Abstract
Background
Given that viral infections can increase the risk of adverse pregnancy outcomes, such as spontaneous miscarriage, preterm premature rupture of membranes, and preterm birth, the effects of COVID-19, a novel emerging coronavirus disease rapidly spreading globally, on pregnancy outcomes have garnered significant attention.
Methods
We conducted a review of studies related to pregnant women infected with SARS-CoV-2 over the past five years (December 2019 to April 2023), utilizing search engines such as PubMed, Web of Science, and the China National Knowledge Infrastructure (CNKI). This study was registered with PROSPERO with ID: CRD42024540849.
Results
A total of 218 articles were screened, with 15 studies meeting the inclusion criteria for this research, including 12 cohort studies, one cross-sectional study, one case-control study, and one case series. Six studies found that the preterm birth rate was higher in the infected group compared to the control group; five studies showed that the cesarean section rate was higher in the infected group; three studies found that the APGAR scores of newborns were higher in the control group than in the infected group; three studies indicated that the mortality rate of newborns in the infected group was higher than that in the control group.
Conclusions
Our retrospective review suggests that compared to pregnant women not infected with SARS-CoV-2, those diagnosed with COVID-19 are more likely to experience adverse outcomes such as preterm birth, cesarean delivery, and low birth weight in newborns.
Keywords: COVID-19, Pregnancy, Viral infections, Pregnancy outcomes, Neonatal outcomes
Background
Coronavirus Disease 2019 (COVID-19), caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a single-stranded RNA virus belonging to the subgenus Sarbecovirus of the β-coronavirus genus, has rapidly spread to numerous countries, promptly triggering a global health emergency. The World Health Organization (WHO) declared the outbreak a pandemic on March 12, 2020 [1]. The virus poses a risk of infection to everyone, from adults and adolescents to children, and notably to pregnant women and newborns [2]. As the pandemic progressed, the primary strains of the virus have evolved and mutated from the original strain to the Alpha, Beta, Gamma, Delta, Omicron, and XBB variant lineages. As of February 2024, a hospital in Beijing reported a positivity rate of 21.1% for COVID-19 through influenza-like illness surveillance, with the JN.1 variant being the predominant strain [3].
Due to physiological changes in the immune and cardiopulmonary systems, pregnant women are at increased risk of acquiring viral respiratory infections and developing severe pneumonia, which can lead to serious outcomes, including the need for tracheal intubation, admission to the Intensive Care Unit (ICU), renal failure, and death [4]. Experiences with viral infections during pregnancy have shown that these infections can lead to increased maternal complications such as spontaneous miscarriage, preterm premature rupture of membranes, and preterm birth. Severe infections may also result in acute respiratory distress syndrome and multiorgan failure among other serious complications. It is well recognized that viral pneumonia, particularly with comorbidities such as chronic cardiovascular and respiratory diseases and/or obesity, can significantly increase morbidity in pregnant women and newborns, raising numerous concerns about the impact of COVID-19 infections on pregnant women [2]. Pregnancy has been reported as an independent risk factor for adverse outcomes in women infected with COVID-19, especially in the presence of comorbidities such as diabetes or preeclampsia. The specific changes in the cardiopulmonary system that occur during pregnancy may partly explain the increased morbidity in pregnant women compared to the general non-pregnant population [5].
In light of the vast array of studies that have emerged since the onset of the pandemic, and considering that these studies form the basis for clinical decision-making for this sensitive patient group, we have decided to conduct a systematic descriptive analysis of existing research. This analysis focuses on the pregnancy and neonatal outcomes of women infected with COVID-19, aiming to assess the impact of COVID-19 infections on pregnancy outcomes in women of reproductive age, thereby providing a basis and reference for further clinical decision-making and accurate medication use.
Methods
Eligibility criteria
Types of studies
All observational studies, retrospective cohort studies, cross-sectional studies, case-control studies, and Randomized Controlled Trials (RCTs) addressing the impact of COVID-19 on pregnancy outcomes in women were included. There were no restrictions on the year of publication, study location, or language.
Inclusion criteria
Based on the inclusion criteria of the studies reviewed, patients who tested positive for SARS-CoV-2 using RT-PCR (Reverse Transcription-Polymerase Chain Reaction) on nasopharyngeal swabs and/or had a positive chest CT scan at any stage of pregnancy and were admitted for delivery, regardless of clinical signs, were considered as COVID-19 infected patients and included in this study.
Exclusion criteria
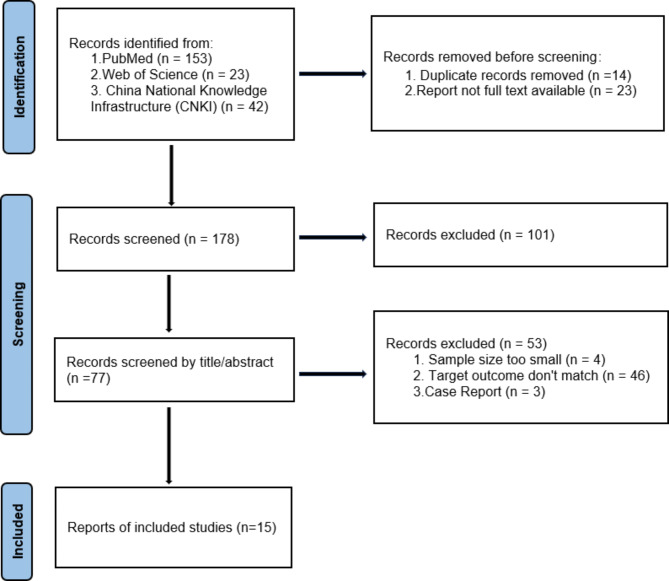
The exclusion criteria were studies with missing data or inaccessible full texts, reports that were not peer-reviewed or unpublished, studies without specified dates and locations, suspected duplicate reports, and studies that did not report pregnancy or neonatal outcomes. Detailed exclusion criteria are illustrated in the research flow diagram (Fig. 1).
Fig. 1.
flow-chart*
*Excluding 12 studies after reading the full article
Outcome measures
The primary outcome measures were maternal pregnancy outcomes (including preterm birth (< 37 weeks), mode of delivery, maternal mortality) and neonatal outcomes (including low birth weight (< 2500 g), APGAR scores, neonatal mortality). Secondary outcome measures included the incidence of pregnancy complications. The original studies included at least one of the following outcomes: maternal pregnancy outcomes, neonatal outcomes, pregnancy complications, and medication treatments.
COVID-19 severity of disease
We categorized the severity of disease according to the clinical symptoms recorded by the women included in the study as follows: (1) Asymptomatic: no clinically significant symptoms were present. (2) Mild-moderate: mild symptoms, such as fever, cough, myalgia, fatigue, shortness of breath, and respiratory distress, etc. (3) Severe: severe pulmonary lesions or other serious complications, admission to ICU, required intubation and invasive ventilation ( admitted to intensive care unit (ICU), required intubation and invasive ventilation) and etc.
Literature search strategy
English databases (including PubMed, Web of Science) and Chinese databases (China National Knowledge Infrastructure (CNKI)) were searched, covering the period from January 2019 to April 2023. A combination of Medical Subject Headings (MeSH) terms and keywords such as “COVID-19,” “Pregnancy,” “Pregnancy Outcomes,” “Neonatal Outcomes,” “Preterm Birth,” “Cesarean Section” was used for full-text searches. After removing duplicates, titles, abstracts, and full texts were screened and checked against the predefined inclusion and exclusion criteria. Extracted data included the first author, country of the study, type of study, sample size, primary outcomes, secondary outcomes, and key findings. This study was registered with PROSPERO with ID: CRD42024540849.
Results
A search and screening of literature from three databases yielded 218 articles, of which 15 studies met the inclusion criteria. Among these included studies, 12 were cohort studies, one was a cross-sectional study, one was a case-control study, and one was a case series study. The studies were conducted across various countries and age groups, each differing in scale. Nine studies (66.7%) were carried out in Asia, four (26.7%) in Europe (with three (20%) in the United Kingdom), one (6.7%) in Africa, and one (6.7%) in North America. All participants were women of reproductive age (≥ 18 years). All 15 studies documented pregnancy complications, with the most common being gestational diabetes mellitus (GDM), preeclampsia, and hypertension. Nine studies reported on the medication treatments for pregnant women infected with COVID-19, including chloroquine, antibiotics, antivirals, and steroids; however, specific details regarding drug dosages and effects were not provided. Moreover, there was a lack of comparative data for pregnant women, particularly in comparison with treatments proven effective in the general population, thus precluding any clinically meaningful interpretation. The characteristics of the included studies are summarized in Table 3-1.
Table 3-1.
Characteristics of the included studies
| Research | Type of Research | Nation | Sample Size | severity of the disease* | complications | Medications | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Pirjani R;2020 [16] | prospective cohort study | Iran | Infected pregnant women: 66 VS Non-infected pregnant women: 133 |
②: 62/66 (93.94%), ③: 4/66 (6.06%) |
GDM, Preeclampsia, PROM |
Chloroquine, lopinavir/ritonavir, Oseltamivir |
PB, Delivery type, LBW, APGAR score, Neonatal death |
A significant difference was found in term of delivery type |
| Adhikari EH;2020 [17] | observational cohort study | America | Infected pregnant women: 245 VS Non-infected pregnant women: 3035 |
①: 107/252 (42.46%), ②: 142/252 (56.35%), ③: 3/252 (1.19%) |
GDM, Preeclampsia | Remdesivir, dexamethasone, interleukin-6 inhibitor |
PB, Delivery type, APGAR score, Neonatal death |
SARS-CoV-2 infection in pregnancy is not associated with adverse pregnancy outcomes |
| Alipour Z;2021 [15] | Retrospective analytical cohort study | Iran | Infected pregnant women: 53 VS Non-infected pregnant women: 135 |
②: 118/133 (88.7%), ③: 15/133 (11.3%) |
GDM, Preeclampsia, Hypertension |
Antibiotic, Antiviral |
PB, Delivery type, Maternal mortality, LBW, APGAR score |
Adverse maternal and fetal outcomes, including caesarean section, preterm birth. |
| Binte Masud S;2021 [14] | group-comparison cross-sectional study | Bangladesh | Infected pregnant women: 70 VS Non-infected pregnant women: 140 | — |
Diabetes, Preeclampsia, Hypertension |
— |
PB, Delivery type, Low Birth LBW, APGAR score, Neonatal death |
Undergoing a CS delivery and giving birth to preterm babie |
| Gupta P;2021 [20] | Retrospective analytical cohort study | India | Infected pregnant women: 108 VS Non-infected pregnant women: 3057 |
①: 93/108 (86.1%), ②: 13/108 (12%), ③: 2/108 (1.8%) |
GDM, Pregnancy-induced Hypertension |
Antibiotics, Dexamethasone |
PB, Delivery type, Maternal Death, APGAR score, Neonatal death |
Unfavorable perinatal outcomes including higher rates of cesarean delivery, preterm birth, low birth weight, and low Apgar score and higher rates of complications |
| Gurol-Urganci I;2021 [6] | present retro-prospective study | England | Infected pregnant women: 3527 VS Non-infected pregnant women: 338,553 | — | Preeclampsia | — |
PB, Delivery type, APGAR score, Neonatal death |
Having higher rates of fetal death and preterm birth, preeclampsia and emergency cesarean delivery |
| Oncel MY;2021 [7] | multicenter cohort study | Turkish | Infected pregnant women: 125 | ③: 8/125 (6.4%) |
GDM, Preeclampsia, Hypertension |
— |
PB, Delivery type, Maternal Death, APGAR score, Neonatal death |
Maternal mortality, higher rates of preterm birth and cesarean section |
| Al Hashmi I;2022 [8] | Multi-Center Case-Control Comparison Study | Middle-Eastern countries | Infected pregnant women: 174 VS Non-infected pregnant women: 87 |
②: 65/87 (74.71%), ③: 12/87 (13.79%) |
Preeclampsia |
Azithromycin, Hydroxychloroquine monotherapy, Amoxacillin-clavulanate, Ceftriaxone, Steroids, Other medication |
PB, Delivery type, Maternal Death, APGAR score, Neonatal death |
Preeclampsia, preterm labor, requiring cesarean section, and low neonatal Apgar score were more common in high-risk pregnant women |
| Yang R;2019 [18] | a population-based cohort study | China | Infected pregnant women: 65 VS Non-infected pregnant women: 11,013 | — |
GDM, Preeclampsia, Hypertension |
— |
PB, Delivery type, |
An increased risk of adverse birth outcomes, including iatrogenic preterm birth and cesarean section delivery |
| Chung Y;2022 [9] | multicenter observational study | Korea | Infected pregnant women: 65 |
②: 37/65 (56.92%), ③: 28/65 (43.08%) |
GDM, Preeclampsia, Hypertension |
Regdanvimab, Remdesivir, Antibiotics, Corticosteroid, Antipyretics, Tocilizumab |
PB, Delivery type, Maternal Death, APGAR score, Neonatal death |
At higher risk of progression to severe disease |
| Faraz S;2022 [10] | Retrospective analytical cohort study | United Arab Emirates | Infected pregnant women: 123 |
②: 93/123 (75.61%), ③: 30/123 (24.39%) |
GDM | — |
PB, Delivery type, Maternal Death, LBW, APGAR score |
High mortality, complications, cesarean section delivery, premature and low birth weights were more common |
| Abedzadeh-Kalahroudi M;2021 [11] | A case series study | Iran | Infected pregnant women: 26 |
②: 24/26 (92.31%), ③: 2/26 (7.69%) |
GDM, Preeclampsia, Hypertension |
Antibiotic, Antibiotic and antiviral |
PB, Delivery type, LBW, APGAR score, Neonatal death |
Increased rate of preterm delivery and cesarean section prematurity and low birth weight |
| Al-Matary A;2021 [19] | retrospective descriptive study | Saudi Arabia | Infected pregnant women: 288 |
②: 176/288 (61.1%), ③: 54/288 (18.75%) |
GDM, Preeclampsia, Hypertension |
Corticosteroid, Antibiotics, Antiviral |
PB, Delivery type, Maternal Death, LBW, APGAR score, Neonatal death |
The adverse pregnancy outcome was premature Preeclampsia |
| Antoun L;2020 [12] | prospective cohort study | England | Infected pregnant women: 19 |
②: 17/19 (89.47%), ③: 2/19 (10.53%) |
GDM, Preeclampsia | Steroids |
PB, Delivery type, Maternal Death, Neonatal death |
High prevalence of preeclampsia, preterm birth, and caesarean section |
| Knight M;2020 [13] | prospective cohort study | England | Infected pregnant women: 427 |
②: 386/427 (90.40%), ③: 41/427 (9.60%) |
Diabetes, Hypertension |
Oseltamivir Lopinavir/ritonavir, Remdesivir, Corticosteroids |
PB, Delivery type, Maternal Death, APGAR score |
The greater risk of severe maternal and neonatal morbidity and mortality |
* SARS-CoV-2 illness severity at initial presentation, ① represents Asymptomatic, ② represents Mild and/or Moderate, ③ represents Severe
Primary outcome measures
The primary outcome measures were maternal pregnancy outcomes (including preterm birth (< 37 weeks), mode of delivery, maternal mortality) and neonatal outcomes (including low birth weight (< 2500 g), APGAR scores, stillbirth/neonatal mortality). Among the 15 studies, preterm birth, mode of delivery, and maternal mortality were described; Preterm births were documented in 15 studies, vaginal deliveries were described in 11 studies, maternal caesarean sections were documented in 14 studies, and maternal mortality rates were documented in 9 studies. 13 studies described low birth weight, APGAR scores, and stillbirth/neonatal mortality; LBW was recorded in 7 studies, neonatal deaths were mentioned in 10 studies and neonatal APGAR scores were recorded in 12 studies.
Maternal pregnancy outcomes
Preterm birth
In the 15 studies addressing preterm birth, the rate of preterm birth ranged from 2.87 to 52.9%. In these studies, six found a higher rate of preterm birth in the infected group compared to the control group (p < 0.05), and six studies reported the probability of preterm birth only in the COVID-19 infected group, ranging from 15.5 to 38.46%. Binte Masud S [14] and others reported that the majority (52.9%) of COVID-19 positive women experienced preterm birth compared to 30.0% of COVID-19 negative women, with a significant association between infection status and gestational age (P = 0.001). A retrospective cohort study in Iran [15] and a national cohort study in the UK [6], after adjusting for potential confounders, found a statistically significant difference in the rate of preterm birth between the two groups (p = 0.0001), with pregnant women in the COVID-19 group having a significantly higher likelihood of preterm birth than the control group. Relevant data are presented in Table 3-2.
Table 3-2.
Mamaternal outcomes of SARS-CoV-2 positive and SARS-CoV-2 negative pregnant women
| Research | Preterm birth (PB) | P-Value | Delivery type | P-Value | Maternal Death | P-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Vaginal delivery | P-Value | cesarean delivery | P-Value | ||||||
| Pirjani R;2020 [16] | — | 0.064/0.689 | — | — | — | 0.030/0.024# | — | — | — |
| Adhikari EH;2020 [17] |
COVID-19: 27/245 (11%) No COVID-19:328/3035 (11%) |
0.92 |
COVID-19:174/245 (71%) No COVID-19:1975/3035 (65%) |
0.09 |
COVID-19: 65/245 (27%) No COVID-19:1011/3035 (33%) |
0.03* | — | — | — |
| Alipour Z;2021 [15] |
COVID-19: 25/53 (47.2%) No COVID-19: 18/135 (13.3%) |
0.000# |
COVID-19: 20/53 (37.7%) No COVID-19:47/135 (34.8%) |
— |
COVID-19: 9/53 (17.0%) No COVID-19: 70/135 (51.9%) |
0.22/0.04* | — |
COVID-19: 4/133 (3.0%) No COVID-19: 0/165(0) |
0.03* |
| Binte Masud S;2021 [14] |
COVID-19: 37/70 (52.9%) No COVID-19: 42/140 (30.0%) |
0.001# |
COVID-19: 20/70 (28.6%) No COVID-19:81/140 (57.9%) |
— |
COVID-19: 50/70 (71.4%) No COVID-19: 59/140 (42.1%) |
— | < 0.001# | — | — |
| Gupta P;2021 [20] |
COVID-19: 31/108 (28.3%) No COVID-19: 447/3057 (14.6%) |
0.0001# |
COVID-19: 45/108(41.6%) No COVID-19: 2143/3057 (70.1%) |
< 0.0001# |
COVID-19: 63/108(58.3%) No COVID-19:914/3057 (29.8%) |
< 0.0001# | — |
COVID-19: 1/108 (0.9%) No COVID-19:7/3057 (0.22%) |
0.1586 |
| Gurol-Urganci I;2021 [6] |
COVID-19: 369/3047 (12.1%) No COVID-19: 18,572/322,494 (5.8%) |
< 0.001# |
COVID-19: 1734/3527 (49.2%) No COVID-19:228382/338,553 (67.5%) |
< 0.001# |
COVID-19: 1355/3527 (38.4%) No COVID-19:109313/338,553 (32.3%) |
< 0.001# | — | — | — |
| Yang R;2019 [18] |
COVID-19: 9/65 (14%) No COVID-19:579/11,013 (5%) |
0.01# |
COVID-19: 13/65 (20%) No COVID-19:4993/11,013 (45%) |
— |
COVID-19: 52/65 (80%) No COVID-19:6020/11,013 (55%) |
— | < 0.001# | — | — |
| Oncel MY;2021 [7] | COVID-19: 33/125 (26.4%) | — | — | — | COVID-19: 89/125 (71.2%) | — | — | COVID-19: 6/125 (4.8%) | — |
| Al Hashmi I;2022 [8] |
COVID-19: 5/174 (2.87%) No COVID-19: 0 (0) |
0.01# | — | — |
COVID-19: 38/174(21.84%) No COVID-19: 1/87 (1.1%) |
< 0.01# | — |
COVID-19: 0/174 (0) No COVID-19:0/87 (0) |
— |
| Chung Y;2022 [9] | COVID-19: 25/65 (38.46%) | — | — | — | COVID-19: 51/65 (78.46%) | — | — | COVID-19: 0/65 (0) | — |
| Faraz S;2022 [10] | COVID-19: 57/123 (46.3%) | — | COVID-19: 61/123 (49.6%) | — | COVID-19: 62/123 (50.4%) | — | — | COVID-19: 6/123 (4.8%) | — |
| Abedzadeh-Kalahroudi M;2021 [11] | COVID-19: 11/26 (38%) | — | COVID-19: 8/26 (30.8%) | — | COVID-19: 18/26 (69.2%) | — | — | — | — |
| Al-Matary A;2021 [19] | COVID-19: 31/204 (15.5%) | — | COVID-19: 131/204 (64.2%) | — | COVID-19: 73/204 (35.8%) | — | — | COVID-19: 1/204 (0.5%) | — |
| Antoun L;2020 [12] | COVID-19: 7/19 (36.8%) | — | COVID-19: 3/19 (15.8%) | — | COVID-19: 16/19 (84%) | — | — | COVID-19: 1/19 (5.3%) | — |
| Knight M;2020 [13] | COVID-19: 70/266 (26%) | — | COVID-19: 106/262 (40.4%) | — | COVID-19: 156/262 (60%) | — | — | COVID-19: 5/427 (1%) | — |
* indicates statistical difference with p value less than 0.05; # indicates statistical difference with p value less than 0.01
Mode of delivery
Among the 14 studies that recorded the mode of delivery, five showed a higher rate of cesarean section in the infected group compared to the control group, and six studies reported the probability of cesarean section only in the COVID-19 infected group, ranging from 15.5 to 38.5%. Studies by Binte Masud S [14] and Reihaneh P [16] showed significant differences in the mode of delivery between infected and non-infected women, with COVID-19 positive women being more likely to undergo cesarean section and deliver preterm infants (P < 0.001). After adjusting for covariates, the likelihood of undergoing a cesarean section for women infected with COVID-19 was 3.27 times that of non-infected women. Studies by Yang R [18] and Antoun L [12] found that the risk of preterm birth (iatrogenic preterm birth) and cesarean section increased in women diagnosed with COVID-19 only when there were indications of SARS-CoV-2 infection in the mother or fetus, such as maternal respiratory distress [18] and related complications, fetal distress, non-progressive labor with pathological cardiotocography (CTG), unsuccessful induction following premature rupture of membranes (PROM), and severe sepsis [12]. These analyses indicate that pregnant women infected with COVID-19 are at a higher risk of undergoing cesarean delivery, with a portion of the increased rate attributable to indications arising from SARS-CoV-2 infection. Relevant data are presented in Table 3-2.
Maternal mortality
Of the included studies, nine recorded data on maternal mortality, with one study showing a higher mortality rate in the infected group compared to the control group (p < 0.05). A study from Iran [15] found a statistically significant difference in maternal mortality rates between the COVID-19 group and the control group (p = 0.03). However, the study by Gupta P [20] and others showed no significant difference in maternal mortality rates between the COVID-19 and control groups (p = 0.1586). A retrospective observational study in Dubai [10] categorized COVID-19 patients into mild/moderate and severe groups, finding that the mortality rate was higher among pregnant women with severe COVID-19. Relevant data are presented in Table 3-2.
Neonatal outcomes
Low birth weight
Seven studies documented instances of low birth weight. Among these, two studies indicated that the probability of low birth weight was higher in the neonates of the infected group compared to the control group; four studies reported an incidence rate of low birth weight in neonates born to infected mothers ranging from 12.8 to 34.6%. A retrospective cohort study from Iran [15] found that neonates born to mothers diagnosed with COVID-19 had a higher probability of low birth weight than those born to mothers not diagnosed with COVID-19, and this association was significantly correlated with COVID-19 (p < 0.05). The study by Saima Faraz et al. [10]. found that neonates born to severe COVID-19 patients had a significantly increased probability of being preterm (p < 0.001) and having low birth weight (p = 0.002). The case series by Abedzadeh-Kalahroudi M et al. [11]. indicated that low birth weight was one of the most common neonatal outcomes. Relevant data are presented in Table 3-3.
Table 3-3.
Neonatal outcomes of SARS-CoV-2 positive and SARS-CoV-2 negative pregnant women
| Research | Low Birth Weight (LBW) | P-Value | APGAR score | Neonatal death | P-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1th-min APGAR score | P-Value | 5th-min APGAR score | P-Value | Low APGAR score | P-Value | |||||
| Pirjani R;2020 [16] | — | 0.245/0.723 | — | 0.178/0.463 | — | 0.136/0.285 | — | — | — | 0.985/0.614 (stilllbirth) |
| Adhikari EH;2020 [17] | — | — | — | — | — | — |
COVID-19: <4 0 (0) No COVID-19:37/3015 (1.2%) |
0.08 |
COVID-19: 0(0) (stillbirth) No COVID-19: 18/3015 (0.6%) |
0.23 |
| Alipour Z;2021 [15] |
COVID-19: 16/53 (30.2%) No COVID-19:20/135 (14.8%) |
0.02* |
COVID-19: 8.2 (1.7%) No COVID-19: 8.8 (0.7%) |
0.003# |
COVID-19: 9.1 (1.5%) No COVID-19: 9.8 (0.8%) |
0.000# | — | — | — | — |
| Binte Masud S;2021 [14] |
COVID-19:17/68 (25.0%) No COVID-19:27/136 (19.9%) |
0.399 |
COVID-19: 7.40 ± 1.88 No COVID-19: 7.79 ± 1.33 |
0.088 |
COVID-19: 9.09 ± 2.32 No COVID-19: 9.50 ± 1.51 |
0.125 | — | — |
COVID-19: 2/70 (2.9%) (stillbirth) No COVID-19: 0 (0) |
0.125 |
| Gupta P;2021 [20] | — | — | — | — | — | — |
COVID-19: 7/108 (6.48%) No COVID-19: 83/3057 (2.7%) |
0.0199* |
COVID-19: 2/108 (1.9%) No COVID-19: 42/3057 (1.3%) |
0.5914 |
| Gurol-Urganci I;2021 [6] | — | — | — | — | — | — |
Composite Adeverse outcomes COVID-19: 222(7.6%) No COVID-19: 16,501 (5.2%) |
< 0.001# |
COVID-19: 30/3527 (0.85%) No COVID-19: 1140/338,553 (0.34%) |
< 0.001# |
| Oncel MY;2021 [7] | COVID-19: 16/125 (12.8%) | — |
COVID-19: 8 (7–8) No COVID-19: 8 (5–8) |
0.177 |
COVID-19: 9 (9–10) No COVID-19: 8 (7–9) |
0.039* | — | — | COVID-19: 1/125 (0.8%) | — |
| Al Hashmi I;2022 [8] | — | — | — | — | — | — |
COVID-19: 52/174 (29.89%) No COVID-19: 0 (0) |
< 0.01# |
COVID-19: 0 (0) No COVID-19: 0 (0) |
— |
| