Abstract
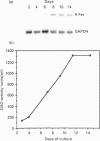
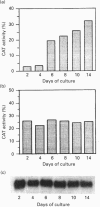
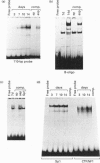
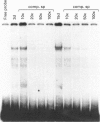
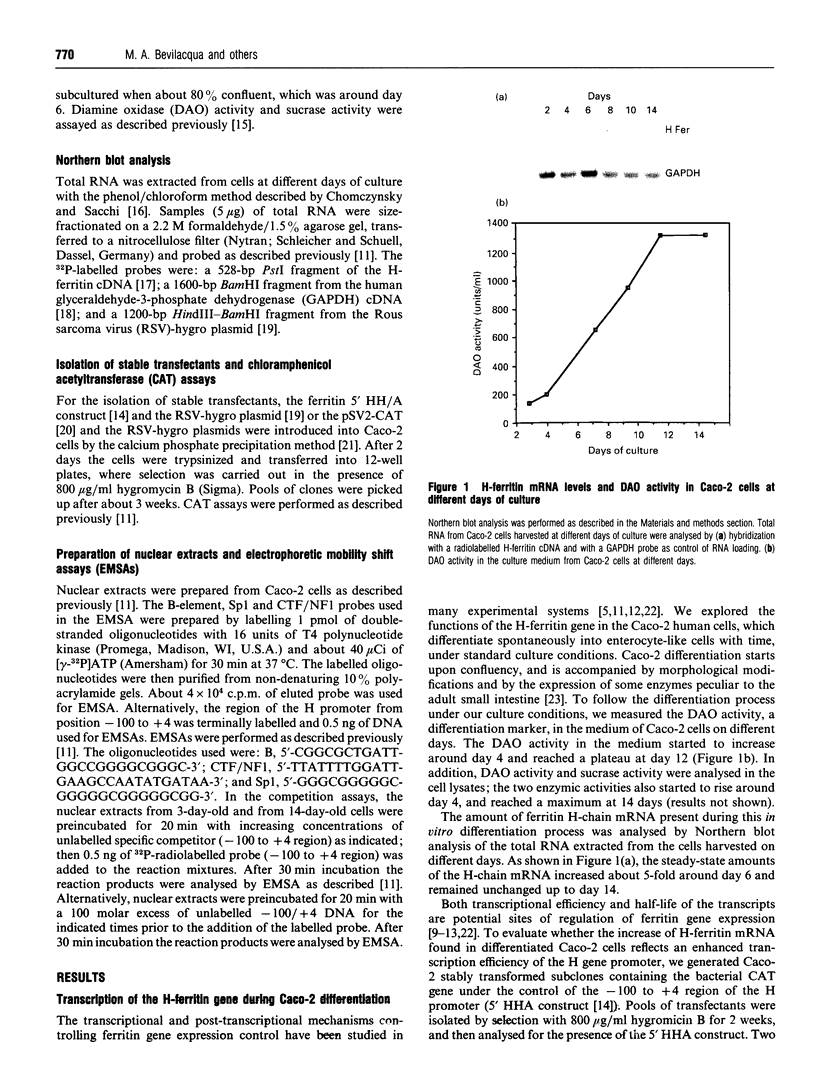
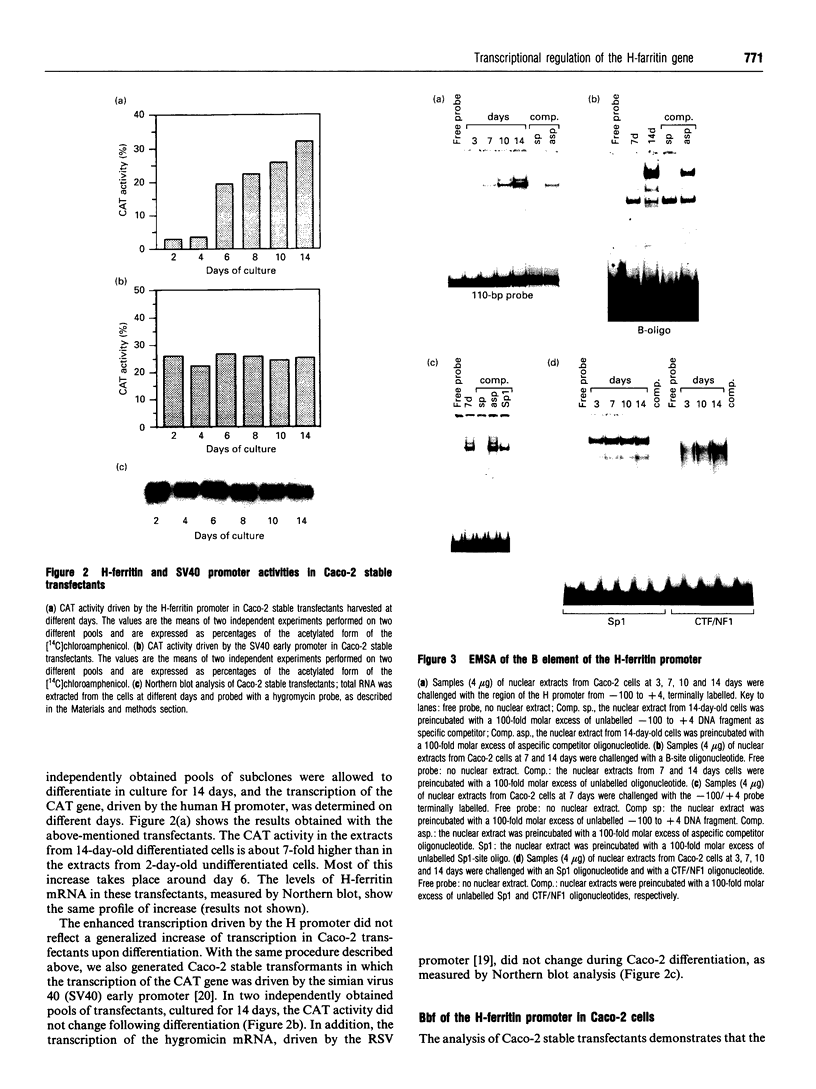
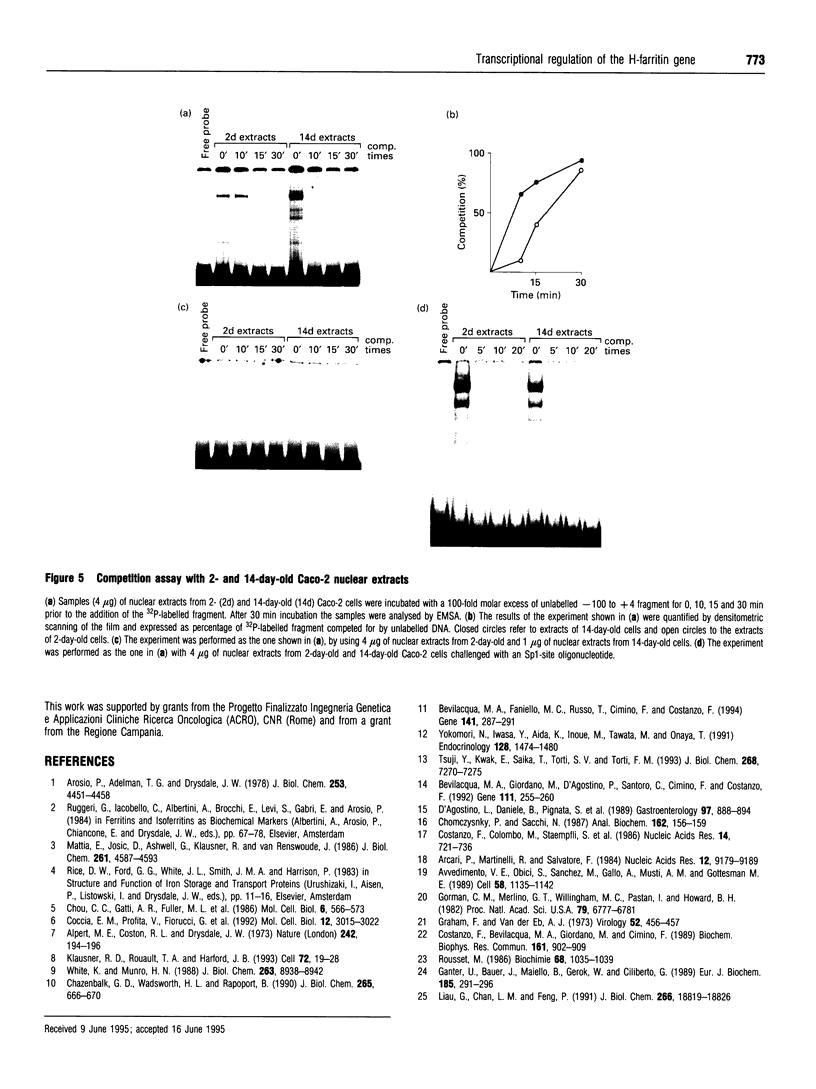
In this paper, we examine the mechanisms that regulate the expression of the heavy (H) ferritin subunit in the colon carcinoma Caco-2 cell line allowed to differentiate spontaneously in vitro. The differentiation process of these cells in continuous culture is accompanied by an accumulation of the mRNA coding for the apoferritin H chain. The analysis of Caco-2 subclones stably transfected with an H-chain promoter-chloramphenicol acetyltransferase (CAT) construct revealed that the mRNA increase is paralleled by an enhanced transcription of the H gene, driven by the -100 to +4 region of the H promoter. The H gene transcriptional activation seems to be a specific feature of differentiated Caco-2 cells, since the activity of other promoters did not change upon differentiation. The -100 to +4 region of the H promoter binds a transcription factor called Bbf (B-box binding factor); electrophoretic-mobility-shift-assay analyses showed that the retarded complex due to Bbf-H promoter interaction is significantly increased in the differentiated cells. We propose that the activation of H-ferritin gene expression may be associated with the establishment of a differentiated phenotype in Caco-2 cells, and that the H-ferritin gene transcriptional up-regulation is accompanied by a modification in the activity of the transcription factor Bbf.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert E., Coston R. L., Drysdale J. W. Carcino-foetal human liver ferritins. Nature. 1973 Mar 16;242(5394):194–196. doi: 10.1038/242194a0. [DOI] [PubMed] [Google Scholar]
