were facilitated by local leaders and a researcher from the Laboratory of Biocultural Ecology, Conservation, and Evolution (LECEB), who had previously interviewed community members. The residents were recruited through door-to-door invitations on the day before the workshop was held.
In the inaugural participatory workshop, participants were asked to list the wild plants they harvested for sale or consumption. They recorded the common names on a piece of cardboard, selecting eleven for further discussion. We then asked which of those species were most important for sale and consumption within the community, and they ranked the top five in order of importance. Additionally, the workshop served to note wood resources tied to food plants and their utilization for consumption and commerce within the community.
Thirteen women and three men, ranging in age from 31 to 82, contributed to this first workshop. While all were identified as gatherers, some also engaged in agriculture and fishing. A follow-up workshop sought to enrich this data with contributions from another set of gatherers (n = 17), including eight newcomers. This session, comprising thirteen women and four men from the same age bracket, validated the initial findings regarding species and harvesting sites.
Participants utilized a detailed satellite image from Google Earth to denote areas frequented for food and wood collection. An overlay of transparent acetate allowed them to make corrections directly on the map.
After pinpointing these areas, we selected those most frequented for the harvesting of both food plants and wood, prioritizing locations where the ranked key species were prevalent. Among the listed key species—Myrciaria floribunda (H. West ex Willd.) O. Berg (“cambuí”), Genipa americana L. (“jenipapo”), Psidium guineense Sw. (“araçá”), Spondias mombin L.(“cajá”), and Tamarindus indica L. (“tamarino”)—only the first three were represented in the forest surveys due to their presence in forests. Although S. mombin and T. indica are also key species in the region, the former occurred at the edges of roads or in backyards, while the last is found in fenced area with wire, with reports of increasing cultivation for pulp production by large landowners. Therefore, our study only included three out of the five key species, as well as the species that co-occur with them.
Three sites were thus chosen for the forest inventory: two with a natural predominance of key species and one characterized by a more generalized distribution of various plant species, including those bearing edible fruits.
Gomes et al. [31], the research design we adopted gave precedence to examining species that occurred alongside the key species; consequently, not all food and wood plants were included in our scope. Notably, Schinus terebinthifolia Raddi, while not a key species within Retiro, is a significant commercial species in the community and the most important commercial plant in neighboring areas, such as the Fazenda Paraíso settlement [31].
2nd data collection stage: forest inventory and field herbarium
The research included a forest inventory as part of a larger investigation of our research group into plant resource utilization within the region, although ecological data is not part of this study. For the purposes of this study, the forest inventory was only used to identify and collect species that co-occur with the key species. Without the forest inventory, we would have no baseline for the field herbarium (notebook with exsiccates of the species used as a visual stimulus during the application of the checklist-interview technique), since we would not know which woody plants co-occur with our key species. Therefore, although it is not our purpose to present results on forest structure and composition, the inventory was fundamental for the research. Exsiccates of these species were included in the field herbarium based on their abundance, as detailed below.
The sites selected for the inventory were privately owned yet accessible to local gatherers. Two of these sites fell within the Marituba do Peixe Environmental Protection Area boundaries in Piaçabuçu, while the third was in the municipality of Penedo, not included in this protection area but still proximal to the community.
We established five permanent plots, each measuring 50 × 20 m, and further divided these into 50 smaller subplots of 10 × 10 m situated within the primary native vegetation gathering sites designated during the workshops. This amounted to 0.5 hectares per area, with a total of 1.5 hectares surveyed across all areas.
During the inventory, we collected at least three reproductive samples of each plant species within the plots for identification and to assemble a field herbarium for use in subsequent interviews. Certain species, commonly referred to as “ingá” and “pau d’arco”, lacked fertile material at the time of collection, leading us to categorize them as ethnospecies for the purposes of this study. Consequently, in our identification records, we referred to these simply as “ingá” and “pau d’arco”, acknowledging that these common names might represent multiple botanical species. Furthermore, the ethnospecies “cambuí”, although biologically uniform—belonging to the species Myrciaria floribunda (H. West ex Willd.) O. Berg—was recognized by some residents as having different ethnovarieties—a distinction not universally acknowledged. In our analysis, we accounted for each mention of “cambuí” by participants, even though the general data summary did not differentiate between ethnovarieties. For instance, if interviewee A identified two types of “cambuí” (Yellow and Red) and Interviewee B referred to one (a general “cambuí”), we recorded two entries for A and one for B in our database.
For the field herbarium, we mounted exsiccates from species with more than 15 individuals in the surveyed areas onto duplex paper of dimensions 42 × 29.7 cm and stored them in folders of matching size. The herbarium included 24 species in total and 2 taxa that were treated as ethnospecies.
Photography of each species was conducted in situ, capturing images that emphasized the plants’ distinguishing features: overall appearance, flowers and/or fruits, branches, and stems. These photographs were compiled into folders on a tablet, which was employed to display the images during interviews. Both the exsiccates and the photo folders were numerically coded to correspond with the identifiers on the interview forms, ensuring that interviewees were unaware of the plant names and assisting the interviewer.
The botanical collection phase commenced in November 2021 and concluded in April 2023, an extended period due to intermittent interruptions from COVID-19 peaks and flooding that hindered fieldwork.
A local guide with extensive knowledge of the vegetation provided assistance for all fieldwork involving local vegetation access. We adhered to standard botanical collection protocols, and the exsiccate samples were deposited at the Dárdano de Andrade Lima herbarium of the Agronomic Institute of Pernambuco.
3rd data collection stage: checklist interview
Before commencing the interviews (third stage), we mapped all Retiro households in May 2023. This mapping was imperative for sample size calculation due to the absence of a census record; the health unit’s data was limited to registered families. We determined that household heads (one per household) aged 18 or older present during our visit would be interviewed. Considering that some individuals reside in the community only for short periods, we established an inclusion criterion that only families living in the area for more than one year would be eligible for the study.
We ascertained the number of residences, including both occupied and vacant, to be 361, initially yielding a sample size of 187 residences based on a 95% confidence level and a 5% margin of error. Subsequently, we conducted a simple random selection.
As every house in the community was recorded, including unoccupied ones, some selected residences were vacant. Additionally, given the research’s focus on potentially harmful wood resource use within the Environmental Protection Area, some families were reluctant to participate. Therefore, from the 187 chosen residences, we could only conduct interviews in 81 interviews of them. To overcome refusals, flood-affected houses, and temporary residents, additional draws were made.
After all draws, we excluded unoccupied houses (n = 82), residences on flood-impacted streets (n = 12), households temporary inhabitants (n = 18) and refusals or unavailability (n = 74) from the sample. After three unsuccessful attempts to locate a household head, we inferred their non-participation.
A notable number of individuals opted out of the study, a figure aligned with expectations for wood use research in protected areas, mirroring findings from Medeiros et al. [3]. The considerable number of unoccupied houses in the community can be primarily attributed to their use as summer residences by individuals from nearby municipalities, taking advantage of the community’s closeness to the beach. Additionally, a number of these houses are situated in areas susceptible to flooding during the rainy season, which also contributes to their vacancy.
