Abstract
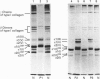
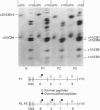
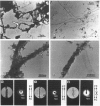
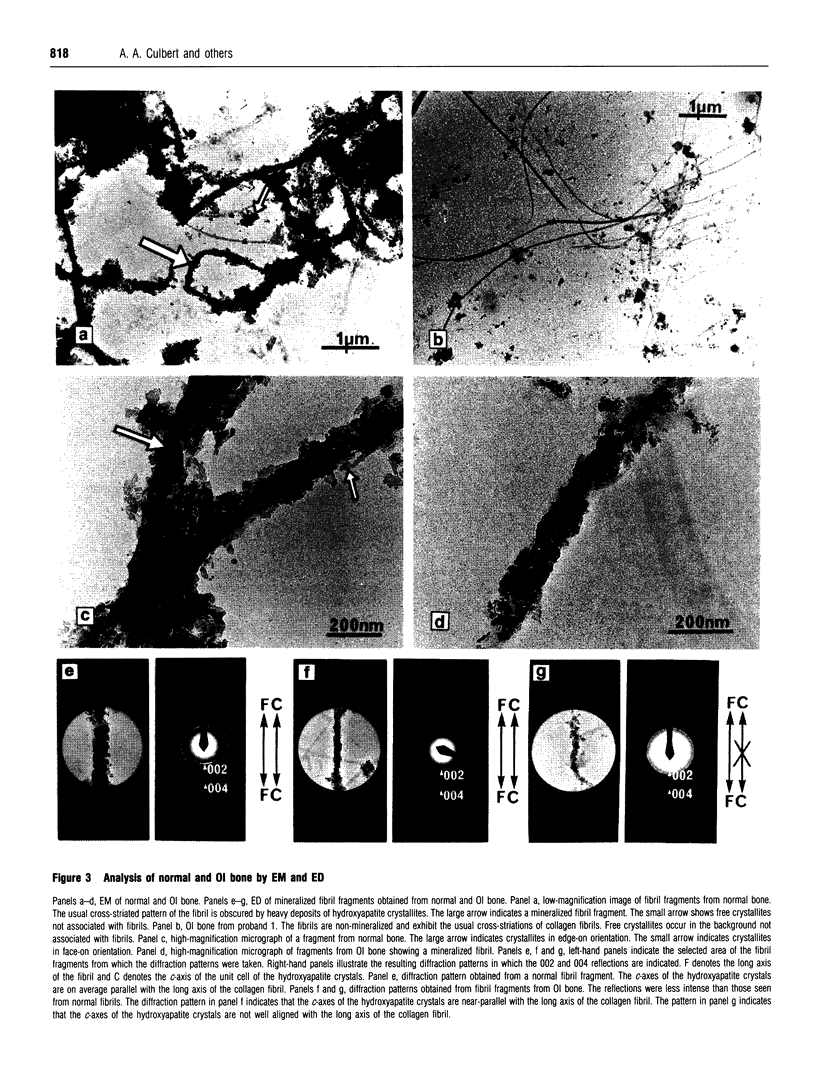
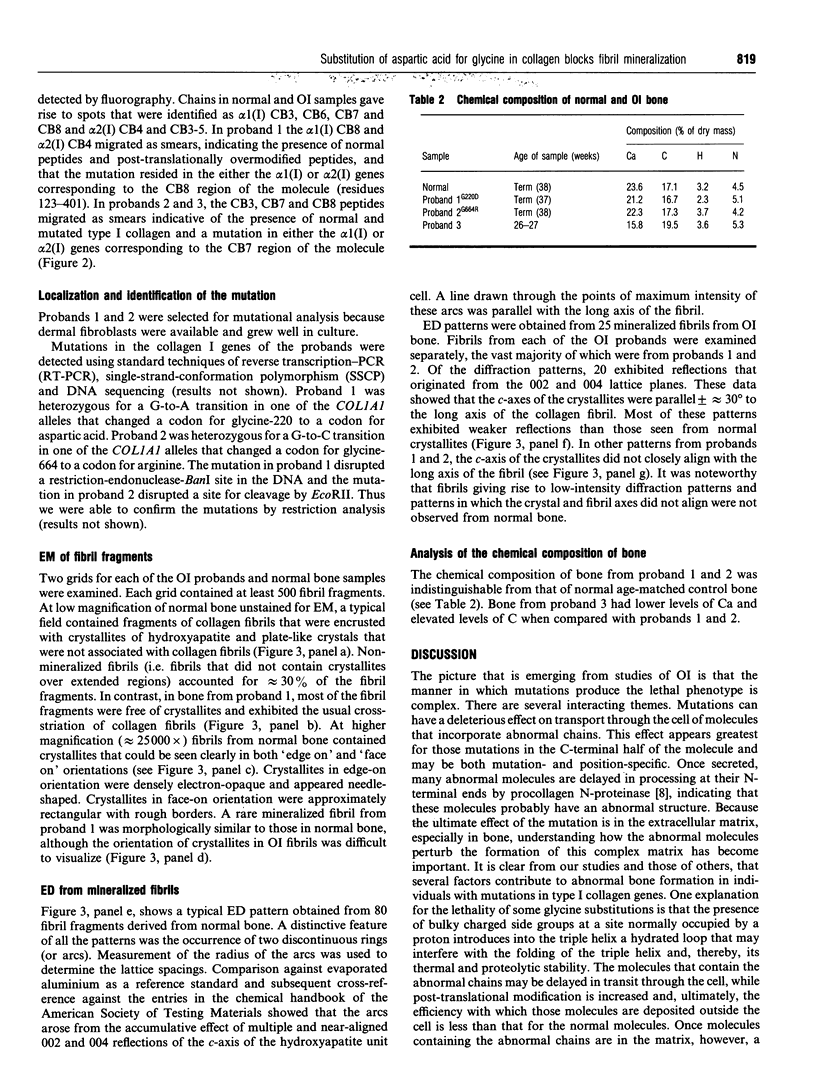
We identified two infants with lethal (type II) osteogenesis imperfecta (OI) who were heterozygous for mutations in the COL1A1 gene that resulted in substitutions of aspartic acid for glycine at position 220 and arginine for glycine at position 664 in the product of one COL1A1 allele in each individual. In normal age- and site-matched bone, approximately 70% (by number) of the collagen fibrils were encrusted with plate-like crystallites of hydroxyapatite. In contrast, approximately 5% (by number) of the collagen fibrils in the probands' bone contained crystallites. In contrast with normal bone, the c-axes of hydroxyapatite crystallites were sometimes poorly aligned with the long axis of fibrils obtained from OI bone. Chemical analysis showed that the OI samples contained normal amounts of calcium. The probands' bone samples contained type I collagen, overmodified type I collagen and elevated levels of type III and V collagens. On the basis of biochemical and morphological data, the fibrils in the OI samples were co-polymers of normal and mutant collagen. The results are consistent with a model of fibril mineralization in which the presence of abnormal type I collagen prevents normal collagen in the same fibril from incorporating hydroxyapatite crystallites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi E., Hayashi T. In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res. 1986;14(4):257–266. doi: 10.3109/03008208609017469. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J., Schuppan D., Rabanus J. P., Rauch R., Niechoy U., Gelderblom H. R. Immunoelectron microscopic localization of collagens type I, V, VI and of procollagen type III in human periodontal ligament and cementum. J Histochem Cytochem. 1991 Jan;39(1):103–110. doi: 10.1177/39.1.1983870. [DOI] [PubMed] [Google Scholar]
- Bella J., Eaton M., Brodsky B., Berman H. M. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994 Oct 7;266(5182):75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Fitch J. M., Babiarz J. P., Doane K. J., Linsenmayer T. F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990 Apr;95(Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Brenner R. E., Vetter U., Nerlich A., Wörsdorfer O., Teller W. M., Müller P. K. Osteogenesis imperfecta: insufficient collagen synthesis in early childhood as evidenced by analysis of compact bone and fibroblast cultures. Eur J Clin Invest. 1989 Apr;19(2):159–166. doi: 10.1111/j.1365-2362.1989.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Chessler S. D., Wallis G. A., Byers P. H. Mutations in the carboxyl-terminal propeptide of the pro alpha 1(I) chain of type I collagen result in defective chain association and produce lethal osteogenesis imperfecta. J Biol Chem. 1993 Aug 25;268(24):18218–18225. [PubMed] [Google Scholar]
- Cohen-Solal L., Zylberberg L., Sangalli A., Gomez Lira M., Mottes M. Substitution of an aspartic acid for glycine 700 in the alpha 2(I) chain of type I collagen in a recurrent lethal type II osteogenesis imperfecta dramatically affects the mineralization of bone. J Biol Chem. 1994 May 20;269(20):14751–14758. [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Timpl R., Olsen B. R. Procollagen intermediates during tendon fibrillogenesis. J Histochem Cytochem. 1988 Nov;36(11):1425–1432. doi: 10.1177/36.11.3049791. [DOI] [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987 Nov 15;262(32):15696–15701. [PubMed] [Google Scholar]
- Kadler K. Extracellular matrix. 1: fibril-forming collagens. Protein Profile. 1994;1(5):519–638. [PubMed] [Google Scholar]
- Lightfoot S. J., Holmes D. F., Brass A., Grant M. E., Byers P. H., Kadler K. E. Type I procollagens containing substitutions of aspartate, arginine, and cysteine for glycine in the pro alpha 1 (I) chain are cleaved slowly by N-proteinase, but only the cysteine substitution introduces a kink in the molecule. J Biol Chem. 1992 Dec 15;267(35):25521–25528. [PubMed] [Google Scholar]
- Pope F. M., Nicholls A. C., Eggleton C., Narcissi P., Hey E. N., Parkin J. M. Osteogenesis imperfecta (lethal) bones contain types III and V collagens. J Clin Pathol. 1980 Jun;33(6):534–538. doi: 10.1136/jcp.33.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic A. M., Adachi E., Kadler K. E., Hojima Y., Prockop D. J. Copolymerization of pNcollagen III and collagen I. pNcollagen III decreases the rate of incorporation of collagen I into fibrils, the amount of collagen I incorporated, and the diameter of the fibrils formed. J Biol Chem. 1991 Jul 5;266(19):12703–12709. [PubMed] [Google Scholar]
- Sokolov B. P., Sher B. M., Hausmann J., Marik I., Deyl Z., Kalinin V. N. Altered ratio of collagen chains in bone of a patient with non-lethal osteogenesis imperfecta. Biochim Biophys Acta. 1992 Feb 14;1138(2):93–96. doi: 10.1016/0925-4439(92)90047-q. [DOI] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Traub W., Arad T., Vetter U., Weiner S. Ultrastructural studies of bones from patients with osteogenesis imperfecta. Matrix Biol. 1994 Aug;14(4):337–345. doi: 10.1016/0945-053x(94)90200-3. [DOI] [PubMed] [Google Scholar]