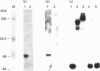
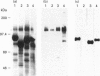
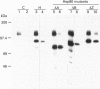
Abstract
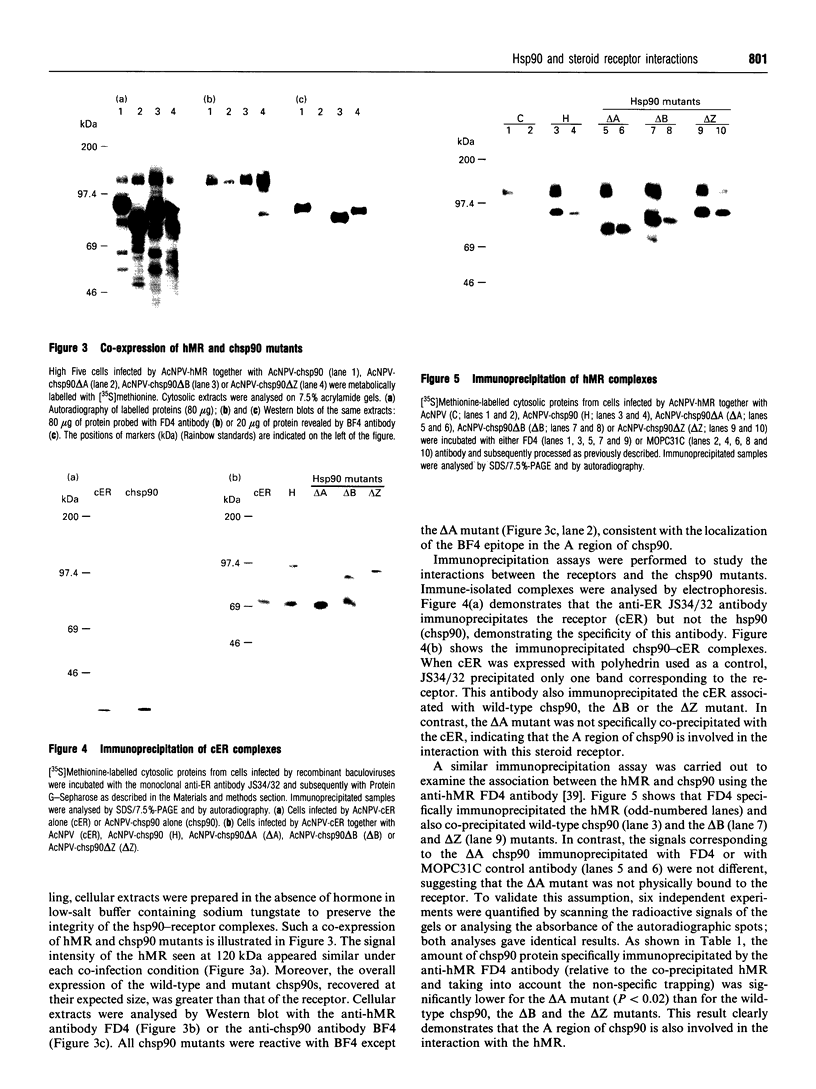
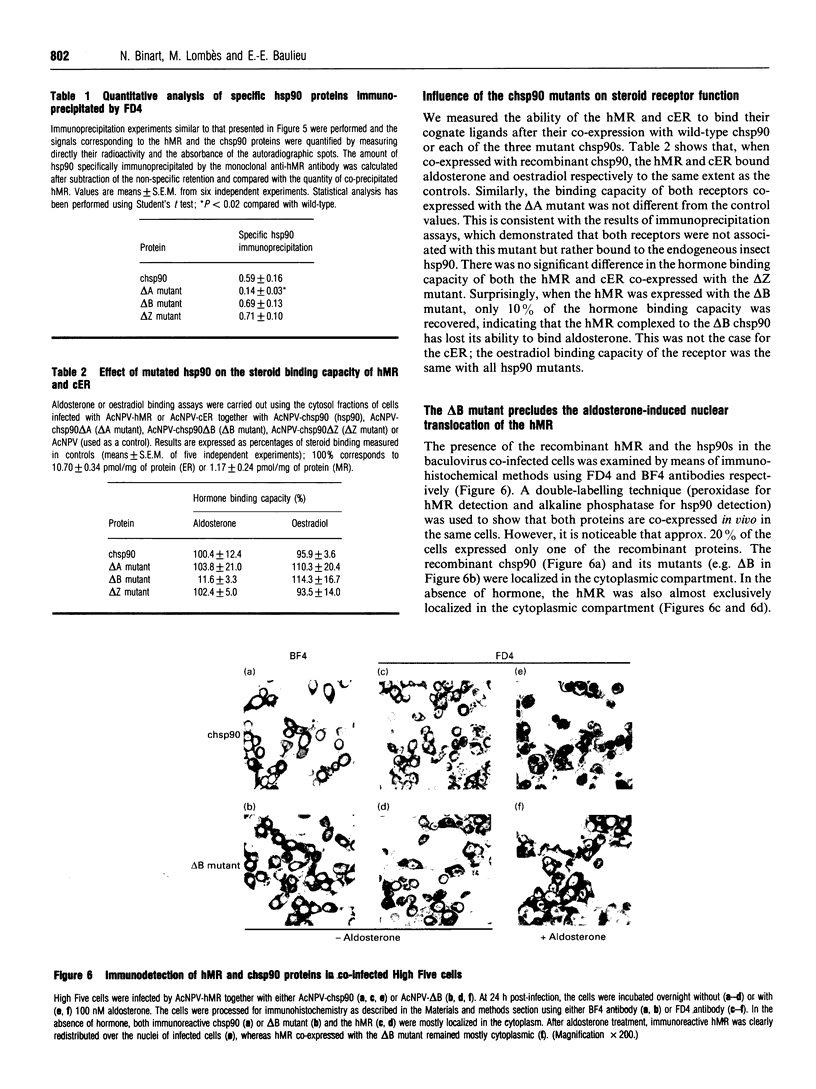
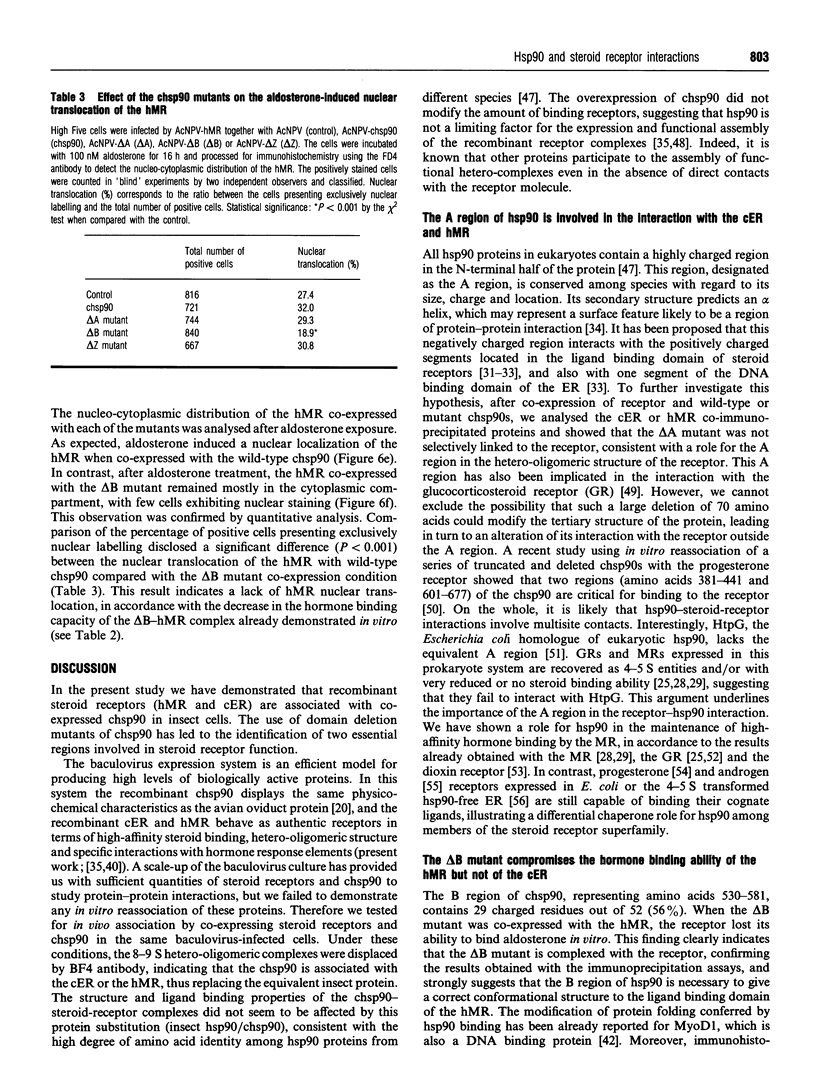
Recent studies have confirmed that the 90 kDa heat-shock protein (hsp90) interacts both in vitro and in vivo with steroid receptors, encouraging further detailed physicochemical and functional analysis of its chaperone role. Thus, to explore the relationship between hsp90 and receptors, the baculovirus system was used to overexpress the chick hsp90 alpha (chsp90) along with the chick oestradiol receptor (cER) or the human mineralocorticosteroid receptor (hMR). These receptors were able to form 9 S complexes with chsp90, demonstrating the association of the co-expressed recombinant proteins. Three mutants of chsp90 (delta A, delta B and delta Z) have been created by deletion of the A (residues 221-290) and B (530-581) regions, rich in charged amino acids, and the Z (392-419) region, a putative leucine zipper. After co-expression, anti-receptor antibodies immunoprecipitated the cER or hMR complexed with the wild-type chsp90, the delta B or the delta Z mutant, but not with the delta A chsp90, indicating that deletion of the A region of chsp90 leads to a lack of interaction with these receptors. The hormone binding capacity of the cER was unaffected after its co-expression with each of the three mutants. In contrast, the hMR co-expressed with the delta B mutant failed to bind aldosterone, a finding confirmed in vivo by the absence of hormone-induced hMR nuclear translocation. Thus the B region is required for high-affinity ligand binding by the hMR. Our results suggest that the A region (but not the B or Z regions) is involved in binding of chsp90 to the cER and hMR, while the B region is essential for hormone binding by the hMR, consistent with a chaperone function for hsp90.
Full text
PDF


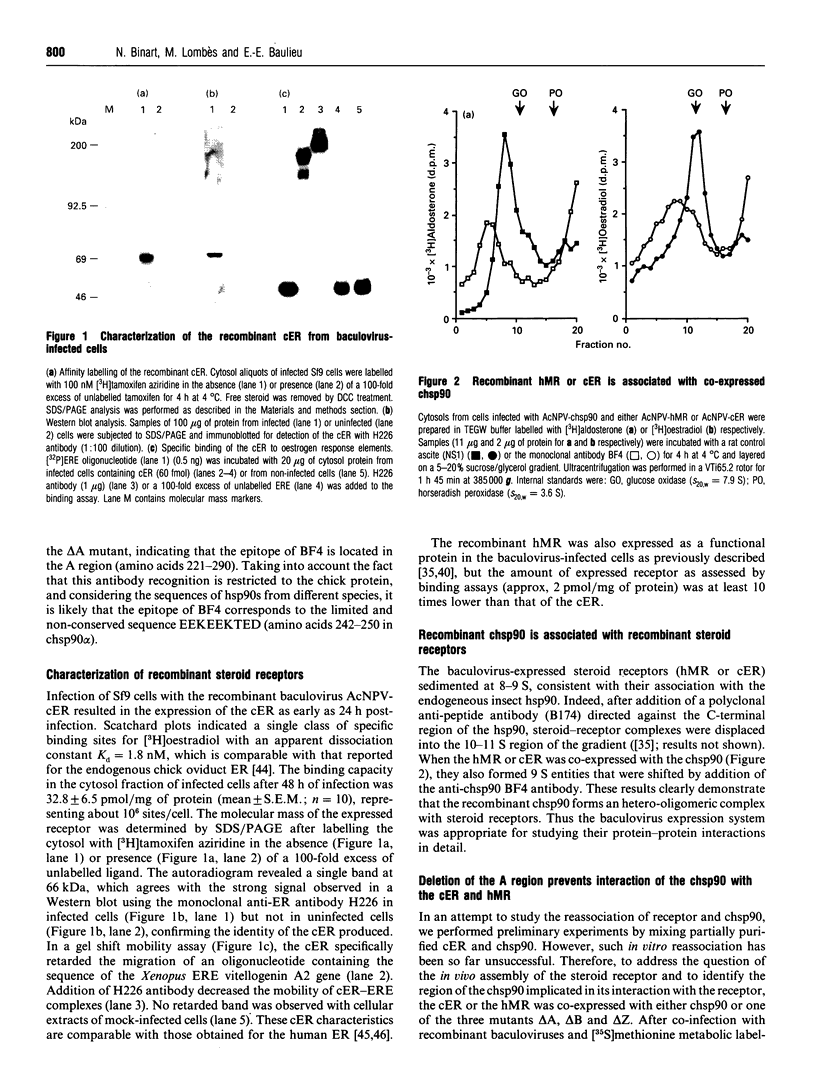

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Litwack G. The steroid binding domain influences intracellular solubility of the baculovirus overexpressed glucocorticoid and mineralocorticoid receptors. Biochemistry. 1993 May 25;32(20):5387–5393. doi: 10.1021/bi00071a014. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Steroid hormone antagonists at the receptor level: a role for the heat-shock protein MW 90,000 (hsp 90). J Cell Biochem. 1987 Oct;35(2):161–174. doi: 10.1002/jcb.240350209. [DOI] [PubMed] [Google Scholar]
- Best-Belpomme M., Mester J., Weintraub H., Baulieu E. E. Oestrogen receptors in chick oviduct. Characterization and subcellular distribution. Eur J Biochem. 1975 Sep 15;57(2):537–547. doi: 10.1111/j.1432-1033.1975.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Binart N., Chambraud B., Dumas B., Rowlands D. A., Bigogne C., Levin J. M., Garnier J., Baulieu E. E., Catelli M. G. The cDNA-derived amino acid sequence of chick heat shock protein Mr 90,000 (HSP 90) reveals a "DNA like" structure: potential site of interaction with steroid receptors. Biochem Biophys Res Commun. 1989 Feb 28;159(1):140–147. doi: 10.1016/0006-291x(89)92415-7. [DOI] [PubMed] [Google Scholar]
- Binart N., Chambraud B., Levin J. M., Garnier J., Baulieu E. E. A highly charged sequence of chick hsp90: a good candidate for interaction with steroid receptors. J Steroid Biochem. 1989;34(1-6):369–374. doi: 10.1016/0022-4731(89)90110-6. [DOI] [PubMed] [Google Scholar]
- Binart N., Lombes M., Rafestin-Oblin M. E., Baulieu E. E. Characterization of human mineralocorticosteroid receptor expressed in the baculovirus system. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10681–10685. doi: 10.1073/pnas.88.23.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Sharp P. A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990 Jul 5;265(19):11238–11243. [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Caamaño C. A., Morano M. I., Patel P. D., Watson S. J., Akil H. A bacterially expressed mineralocorticoid receptor is associated in vitro with the 90-kilodalton heat shock protein and shows typical hormone- and DNA-binding characteristics. Biochemistry. 1993 Aug 24;32(33):8589–8595. doi: 10.1021/bi00084a028. [DOI] [PubMed] [Google Scholar]
- Cadepond F., Binart N., Chambraud B., Jibard N., Schweizer-Groyer G., Segard-Maurel I., Baulieu E. E. Interaction of glucocorticosteroid receptor and wild-type or mutated 90-kDa heat shock protein coexpressed in baculovirus-infected Sf9 cells. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10434–10438. doi: 10.1073/pnas.90.22.10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambraud B., Berry M., Redeuilh G., Chambon P., Baulieu E. E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J Biol Chem. 1990 Nov 25;265(33):20686–20691. [PubMed] [Google Scholar]
- Denis M., Gustafsson J. A., Wikström A. C. Interaction of the Mr = 90,000 heat shock protein with the steroid-binding domain of the glucocorticoid receptor. J Biol Chem. 1988 Dec 5;263(34):18520–18523. [PubMed] [Google Scholar]
- Denis M., Wikström A. C., Gustafsson J. A. The molybdate-stabilized nonactivated glucocorticoid receptor contains a dimer of Mr 90,000 non-hormone-binding protein. J Biol Chem. 1987 Aug 25;262(24):11803–11806. [PubMed] [Google Scholar]
- Eul J., Meyer M. E., Tora L., Bocquel M. T., Quirin-Stricker C., Chambon P., Gronemeyer H. Expression of active hormone and DNA-binding domains of the chicken progesterone receptor in E. coli. EMBO J. 1989 Jan;8(1):83–90. doi: 10.1002/j.1460-2075.1989.tb03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hutchison K. A., Czar M. J., Scherrer L. C., Pratt W. B. Monovalent cation selectivity for ATP-dependent association of the glucocorticoid receptor with hsp70 and hsp90. J Biol Chem. 1992 Jul 15;267(20):14047–14053. [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Kang K. I., Devin J., Cadepond F., Jibard N., Guiochon-Mantel A., Baulieu E. E., Catelli M. G. In vivo functional protein-protein interaction: nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):340–344. doi: 10.1073/pnas.91.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Nishida E., Kadowaki T., Matsuzaki F., Iida K., Harada F., Kasuga M., Sakai H., Yahara I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8054–8058. doi: 10.1073/pnas.83.21.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombès M., Binart N., Delahaye F., Baulieu E. E., Rafestin-Oblin M. E. Differential intracellular localization of human mineralocorticosteroid receptor on binding of agonists and antagonists. Biochem J. 1994 Aug 15;302(Pt 1):191–197. doi: 10.1042/bj3020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombès M., Binart N., Oblin M. E., Joulin V., Baulieu E. E. Characterization of the interaction of the human mineralocorticosteroid receptor with hormone response elements. Biochem J. 1993 Jun 1;292(Pt 2):577–583. doi: 10.1042/bj2920577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Moncharmont B., Su J. L., Parikh I. Monoclonal antibodies against estrogen receptor: interaction with different molecular forms and functions of the receptor. Biochemistry. 1982 Dec 21;21(26):6916–6921. doi: 10.1021/bi00269a046. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Denis M., Gustafsson J. A. The transformed glucocorticoid receptor has a lower steroid-binding affinity than the nontransformed receptor. Biochemistry. 1990 Feb 20;29(7):1880–1886. doi: 10.1021/bi00459a031. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Ota M. Association of the 90-kDa heat shock protein does not affect the ligand-binding ability of androgen receptor. J Steroid Biochem Mol Biol. 1992 Sep;42(8):803–812. doi: 10.1016/0960-0760(92)90088-z. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Sato N., Ota M. Dual roles of 90-kDa heat shock protein in the function of the mineralocorticoid receptor. J Biochem. 1993 Jun;113(6):769–775. [PubMed] [Google Scholar]
- Obourn J. D., Koszewski N. J., Notides A. C. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993 Jun 22;32(24):6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pongratz I., Mason G. G., Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992 Jul 5;267(19):13728–13734. [PubMed] [Google Scholar]
- Pratt W. B., Czar M. J., Stancato L. F., Owens J. K. The hsp56 immunophilin component of steroid receptor heterocomplexes: could this be the elusive nuclear localization signal-binding protein? J Steroid Biochem Mol Biol. 1993 Sep;46(3):269–279. doi: 10.1016/0960-0760(93)90216-j. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Jolly D. J., Pratt D. V., Hollenberg S. M., Giguere V., Cadepond F. M., Schweizer-Groyer G., Catelli M. G., Evans R. M., Baulieu E. E. A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem. 1988 Jan 5;263(1):267–273. [PubMed] [Google Scholar]
- Radanyi C., Joab I., Renoir J. M., Richard-Foy H., Baulieu E. E. Monoclonal antibody to chicken oviduct progesterone receptor. Proc Natl Acad Sci U S A. 1983 May;80(10):2854–2858. doi: 10.1073/pnas.80.10.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanyi C., Renoir J. M., Sabbah M., Baulieu E. E. Chick heat-shock protein of Mr = 90,000, free or released from progesterone receptor, is in a dimeric form. J Biol Chem. 1989 Feb 15;264(5):2568–2573. [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Couette B., Radanyi C., Lombes M., Baulieu E. E. Mineralocorticosteroid receptor of the chick intestine. Oligomeric structure and transformation. J Biol Chem. 1989 Jun 5;264(16):9304–9309. [PubMed] [Google Scholar]
- Redeuilh G., Moncharmont B., Secco C., Baulieu E. E. Subunit composition of the molybdate-stabilized "8-9 S" nontransformed estradiol receptor purified from calf uterus. J Biol Chem. 1987 May 25;262(15):6969–6975. [PubMed] [Google Scholar]
- Rose D. W., Welch W. J., Kramer G., Hardesty B. Possible involvement of the 90-kDa heat shock protein in the regulation of protein synthesis. J Biol Chem. 1989 Apr 15;264(11):6239–6244. [PubMed] [Google Scholar]
- Sabbah M., Redeuilh G., Baulieu E. E. Subunit composition of the estrogen receptor. Involvement of the hormone-binding domain in the dimeric state. J Biol Chem. 1989 Feb 15;264(5):2397–2400. [PubMed] [Google Scholar]
- Sanchez E. R., Redmond T., Scherrer L. C., Bresnick E. H., Welsh M. J., Pratt W. B. Evidence that the 90-kilodalton heat shock protein is associated with tubulin-containing complexes in L cell cytosol and in intact PtK cells. Mol Endocrinol. 1988 Aug;2(8):756–760. doi: 10.1210/mend-2-8-756. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Scherrer L. C., Dalman F. C., Massa E., Meshinchi S., Pratt W. B. Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J Biol Chem. 1990 Dec 15;265(35):21397–21400. [PubMed] [Google Scholar]
- Scherrer L. C., Picard D., Massa E., Harmon J. M., Simons S. S., Jr, Yamamoto K. R., Pratt W. B. Evidence that the hormone binding domain of steroid receptors confers hormonal control on chimeric proteins by determining their hormone-regulated binding to heat-shock protein 90. Biochemistry. 1993 May 25;32(20):5381–5386. doi: 10.1021/bi00071a013. [DOI] [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Shaknovich R., Shue G., Kohtz D. S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84). Mol Cell Biol. 1992 Nov;12(11):5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Toft D. O. Mutational analysis of hsp90 binding to the progesterone receptor. J Biol Chem. 1993 Sep 25;268(27):20373–20379. [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]