The final sample consisted of 115 individuals—81 women and 34 men. Interviews were conducted from May to July 2023. During interviews, we applied the checklist-interview technique [27] to ensure uniform visual stimuli across all informants, enhancing recall of all plant-associated uses.
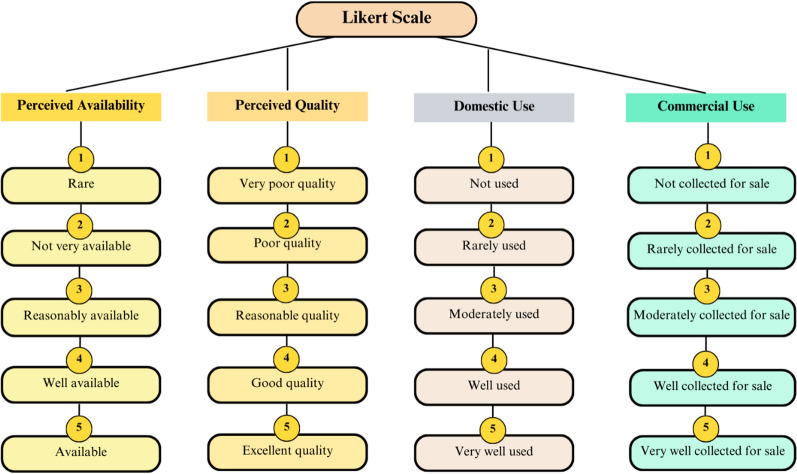
Interviewees were shown photos of each species and queried on whether they recognized the species. Affirmative responses led to further questions on the plant’s name, its uses (food and wood), whether the interviewee actually used the species, parts utilized, commercial harvesting, and collection and sale sites. For recognized plants, a Likert scale rated: perceived availability (only for those interviewees that often frequent vegetation areas), wood quality by use category (fuel, construction, technology), domestic use for wood and food, and commercial use.
In the fuel category, wood is used as firewood or charcoal for generating energy, cooking food, and heating water or spaces. The construction category encompasses the use of wood in structures for territorial demarcation, building homes, shelters for animals, and storage of items (e.g., fences, posts, house lines, rafters, battens, doors, windows). Technology refers to the use of wood in manipulated items that are not intended for demarcating spaces, such as tool handles, benches, tables, chairs, canoes, and oars, among others [33].
The ratings and responses in Likert scale are presented in Fig. 2.
Fig. 2.
Information collected using a Likert scale on the variables considered in this study
This classification facilitated the synthesis of scoring for perceived wood quality, allowing individuals to assign ratings by category rather than for each specific use. If a participant identified a plant as useful for wood but did not personally use it, we probed for the reasons behind this choice. We also asked if there were any of the mentioned plants that, despite being good for wood uses, the interviewee did not harvested. These questions were included to gather information on self-conscious protective behaviors associated with the food use of woody species.
Only for the ethnospecies “ingá” and “pau d’arco”, instead of showing the photos and exsiccate, we asked directly if the person knew them for food or wood uses. In case of a positive answer, we asked the same cycle of questions conducted for the other species. This was done because we did not obtain sufficient fertile material for the taxonomic identification of all species of “ingá” and “pau d’arco” during the various collection events.
Additionally, we gathered socio-economic data from all informants through structured interviews, including gender, age, occupation, income, place of origin, education, and length of residence. This information enabled the characterization of the socio-economic profile of the interviewees.
In this sense, the primary livelihoods include gathering, particularly collecting edible fruits, as well as pension, fishing, and agriculture, with some engaging in multiple occupations. A variety of other professions are represented to a lesser extent. The age of interviewees spans ages 18 to 82, with an average age of 48.14 years.
Most interviewees are literate (76.65%) are literate, of whom 73.91% have completed or partially completed basic education, and 1.74% have higher education qualifications.
The number of people occupying the residences ranges from one to seven residents. However, the majority of houses are occupied by: two or three residents (29.57%), followed by one or four resident(s) (15.65%).
Household incomes show substantial variation: (a) under one minimum wage (28.70%), (b) exactly one minimum wage (14.78%), (c) one and a half to two minimum wages (41.74%), (d) up to three minimum wages (13.04%), with a minority exceeding five minimum wages (1.74%).
Data analysis
For statistical analyses, we removed from the database any instances where species were identified for food purposes but not for wood purposes, as the focus of the research was on criteria for selecting wood plants. Consequently, non-wood plants were disregarded. Similarly, we excluded data from individuals who did not frequent forest environments to ensure that our information on species availability came from realistic assessments.
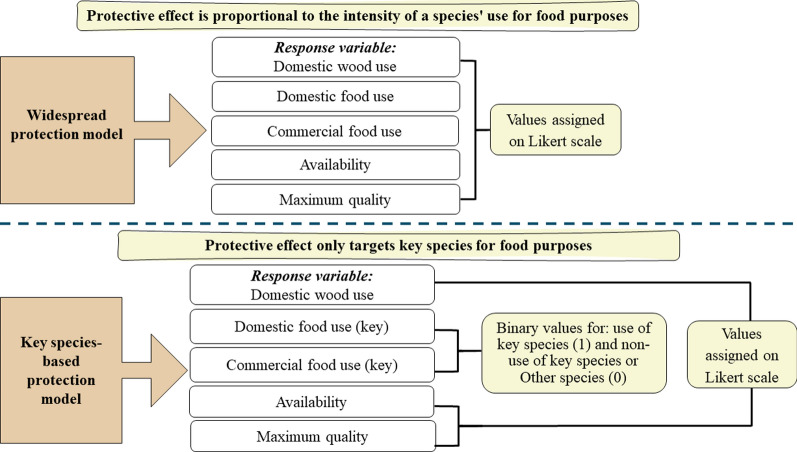
Our response variable, domestic wood use, was ordinal, as depicted in Fig. 3. Therefore, we utilized Cumulative Link Mixed Models (CLMMs), incorporating the interviewee as a random effect to account for the non-independence of information from the same individual. The CLMMs were executed using the clmm function from the R package ordinal.
Fig. 3.
Widespread species model and key species-based model with their variables and respective measures
To evaluate the stability of our models and check for multicollinearity, we used the omcdiag function from the mctest package in R. We determined an absence of multicollinearity if none or at most one of the six indicators were positive. To circumvent multicollinearity, we constructed two models. The first model, termed the widespread protection model, assigned domestic and commercial food use values on a 5-point Likert scale based on reported usage intensity. For the key species-based protection model, food use was a binary variable: it took the value of 1 if the mention included the use of a key species, and 0 if the mention involved a key species only known but not used, or non-key species, regardless of usage.
Model selection was based on the most parsimonious option, as indicated by the lowest Akaike Information Criterion corrected for small sample sizes (AICc). We interpreted a ΔAICc (difference from the lowest AICc) of less than 2 as substantial support for the model’s inclusion among the best set of models, following Burnham and Anderson [34]. Following model selection, we computed a model average, which considered the average beta of all variables within the parsimonious models. Since the variables were standardized via z-standardization, we compared the relative effect sizes of all variables.
The variable ‘commercial wood use’ was not included in the models due to its limited mentions (n = 5) within the community and only six citations of species that are commercially traded for wood, exclusive of domestic use.
In addition to the explanatory variables related to food use, both models incorporated control variables for availability and quality, as previously identified in the literature as predictors of wood use [20, 24, 25]. Our quality indicator was the maximum perceived quality. It was determined by the highest Likert scale quality rating given by an interviewee for a species across the three categories of wood use. For example, if, for a given species, values of 3, 4, and 5 were assigned by an interviewee to the categories of construction, technology, and fuelwood, respectively, the maximum perceived quality would be recorded as 5.
To analyze the qualitative data on protection behaviors, we examined the responses, categorized them, and used descriptive statistics.
Results
Wood and food uses: general aspects
All plants were recognized to varying extents by the interviewees. The most recognized species/ethnospecies were: Genipa americana L. (“jenipapo"), Inga spp. (“ingá”), Myrciaria floribunda (H. West ex Willd.) O. Berg (“cambuí”), Manilkara salzmannii (A.DC.) H.J.Lam (Massaranduba), Psidium guineense Sw. (“araçá”), Mouriri sp. (“cruirí"), and Bignoniaceae spp. (“pau d’arco”), with recognition rates of 56.5% or higher during interviews. The first five species achieved high recognition levels, exceeding 80%. Notably, G. americana, M. floribunda, and P. guineeense were identified as key species during the workshops. A comprehensive list of all species included in the checklist, along with their recognition and citation frequencies, is presented in Table 1.
Table 1.
Plants that were part of the checklist interview, their citation percentages (general and by use), and occurrence areas
| Popular name | Family | Scientific name | Areasa | Voucher | No of citations | % general | % Food use | % Wood use |
|---|---|---|---|---|---|---|---|---|
| Jenipapo | Rubiaceae | Genipa americana L | C | 94,826 | 114 | 99.1 | 99.1 | 57.4 |
| Ingá* | Leg. Mim | Inga spp. | A, B and C | – | 112 | 97.4 | 97.4 | 67.8 |
| Cambuí | Myrtaceae | Myrciaria floribunda (H. West ex Wild.) O. Berg | A and B | 94,056 | 103 | 89.6 | 89.6 | 41.7 |
| Massaranduba | Sapotaceae | Manilkara salzmannii (A.DC.) H.J.Lam | A and B | 93,971 | 96 | 83.5 | 82.6 | 77.4 |
| Araçá | Myrtaceae | Psidium guineense Sw | C | 94,807 | 93 | 80.9 | 80.9 | 30.4 |
| Cruiri | Melastomataceae | Mouriri sp. | C | 94,822 | 75 | 65.2 | 64.3 | 51.3 |
| Pau d'arco* | Bignoniaceae | Bignoniaceae spp. | A and B | – | 66 | 57.4 | 0 | 57.4 |
| Carrapatinho | Rutaceae | Esenbeckia grandiflora Mart | A and B | 93,988 | 27 | 23.5 | 0 | 23.5 |
| Banana de papagaio | Clusiaceae | Kielmeyera rugosa Choisy | A and B | 93,982 | 26 | 22.6 | 0 | 22.6 |
| Murici comum | Malpighiaceae | Byrsonima sericea DC | A, B and C | 94,066 | 22 | 19.1 | 13.9 | 18.3 |
| Murta roxa | Myrtaceae | Neomitranthes obtusa Sobral & Zambom | A and B | 94,767 | 18 | 15.7 | 10.4 | 15.7 |
| Peroba | Bignoniaceae | Tabebuia elliptica (DC.) Sandwith | A and B | 94,765 | 18 | 15.7 | 0 | 15.7 |
| Orelha d'onça | Polygonaceae | Coccoloba laevis Casar | A | 94,000 | 18 | 15.7 | 7.8 | 15.7 |
| Louro | Lauraceae | Ocotea notata (Nees & Mart.) Mez | A and B | 94,064 | 16 | 13.9 | 0.9 | 13.9 |
| Sicupira | Leg. Caes | Diptychandra aurantiaca Tul | A | 93,999 | 14 | 12.2 | 0 | 12.2 |
| Espinho branco | Rubiaceae | Machaonia acuminata Bonpl | C | 94,813 | 12 | 10.4 | 0 | 10.4 |
| Meiru | Annonaceae | Xylopia laevigata (Mart.) R.E.Fr | A and B | 94,067 | 9 | 7.8 | 0 | 7.8 |
| Murici de vaqueiro | Malpighiaceae | Byrsonima bahiana W.R.Anderson | A | 93,989 | 9 | 7.8 | 7.8 | 6.1 |
| Pirunga | Myrtaceae | Myrcia arenaria L.L.Santos et al | A and B | 93,995 | 9 | 7.8 | 7.0 | 6.1 |
| Murta branca | Myrtaceae | Eugenia punicifolia (Kunth) DC | A and B | 94,068 | 8 | 7.0 | 4.3 | 7.0 |
| Açoita égua | Myrtaceae | Myrcia loranthifolia (DC.) G.Burton & E.Lucas | A, B and C | 93,994 | 7 | 6.1 | 3.5 | 6.1 |
| Camarão | Flacourtiaceae | Casearia Sylvestris Sw | A and C | 94,821 | 7 | 6.1 | 0 | 6.1 |
| Piranha | Nyctaginaceae | Guapira opposita (Vell.) Reitz | A and B | 93,993 | 5 | 4.3 | 0.9 | 4.3 |
| Rompe gibão | Euphorbiaceae | Phyllanthus sp. | C | – | 4 | 3.5 | 0 | 3.5 |
| Sete casco | Euphorbiaceae | Pera glabrata (Schott) Baill | A and B | 93,976 | 3 | 2.6 | 0 | 2.6 |
| Murta amarela | Myrtaceae | Neomitranthes sp. | A, B and C | 93,991 | 1 | 0.9 | 0 | 0.9 |
*Ethnospecies
aOccurrence areas of plant species—local denominations: (A)—Carrasco, (B)—Zé Marinho, (C)—Lalo (Patos)
Over half of the species on the checklist (57.69% or n = 15) were recognized for both food and wood uses. Within the three categories of wood use addressed in this study, fuelwood (37.89%) and construction (36.93%) had the highest citation percentages. Technology accounted for only 25.18% of wood citations. Within the fuelwood category, firewood led with the highest percentage (62.82%) of citations relative to the total uses in the category, followed by charcoal (37.18%). The construction category comprised 25 wood uses, with over half (50.55%) the citations pertaining to fences, and the remainder divided among uses such as line (11.98%) and rafter (10.74%). The technology category included 67 wood uses, featuring lower usage percentages compared to the other categories. Uses such as hoe handle (11.92%) and hoe shaft (10.30%) were the only applications exceeding 10% of citations in relation to the total uses within this category. All wood uses attributed to the species are detailed in Supplementary Material 1.
Widespread protection model
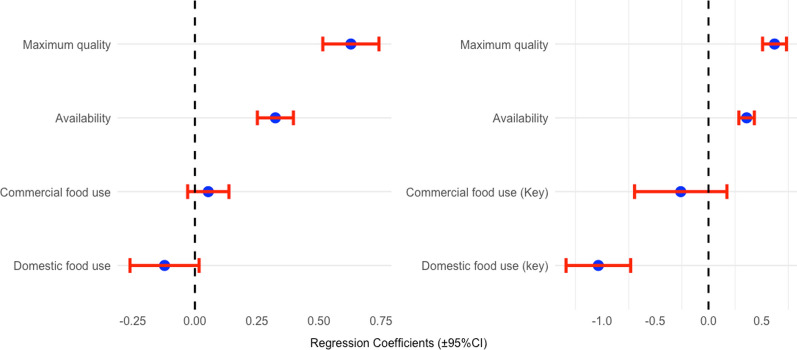
In the widespread protection model, domestic and commercial food use did not significantly influence domestic wood use when controlling for availability and quality variables (Fig. 4). This means that there is no linear relationship between food use and domestic wood use.
Fig. 4.
Impact of Predictor Parameters (quality, availability, domestic food use, commercial food use, domestic use of key species, and commercial use of key species) on domestic wood utilization of wild edible plants. Left: widespread protection model. Right: key species-based protection model. The central circles indicate the median coefficient estimates of the associations, and the horizontal lines delineate the 95% credibility intervals. The parameter coefficient estimates are plotted along the x-axis, while the predictor levels are represented on the y-axis. The vertical line intersecting the zero point on the x-axis (indicating no effect) facilitates comparison of the sizes of positive, negative, and null effect coefficients. In the parameter level grouping, non-overlapping horizontal bars denote significant differences. Horizontal bars intersecting the zero line on the x-axis signify a non-significant effect
Quality and availability were significant predictors of domestic wood use in the model. This suggests that, within the local context, there is a tendency to use woody plants for wood purposes based on their higher quality and greater availability.
Key species-based protection model
We observed a pronounced protective effect on key species, where the domestic use variable was more influential than both perceived availability and wood quality (see Fig. 4). However, the variable indicating commercial use did not significantly affect the use of wood for domestic purposes.
Within the model focusing on key species, both availability and wood quality (considered as control variables) had a significant impact on wood use. Consequently, our findings imply the existence of a threshold level of importance for the protective effect of food use on wood uses. This indicates that only those plants with substantial domestic food importance are shielded from being utilized for wood by the local population. The complete statistical results are available in the Supplementary Material 2.
Evidence of protection based on qualitative data
When inquiring whether individuals refrained from using any of the recognized plants for wood purposes, despite acknowledging their suitability for such use, we gathered responses that support a tendency to protect certain species with dual edible and wood functions. The key species identified during the participatory workshop as significant to the local community, and which garnered substantial recognition in the checklist, were notably prominent in this context.
Out of the 60 respondents to this question, 37 reported no restraint in using plants suitable for wood purposes. Among the 23 participants that chose not to collect certain plants, 12 indicated not collecting species had both edible and wood uses.
Of all mentions of plants with both edible and wood applications, seven pertained to key species (as shown in Table 2). The primary rationale for sparing these species from wood harvesting, or only using their dry branches, is their provision of edible fruits valued within the community. This rationale is illustrated by the testimonies concerning P. guineense, G. americana, and M. floribunda.
Table 2.
Responses from interviewees indicating the protection of key species regarding wood uses
| Key species | Explanations |
|---|---|
| Psidium guineense Sw | “Because if you take the wood, it will dry, and the plant will stop bearing fruits” |
| “They are good (as wood), but they are also fruits.” | |
| Genipa americana L | “Because the fruit is good and sought after by the people, if it's green, I don’t take it, only if it’s dead and dry.” |
| “I don’t take the female one because it bears fruit.” | |
| Myrciaria floribunda H. West ex Wild | “Because I really like the fruit, and I find it very beautiful.” |
| “Because it’s a nostalgic, good fruit.” | |
| “I avoid taking them because they are fruits. I only collect the dry branches.” |
Manilkara salzmannii though having a limited role in commerce, garners mixed views on its suitability for consumption within the community. Nonetheless, six interviewees mentioned the species, with two specifically expressing their intent to conserve it from being used for wood purposes: (1) “I don’t take it, thinking about the fruits and the future. I don’t like to take it (wood) while it’s still green, I only pick up the dry branches that have fallen on the ground.” (2) "Because it’s a plant that bears fruit, and it doesn’t sprout again if you cut it."
Despite M. salzmannii not being designated as a key species during the participatory workshop, it nonetheless received noteworthy acknowledgment in the checklist-interview. This suggests that M. salzmannii may possess a certain degree of importance for food-related uses within the community.
Discussion
In our widespread protection model, neither commercial nor domestic food use significantly explains domestic wood use. By contrast, in the key species-based protection model, domestic use emerges as the primary explanatory variable. In both models, perceived availability and quality significantly explain wood use, with quality being more important than availability.
Consistent with our hypothesis, we identify a protective effect of food use on wood use. This effect is not directly proportionate to the food use of the species but is confined to plants with considerable domestic food importance. Research conducted in the Brazilian Caatinga region, which initially tested the protection hypothesis using medicinal (specialized) and wood (generalist) use, suggested this possibility [26]. Although they observed a modest yet significant linear trend supporting the hypothesis, the authors graphically demonstrated that the protective effect intensified specifically among highly valued medicinal plants. This study furnishes statistical substantiation for what was previously inferred graphically.
Given that the protective effect is selective for key species, it indicates that merely having intermediate or low food importance is insufficient for wild food plants to evade wood use. Protection is afforded only to those species recognized as highly important. Indeed, key species not only receive high acknowledgment in the checklist (> 80%) but are also extensively consumed and increasingly traded within the community, in forms such as fresh fruit, juice pulp, and in the manufacture of alcoholic beverages, ice pops, among other products. Literature highlights that elevating the value of non-timber forest products for local populations acts as an incentive for forest species conservation [12, 35].
Our findings suggest that protection is predominantly correlated with domestic consumption. The domestic use of non-timber forest products can be a way for poorer local populations to save money [36], as is the case with wild fruits that can replace commercially purchased foods. Although wild food plants serve only as supplementary food resources within the community—with staple crops like rice and beans constituting the primary plant food intake—the importance of key wild food plants likely motivates the observed protection behaviors. Moreover, the emotional connection with natural environments resulting from direct experiences with nature can lead to pro-environmental behaviors (actions that reduce negative environmental impacts or enhance the sustainable use of natural resources) or intentions to engage in nature protection, as environmental psychology research has demonstrated [37, 38]. For example, Hinds and Sparks [39] found that individuals who grew up in rural areas tend to report more positive emotional connections, a stronger sense of identification, and more intense behavioral intentions regarding engagement with nature compared to those who were raised in urban environments. In this sense, the protective behaviors associated with the domestic consumption of key species may be related to the emotional bond linked to positive emotions built over a lifetime and across generations.
The low adherence to protective behaviors reported in interviews (see Evidence of protection based on qualitative data) could stem from various factors. Not all protective actions are necessarily conscious. Additionally, individuals may inadvertently omit mention of such behaviors in response to indirect inquiries like those posed in our study. Furthermore, protection may not be universally practiced within the community, and while the pressure to use wood from wild food plants may not be entirely eliminated, it could be reduced by fewer community members intensively exploiting key food plants for wood purposes.
Wood quality and species availability are significant determinants of wood use. It appears that, aside from key food species—whose utilization for wood is limited due to their value as food—other species are more likely to be used for wood purposes when they offer better trade-offs between availability and quality. Most studies that investigate the drivers of wood use tend to analyze quality or availability indicators separately, rather than in combination. These studies have found that either quality or availability can influence wood use [20, 24, 25].
Studies that consider multiple predictors of wood use have yielded divergent results. While availability seems to be a consistent predictor across different contexts, quality may or may not be a determinant of wood use [19, 40].
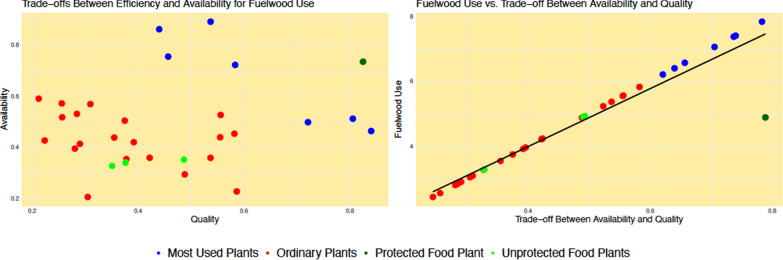
In various social-ecological contexts, research has indicated that trade-offs between multiple variables act as drivers of plant resource use [19, 41]. However, these trade-offs are often considered within a single use-category (e.g., the trade-off between quality and availability to explain fuelwood use). Therefore, the evidence of protection underscores the necessity of considering interactions between use-categories when evaluating criteria for plant resource selection (Fig. 5).
Fig. 5.
Hypothetical example of a trade-off between availability and quality explaining fuelwood use. In a simplified scenario where these are the only predictors of plant use within the fuelwood category, the most utilized species would be those exhibiting the highest trade-offs between availability and quality (represented by the blue dots in the right and left graphs). When considering the interaction with the food use-category under the key-species based protection model, the use of wood species with low to intermediate food importance would be proportional to the trade-off between quality and availability (graph on the right). However, for species that are considered key food plants (indicated by the dark green dot), their utilization for fuelwood would be less than what is predicted by their quality and availability alone
Recommendations for conservation strategies for plant species
The practical implication of a protective effect that acts solely on species of high food importance is that species recognized as having intermediate or lower importance remain unprotected, as do wood species without any associated food use. Moreover, if only a few species are highly valued for food, they might experience intense pressure from their use as food or be protected at the expense of other species. Therefore, we recommend that conservation strategies take into account the interactions between food and wood use-categories, i.e., the effects of one category on the other.
For species with intermediate or lower food importance, popularization strategies could prove beneficial to enhance their perceived value. Programs aimed at popularizing such species are crucial, as they may significantly contribute to food and nutritional security, while their use as food might concurrently protect them from being exploited for wood. These programs should establish incentives that encourage community members to use these resources sustainably. However, the effectiveness of this approach should be continuously monitored, as if importance is the primary factor driving protective behaviors, integrating other wild food plants into the set of key species may prove challenging.
Although the commercial importance of key species did not lead to protection in this study, the inclusion of certain species in local markets could also positively influence domestic use. Thus, popularization strategies could extend beyond local communities, emphasizing the importance of these plants for diet diversification and their potential nutritional value to generate demand for products sourced from local communities. One method to achieve this is through marketing campaigns that raise awareness about the significance of these plants in local markets and across social and conventional media platforms [42].
However, it is crucial to approach the popularization of highly important food species with caution to prevent the oversimplification of the plant community, as observed with açaí (Euterpe oleracea Mart.), where management practices have simplified estuarine communities in the Amazon Rainforest [43].
For wood species that lack an associated food use, conservation strategies must be implemented to mitigate the pressure on their exploitation. Considering that the primary wood uses in the community are for fuel (firewood) and construction (fencing), conservation efforts should be tailored to these applications. Firewood is the most commonly cited use in the Retiro community, and due to its characteristics regarding short replenishment time and large volume of wood used, it poses a significant threat to species conservation, depending on the collection method (green or dry). For people with greater social vulnerability, firewood is an important resource for cooking. To address this, we recommend the use of efficient wood stoves. These stoves, through their structural configuration, reduce cooking time and, consequently, the daily volume of wood used and deforestation compared to traditional stoves [44].
Although there is controversy in the literature regarding the long-term economic costs and benefits of improved stove use in developing countries [45] and their efficiency[46], several studies have shown significant reductions in firewood use with the adoption of this technology [44, 47, 48]. For instance, a study based on an improved stove intervention in the Chalaco District, Northern Andes of Peru, recorded a 46% reduction in firewood consumption (approximately 650 kg of firewood per household throughout the rainy season) among households that properly used improved stoves during winter [48]. Similarly, Bensch and Peters [47], who evaluated the impact of these stoves in rural Senegal through a randomized clinical trial, found a total 31% reduction in firewood consumption over one week. Additionally, the use of efficient stoves can contribute to a higher quality of life for users by reducing smoke from wood combustion, which can cause respiratory diseases [49]. However, for successful implementation of efficient stoves, besides local community interest, factors influencing long-term adoption, such as maintenance costs, need to be considered.
An alternative to replacing firewood use is increased investment in public policies that ensure access to Liquefied Petroleum Gas (LPG). While families receiving the gas voucher through the federal government program (Bolsa Família) still use a mix of LPG and firewood in the community, education, health, and human well-being initiatives, combined with these public policies, may have a better response in the community during the transition from firewood to LPG use. This is especially important considering that the use of firewood, for the most part, spans generations. The same applies to the transition from traditional or makeshift brick stoves to efficient stoves.
To reduce the use of species employed in the construction of dead fences—where trunks and branches of woody plants are cut green for use—we recommend a gradual replacement with species used as living fences, which are kept alive. This strategy has been indicated as effective as it represents a gene bank of native species and contributes to the maintenance of these species [50]. “rompe gibão” (Phyllanthus sp.) and “cruirí” (Mouriri sp.) were mentioned by some interviewees as species used for living fences, and “peroba” (Tabebuia elliptica (DC.) Sandwith) was mentioned as having the ability for its stake to remain green in a humid environment. They are considered hard and resistant woods (“fixe”) by the interviewees who recognized them on the checklist. These species could potentially be used for this purpose, but they need to be evaluated in terms of their characteristics and ecological status.
Finally, although our results admit that there is a protective effect on species with high food importance (key species) regarding wood uses, it is necessary to investigate the ecological status of these species to assess whether harvesting is being done sustainably and if overexploitation of these species is not occurring, as has been identified in other studies with non-timber forest products [5, 14, 15].
Recommendations for future ethnobiological studies
Some challenges for testing the protection hypothesis in future studies include:
Studies should account for the interactions not only between two use-categories but also among all use-categories associated with the plant species. For instance, a plant might be protected from wood uses not solely due to its food or medicinal value, but because it serves multiple purposes. Thus, protection may only become apparent when evaluating the full spectrum of plant use dynamics.
Gender and age variables ought to be incorporated into the tests of the protection hypothesis, given that individuals of different ages or genders may protect plants for varied purposes.
Studies could delve into the affective aspects of protection, as these may inspire individuals to spare certain species from wood uses due to resources that evoke positive affective memories. For example, a fruit that was greatly cherished during one’s childhood or that constituted the main sustenance for a person’s family might be protected. While affective reasons are personal, common patterns may surface, especially among individuals with similar cultural or community backgrounds who may share collective memories.
It is necessary to further investigate the influence of social organization on the protective behavior of local peoples toward wild food species. For instance, in contexts where there are associations of fruit gatherers or cooperatives, protective behavior may increase compared to rural communities where social organization is poorly established or absent. Alternatively, protective behavior may be directed on an individual basis.
Research designs should enhance the methodological approach concerning qualitative evidence for protection. The questioning should be crafted to elicit precise responses without leading the participant, yet still addressing the core issue effectively. Our study utilized indirect questions that may not have fully captured our main objective. We propose that future research adopting discourse analysis techniques (underpinned by multiple theoretical frameworks) would yield valuable insights.
Limitations of this study
For two groups of plants treated in this study as ethnospecies (“pau d’arco” and “ingá”), we were unable to elucidate their taxonomies despite our efforts. Our results suggest that these ethnospecies are not under the protective effect of food use, and the lack of botanical identification complicates the targeting of conservation strategies, especially for future studies in this region. Although we do not know the quantity and specific species, we suspect they are at risk of threat due to logging, especially for “pau d’arco” (Bignoniaceae spp.). At least two species of “pau d’arco” are listed in the International Union for Conservation of Nature Red List with concerning ecological statuses: Handroanthus impetiginosus (Mart. ex DC.) Mattos (“pau d’arco rosa”) listed as near-threatened and Handroanthus serratifolius (Vahl) S. Grose (“pau d’arco amarelo”), listed as endangered [51]. Both were assessed for the list in 2020. As respondents mentioned three types of “pau d’arco” (“roxo”, “amarelo”, and “branco"), it is possible that species of this genus are included. Through botanical identification, we identified that a plant known in the community as “peroba” is the species Tabebuia elliptica (DC.) Sandwith (“pau d’arco branco”), specified on the Red List with a status of least concern. This makes this area an interesting occurrence for this plant group. In light of this, we acknowledge this limitation in our study and invite other researchers specializing in these plant groups to direct research efforts in this region and clarify the taxonomy of these species.
Our data on the perceived quality of wood were collected from a single Likert scale value considering all wood uses of the plant reported by the interviewee for each wood use category, instead of considering the quality for each reported wood use in each category. This optimized data collection. However, the heterogeneous nature of categories such as technology, where the wood quality of the plant can vary significantly among uses (e.g., tools, furniture, boat), can be challenging for the interviewee to assign a single rating considering various distinct uses. This may have biased our results with very generic perceptions of species quality. Given that wood use is diverse, future studies could consider a more meticulous design, such as focusing on the most relevant uses within each category in the local community and assessing their perceived quality independently.
In this study, we did not monitor the collection activity, so we were unable to differentiate between wood collected from fallen stems and branches (with less impact on plants) and wood removed directly from the plants (with greater impact). However, this does not compromise our results, as the aim was to assess general usage behaviors, and other studies have already recorded the predominance of cutting practices, whether for dry or green, live or dead wood [3, 21].
The inclusion of species for the composition of the checklist interview was based on their availability in areas of co-occurrence with key species. Although greater availability of species is a potential indicator of higher use, it is not universal. There may be species in the sampled vegetation areas that are less available precisely because they are under greater use pressure or due to other environmental or intrinsic factors not considered in this study. Therefore, it is essential to also consider ecological approaches in research to have an overall assessment of the impact of such uses on the plant community structure, even if the focus of the research is on the most important plant species.
Conclusion
Overall, we found that there is a protective effect that acts primarily on plant species of high food importance (key species), rather than proportionally to the importance of the species. Consequently, we encourage future studies to test the protection hypothesis within various socio-environmental contexts and we suggest considering two distinct possibilities: generalized protection and protection targeted at key species.
In light of our findings, we advise that species demonstrating an overlap between food and wood uses, yet possessing intermediate or lower food importance should be prioritized in popularization strategies to raise their significance. Moreover, species solely used for timber, which do not benefit from food-related protection, also require attention through biocultural conservation strategies. Given that the protective effect is limited to a select number of plant species, these species warrant further ecological investigation to determine their conservation status within their natural habitats, to identify whether they face increased pressure from their use as food, and to ascertain if their prominence is leading to a reduction in plant diversity.
Supplementary Information
Acknowledgements
We extend our gratitude to the residents of the Retiro community for their support in this research, particularly to Mr. José Antônio and Mrs. Maria das Dores, who warmly welcomed us and facilitated our requests within the community. Our appreciation also goes to Dr. Marcos Sobral, a Myrtaceae expert, who graciously identified the species of this family in our study. Likewise, we express our thanks to the researcher from the Agronomic Institute of Pernambuco, Maria Olivia de Oliveira Cano, for her receptiveness and efficiency in assisting us during all visits throughout the COVID-19 pandemic. We also extend our heartfelt thanks to the members of the Laboratory of Biocultural Ecology, Conservation and Evolution for their support in fieldwork activities.
Abbreviations
- CLMMs
Cumulative link mixed models
- NTFPs
Non-timber forest products
- UFAL
Universidade Federal de Alagoas (Federal University of Alagoas)
- SISBIO
Sistema de Autorização e Informação em Biodiversidade (Biodiversity Authorization and Information System)
- ICMBio
Instituto Chico Mendes de Conservação da Biodiversidade (Chico Mendes Institute for Biodiversity Conservation)
- ICF
Informed consent form
- LECEB
Laboratory of Biocultural Ecology, Conservation, and Evolution
- AICc
Akaike information criterion
- LPG
Liquefied Petroleum Gas
Author contributions
RAC—Conceptualization; Investigation; Methodology; Writing—original draft and final. ELGS—Writing—revision and editing. LFCN—Writing—revision and editing; Data compilation; RRVS and ARC—Supervision; Writing—revision and editing. PMM—Conceptualization; Methodology; Supervision and Writing—final version and editing. All authors read and approved the final version.
Funding
This study was funded by the Brazilian Biodiversity Fund—FUNBIO, the HUMANIZE and Eurofins Foundations (FUNBIO—Fellowships Conserving the Future, granted to RAC, No. 025/2022), the National Council for Scientific and Technological Development (CNPq) (Doctoral fellowship for RAC, No 141873/2020-5 and productivity grant to PMM, No. 304866/2020-2) and the Research Support Foundation of the State of Alagoas (FAPEAL) (Granted to PMM, No. APQ2022021000027).
Availability of data and materials
Data are provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee in research by Federal University of Alagoas (UFAL), No. 1998673. Furthermore, all participants previously signed the Informed Consent Form (ICF).
Consent for publication
Not applicable.
Competing interests
I declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Footnotes
Here, we consider destructive applications, those that compromise the individual plant and its population in the short term. For example, a shallow cut or a substantial cut of the branches of a tree, which can hinder its growth. In contrast to the fruit, which is collected, and the individual plant remains intact, it can cause damage to the population in the long term.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/20/2024
A Correction to this paper has been published: 10.1186/s13002-024-00731-7
Contributor Information
Roberta de Almeida Caetano, Email: robertacaetano1991@gmail.com.
Patrícia Muniz de Medeiros, Email: patricia.medeiros@ceca.ufal.br.
References
- 1.Ribeiro EMS, Arroyo-Rodríguez V, Santos BA, Tabarelli M, Leal IR. Chronic anthropogenic disturbance drives the biological impoverishment of the Brazilian Caatinga vegetation. J Appl Ecol. 2015;52(3):611–20. [Google Scholar]
- 2.Ribeiro DA, de Macedo DG, de Oliveira LGS, de Oliveira Santos M, de Almeida BV, Macedo JGF, et al. Conservation priorities for medicinal woody species in a cerrado area in the Chapada do Araripe, northeastern Brazil. Environ Dev Sustain. 2019;21(1):61–77. [Google Scholar]
- 3.Medeiros PM, De Almeida ALS, Da Silva TC, De Albuquerque UP. Pressure indicators of wood resource use in an atlantic forest area, Northeastern Brazil. Environ Manag. 2011;47(3):410–24. [DOI] [PubMed] [Google Scholar]
- 4.Ros-Tonen MAF. The role of non-timber forest products in sustainable tropical forest management. Holz Roh Werkstoff. 2000;58(3):196–201. [Google Scholar]
- 5.Brokamp G, Borgtoft Pedersen H, Montúfar R, Jácome J, Weigend M, Balslev H. Productivity and management of Phytelephas aequatorialis (Arecaceae) in Ecuador. Ann Appl Biol. 2014;164(2):257–69. [Google Scholar]
- 6.Gaoue OG, Ticktin T. Patterns of harvesting foliage and bark from the multipurpose tree Khaya senegalensis in Benin: variation across ecological regions and its impacts on population structure. Biol Conserv. 2007;137(3):424–36. [Google Scholar]
- 7.Stanley D, Voeks R, Short L. Is non-timber forest product harvest sustainable in the less developed world? A systematic review of the recent economic and ecological literature. Ethnobiol Conserv. 2012;2012(1):1–39. [Google Scholar]
- 8.Shackleton CM. Non-timber forest products in livelihoods. In: Ecological Sustainability for Non-timber Forest Products. Milton Park: Routledge; 2015. p. 12–30. [Google Scholar]
- 9.Jacob MCM, de Medeiros MFA, Albuquerque UP. Biodiverse food plants in the semiarid region of Brazil have unknown potential: A systematic review. PLoS ONE. 2020;15(5):e0230936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacchetta L, Visioli F, Cappelli G, Caruso E, Martin G, Nemeth E, et al. A manifesto for the valorization of wild edible plants. J Ethnopharmacol. 2016;191:180–7. 10.1016/j.jep.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 11.Delang CO. The role of wild food plants in poverty alleviation and biodiversity conservation in tropical countries. Prog Dev Stud. 2006;6:275–86. [Google Scholar]
- 12.Lowore J. Understanding the livelihood implications of reliable honey trade in the miombo Woodlands in Zambia. Front For Glob Change. 2020;3(March):1–16. [Google Scholar]
- 13.Casas A, Caballero J, Mapes C, Zárate S. Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Bot Sci. 2017;47(61):31. [Google Scholar]
- 14.Almeida A. Avaliação ecológica do extrativismo do pequi (Caryocar coriaceum Wittm.) na Floresta Nacional do Araripe, Ceará: informações para um plano de uso sustentável. Tese de Doutorado. 2014;164.
- 15.Avocèvou-Ayisso C, Sinsin B, Adégbidi A, Dossou G, Van Damme P. Sustainable use of non-timber forest products: impact of fruit harvesting on Pentadesma butyracea regeneration and financial analysis of its products trade in Benin. For Ecol Manag. 2009;257(9):1930–8. [Google Scholar]
- 16.Ribeiro JPO, Carvalho TKN, da Silva Ribeiro JE, de Sousa RF, de Farias Lima JR, de Oliveira RS, et al. Can ecological apparency explain the use of plant species in the semi-arid depression of Northeastern Brazil? Acta Bot Brasilica. 2014;28(3):476–83. [Google Scholar]
- 17.Soldati GT, de Medeiros PM, Duque-Brasil R, Coelho FMG, Albuquerque UP. How do people select plants for use? Matching the ecological apparency hypothesis with optimal foraging theory. Environ Dev Sustain. 2017;19(6):2143–61. [Google Scholar]
- 18.Gonçalves PHS, Albuquerque UP, De Medeiros PM. The most commonly available woody plant species are the most useful for human populations: A meta-analysis. Ecol Appl. 2016;26(7):2238–53. [DOI] [PubMed] [Google Scholar]
- 19.Hora JSL, Feitosa IS, Albuquerque UP, Ramos MA, Medeiros PM. Drivers of species’ use for fuelwood purposes: a case study in the Brazilian semiarid region. J Arid Environ. 2021;185:104324. [Google Scholar]
- 20.Cardoso MB, Ladio AH, Dutrus SM, Lozada M. Preference and calorific value of fuelwood species in rural populations in northwestern Patagonia. Biomass Bioenergy. 2015;81(October):514–20. 10.1016/j.biombioe.2015.08.003. [Google Scholar]
- 21.Gonçalves PHS, Medeiros PMD, Albuquerque UP. Effects of domestic wood collection on tree community structure in a human-dominated seasonally dry tropical forest. J Arid Environ. 2021;193:104554. [Google Scholar]
- 22.Cruz MP, Medeiros PM, Combariza IS, Peroni N, Albuquerque UP. “I eat the manofê so it is not forgotten”: local perceptions and consumption of native wild edible plants from seasonal dry forests in Brazil. J Ethnobiol Ethnomed. 2014;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.do Nascimento VT, de Lucena RFP, Maciel MIS, de Albuquerque UP. Knowledge and use of wild food plants in areas of dry seasonal forests in Brazil. Ecol Food Nutr. 2013;52(4):317–43. [DOI] [PubMed] [Google Scholar]
- 24.Lucena RFP, Araújo EDL, De Albuquerque UP. Does the local availability of woody Caatinga plants (Northeastern Brazil) explain their use value? Econ Bot. 2007;61(4):347–61. [Google Scholar]
- 25.Lucena RFP, Lucena CM, Araújo EL, Alves ÂGC, de Albuquerque UP. Conservation priorities of useful plants from different techniques of collection and analysis of ethnobotanical data. An Acad Bras Cienc. 2013;85(1):169–86. [DOI] [PubMed] [Google Scholar]
- 26.da Silva JPC, Gonçalves PH, Albuquerque UP, da Silva RRV, de Medeiros PM. Can medicinal use protect plant species from wood uses? Evidence from Northeastern Brazil. J Environ Manag. 2021;279:111800. [DOI] [PubMed] [Google Scholar]
- 27.Alexiades MN, Sheldon JWS. Selected guidelines for ethnobotanical research: a field manual. New York: New York Botanical Garden; 1996. [Google Scholar]
- 28.IBGE. Instituto Brasileiro de Geografia e Estatísticas. Cidades [Internet]. 2022 [cited 2023 Aug 19]. Available from: http://cidades.ibge.gov.br/download/mapa_e_municipios.php?lang=&uf=al
- 29.CLIMATE-DATA.ORG. Climate data, 2020. <https://pt.climate-data.org/america-do-sul/brasil.
- 30.IMA. Instituto do Meio Ambiente. APA Marituba do Peixe [Internet]. 2023 [cited 2023 May 1]. Available from: https://dados.al.gov.br/catalogo/dataset/apa-da-marituba-do-peixe
- 31.Gomes DL, Ferreira RPDS, Santos ÉMDC, Da SRRV, De MPM. Local criteria for the selection of wild food plants for consumption and sale in Alagoasm, Brazil. Ethnobiol Conserv. 2020;9(May):1–15. [Google Scholar]
- 32.Cabral SAS, de Azevedo Júnior SM, de Larrazábal ME. Abundância sazonal de aves migratórias na Área de Proteção Ambiental de Piaçabuçu, Alagoas, Brasil. Rev Bras Zool. 2006;23(3):865–9. [Google Scholar]
- 33.Ramos MA, Medeiros PM, Albuquerque UP. Methods and techniques applied to ethnobotanical studies of timber resources. In: de Albuquerque UP, Cunha LVFC, editors. Methods and techniques in ethnobiology and ethnoecology. New York: Springer; 2014. p. 15–23. [Google Scholar]
- 34.Burnham KP, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304. [Google Scholar]
- 35.Evans, M.I. (1993). Conservation by commercialization. In: Hladik CM, Hladik A, Linares OF, Pagezy H, Semple A, Hadley M, editors. Tropical forests, people and food: biocultural interactions and applications to development, vol. 13. 1993. Pp. 815–822.
- 36.Shackleton CM, Garekae H, Sardeshpande M, Sinasson Sanni G, Twine WC. Non-timber forest products as poverty traps: Fact or fiction? For Policy Econ. 2024;158:103114. 10.1016/j.forpol.2023.103114. [Google Scholar]
- 37.Collado S, Staats H, Corraliza JA. Experiencing nature in children’s summer camps: affective, cognitive and behavioural consequences. J Environ Psychol. 2013;33:37–44. 10.1016/j.jenvp.2012.08.002. [Google Scholar]
- 38.Cheng JCH, Monroe MC. Connection to nature: children’s affective attitude toward nature. Environ Behav. 2012;44(1):31–49. [Google Scholar]
- 39.Hinds J, Sparks P. Engaging with the natural environment: the role of affective connection and identity. J Environ Psychol. 2008;28(2):109–20. [Google Scholar]
- 40.Marquez-Reynoso MI, Ramírez-Marcial N, Cortina-Villar S, Ochoa-Gaona S. Purpose, preferences and fuel value index of trees used for firewood in El Ocote Biosphere Reserve, Chiapas, Mexico. Biomass Bioenergy. 2017;100:1–9. 10.1016/j.biombioe.2017.03.006. [Google Scholar]
- 41.Bahru T, Kidane B, Tolessa A. Prioritization and selection of high fuelwood producing plant species at Boset District, Central Ethiopia: an ethnobotanical approach. J Ethnobiol Ethnomed. 2021;17(1):1–15. 10.1186/s13002-021-00474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbosa DM, dos Santos GMC, Gomes DL, Santos EMD, da Silva RRV, de Medeiros PM. Does the label “unconventional food plant” influence food acceptance by potential consumers? A first approach. Heliyon. 2021;7(4):E0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freitas MAB, Magalhães JLL, Carmona CP, Arroyo-Rodríguez V, Vieira ICG, Tabarelli M. Intensification of açaí palm management largely impoverishes tree assemblages in the Amazon estuarine forest. Biol Conserv. 2021;261:109251. [Google Scholar]
- 44.Nazmul Alam SM, Chowdhury SJ. Improved earthen stoves in coastal areas in Bangladesh: economic, ecological and socio-cultural evaluation. Biomass Bioenergy. 2010;34(12):1954–60. 10.1016/j.biombioe.2010.08.007. [Google Scholar]
- 45.Jeuland MA, Pattanayak SK. Benefits and costs of improved cookstoves: assessing the implications of variability in health, forest and climate impacts. PLoS ONE. 2012;7(2):e30338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanna R, Esther D, Greenstone M. Up in smoke: the influence of household behavior on the long-run impact of improved cooking stoves. Am Econ J Econ Policy 2016;8(1):80–114. http://www.jstor.org/stable/247391. Am Econ J Econ Policy ;2012;66:37–9.
- 47.Bensch G, Peters J. A recipe for success? Randomized free distribution of improved cooking stoves in Senegal. SSRN Electron J 2012.
- 48.Agurto AM. Improved cooking stoves and firewood consumption: quasi-experimental evidence from the Northern Peruvian Andes. Ecol Econ. 2013;89:135–43. 10.1016/j.ecolecon.2013.02.010. [Google Scholar]
- 49.Alam SMN, Chowdhury SJ, Begum A, Rahman M. Effect of improved earthen stoves: improving health for rural communities in Bangladesh. Energy Sustain Dev. 2006;10(3):46–53. 10.1016/S0973-0826(08)60543-8. [Google Scholar]
- 50.Nascimento VT, Sousa LG, Alves AGC, Araújo EL, Albuquerque UP. Rural fences in agricultural landscapes and their conservation role in an area of caatinga (dryland vegetation) in northeast Brazil. Environ Dev Sustain. 2009;11(5):1005–29. [Google Scholar]
- 51.IUCN. The red list of threatened species (IUCN). Version 2022-2. 2022 [cited 2022 Oct 24]. Available from: https://www.iucnredlist.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided within the manuscript or supplementary information files.