- Arcari P., Martinelli R., Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984 Dec 11;12(23):9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Avvedimento E. V., Obici S., Sanchez M., Gallo A., Musti A., Gottesman M. E. Reactivation of thyroglobulin gene expression in transformed thyroid cells by 5-azacytidine. Cell. 1989 Sep 22;58(6):1135–1142. doi: 10.1016/0092-8674(89)90511-4. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. A., Faniello M. C., Russo T., Cimino F., Costanzo F. Transcriptional regulation of the human H ferritin-encoding gene (FERH) in G418-treated cells: role of the B-box-binding factor. Gene. 1994 Apr 20;141(2):287–291. doi: 10.1016/0378-1119(94)90587-8. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. A., Giordano M., D'Agostino P., Santoro C., Cimino F., Costanzo F. Promoter for the human ferritin heavy chain-encoding gene (FERH): structural and functional characterization. Gene. 1992 Feb 15;111(2):255–260. doi: 10.1016/0378-1119(92)90696-m. [DOI] [PubMed] [Google Scholar]
- Chazenbalk G. D., Wadsworth H. L., Rapoport B. Transcriptional regulation of ferritin H messenger RNA levels in FRTL5 rat thyroid cells by thyrotropin. J Biol Chem. 1990 Jan 15;265(2):666–670. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou C. C., Gatti R. A., Fuller M. L., Concannon P., Wong A., Chada S., Davis R. C., Salser W. A. Structure and expression of ferritin genes in a human promyelocytic cell line that differentiates in vitro. Mol Cell Biol. 1986 Feb;6(2):566–573. doi: 10.1128/mcb.6.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E. M., Profita V., Fiorucci G., Romeo G., Affabris E., Testa U., Hentze M. W., Battistini A. Modulation of ferritin H-chain expression in Friend erythroleukemia cells: transcriptional and translational regulation by hemin. Mol Cell Biol. 1992 Jul;12(7):3015–3022. doi: 10.1128/mcb.12.7.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Bevilacqua M. A., Giordano M., Cimino F. Expression of genes of ferritin subunits in human hepatoma cell lines. Biochem Biophys Res Commun. 1989 Jun 15;161(2):902–909. doi: 10.1016/0006-291x(89)92684-3. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino L., Daniele B., Pignata S., Gentile R., Tagliaferri P., Contegiacomo A., Silvestro G., Polistina C., Bianco A. R., Mazzacca G. Ornithine decarboxylase and diamine oxidase in human colon carcinoma cell line CaCo-2 in culture. Gastroenterology. 1989 Oct;97(4):888–894. doi: 10.1016/0016-5085(89)91493-5. [DOI] [PubMed] [Google Scholar]
- Ganter U., Bauer J., Majello B., Gerok W., Ciliberto G. Characterization of mononuclear-phagocyte terminal maturation by mRNA phenotyping using a set of cloned cDNA probes. Eur J Biochem. 1989 Nov 6;185(2):291–296. doi: 10.1111/j.1432-1033.1989.tb15114.x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Liau G., Chan L. M., Feng P. Increased ferritin gene expression is both promoted by cAMP and a marker of growth arrest in rabbit vascular smooth muscle cells. J Biol Chem. 1991 Oct 5;266(28):18819–18826. [PubMed] [Google Scholar]
- Mattia E., Josic D., Ashwell G., Klausner R., van Renswoude J. Regulation of intracellular iron distribution in K562 human erythroleukemia cells. J Biol Chem. 1986 Apr 5;261(10):4587–4593. [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986 Sep;68(9):1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Tsuji Y., Kwak E., Saika T., Torti S. V., Torti F. M. Preferential repression of the H subunit of ferritin by adenovirus E1A in NIH-3T3 mouse fibroblasts. J Biol Chem. 1993 Apr 5;268(10):7270–7275. [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- Yokomori N., Iwasa Y., Aida K., Inoue M., Tawata M., Onaya T. Transcriptional regulation of ferritin messenger ribonucleic acid levels by insulin in cultured rat glioma cells. Endocrinology. 1991 Mar;128(3):1474–1480. doi: 10.1210/endo-128-3-1474. [DOI] [PubMed] [Google Scholar]