Abstract
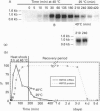
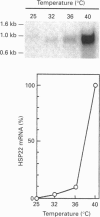
A 3 h treatment at 40 degrees C of pea (Pisum sativum var. Douce Provence) plants induces production and accumulation of a small heat-shock protein of 22 kDa apparent molecular mass, designated HSP22, in the matrix compartment of mitochondria [Lenne and Douce (1994) Plant Physiol. 105, 1255-1261]. We show here that the HSP22 precursor (i.e. the mature protein plus the transit peptide) has an apparent molecular mass of 26 kDa after in vitro translation of mRNA extracted from heat-stressed pea plants and immunodetection. We have isolated, cloned and sequenced the full-length cDNA encoding the precursor of the mitochondrial HSP22. An analysis of the amino acid sequence of the mitochondrial HSP22 reveals that this protein is a representative member of the low-molecular-mass heat shock protein (HSP) superfamily, exhibiting the specific consensus regions that are typical of the small HSPs. Most importantly, comparison of the mitochondrial HSP22 sequence with that of chloroplast small HSPs indicates that HSP22 does not contain the typical chloroplast consensus region III. We have also analysed the kinetics of HSP22 induction, and report results on the temporal expression of HSP22 at the transcriptional level. HSP22 mRNA was detected as soon as 10 min after the temperature was raised to a high temperature of 40 degrees C. Then the amount of HSP22 mRNA declined considerably even though pea plants were still submitted to the heat treatment. These results are discussed in light of the translation data previously published [Lenne and Douce (1994) Plant Physiol. 105, 1255-1261], particularly concerning the physiological behaviour of mitochondria when plants are heat-stressed. Furthermore, we have studied the dependence of HSP22 accumulation with temperature and demonstrate that the pea mitochondrial heat-shock response is only developed under extreme environmental growth conditions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988 Dec;8(12):5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bourguignon J., Macherel D., Neuburger M., Douce R. Isolation, characterization, and sequence analysis of a cDNA clone encoding L-protein, the dihydrolipoamide dehydrogenase component of the glycine cleavage system from pea-leaf mitochondria. Eur J Biochem. 1992 Mar 1;204(2):865–873. doi: 10.1111/j.1432-1033.1992.tb16706.x. [DOI] [PubMed] [Google Scholar]
- Chen Q., Vierling E. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol Gen Genet. 1991 May;226(3):425–431. doi: 10.1007/BF00260655. [DOI] [PubMed] [Google Scholar]
- Derocher A. E., Helm K. W., Lauzon L. M., Vierling E. Expression of a Conserved Family of Cytoplasmic Low Molecular Weight Heat Shock Proteins during Heat Stress and Recovery. Plant Physiol. 1991 Aug;96(4):1038–1047. doi: 10.1104/pp.96.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goping I. S., Frappier J. R., Walden D. B., Atkinson B. G. Sequence, identification and characterization of cDNAs encoding two different members of the 18 kDa heat shock family of Zea mays L. Plant Mol Biol. 1991 Apr;16(4):699–711. doi: 10.1007/BF00023434. [DOI] [PubMed] [Google Scholar]
- Helm K. W., LaFayette P. R., Nagao R. T., Key J. L., Vierling E. Localization of small heat shock proteins to the higher plant endomembrane system. Mol Cell Biol. 1993 Jan;13(1):238–247. doi: 10.1128/mcb.13.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Soltanifar N., Goldschmidt-Clermont M., Rochaix J. D., Schatz G. The cleavable pre-sequence of an imported chloroplast protein directs attached polypeptides into yeast mitochondria. EMBO J. 1986 Jun;5(6):1343–1350. doi: 10.1002/j.1460-2075.1986.tb04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel J. A., Nagao R. T., Goekjian V., Key J. L. Regulation of the heat shock response in soybean seedlings. Plant Physiol. 1990 Nov;94(3):988–995. doi: 10.1104/pp.94.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knack G., Kloppstech K. cDNA sequence of a heat-inducible protein of Chenopodium sharing little homology with other heat-shock proteins. Nucleic Acids Res. 1989 Jul 11;17(13):5380–5380. doi: 10.1093/nar/17.13.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knack G., Liu Z., Kloppstech K. Low molecular mass heat-shock proteins of a light-resistant photoautotrophic cell culture. Eur J Cell Biol. 1992 Oct;59(1):166–175. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lauzon L. M., Helm K. W., Vierling E. A cDNA clone from Pisum sativum encoding a low molecular weight heat shock protein. Nucleic Acids Res. 1990 Jul 25;18(14):4274–4274. doi: 10.1093/nar/18.14.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne C., Douce R. A Low Molecular Mass Heat-Shock Protein Is Localized to Higher Plant Mitochondria. Plant Physiol. 1994 Aug;105(4):1255–1261. doi: 10.1104/pp.105.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J., Vierling E., Ho T. H. Cloning, sequence analysis, and expression of a cDNA encoding a plastid-localized heat shock protein in maize. Plant Physiol. 1990 Aug;93(4):1321–1328. doi: 10.1104/pp.93.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992 May 15;203(1):173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Raschke E., Nagao R. T. The DNA sequence analysis of soybean heat-shock genes and identification of possible regulatory promoter elements. EMBO J. 1984 Nov;3(11):2491–2497. doi: 10.1002/j.1460-2075.1984.tb02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. L. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. doi: 10.1128/mcb.9.11.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Vierling E., Nagao R. T., DeRocher A. E., Harris L. M. A heat shock protein localized to chloroplasts is a member of a eukaryotic superfamily of heat shock proteins. EMBO J. 1988 Mar;7(3):575–581. doi: 10.1002/j.1460-2075.1988.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Arizmendi J. M., Dupuis A., Fearnley I. M., Finel M., Medd S. M., Pilkington S. J., Runswick M. J., Skehel J. M. Sequences of 20 subunits of NADH:ubiquinone oxidoreductase from bovine heart mitochondria. Application of a novel strategy for sequencing proteins using the polymerase chain reaction. J Mol Biol. 1992 Aug 20;226(4):1051–1072. doi: 10.1016/0022-2836(92)91052-q. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]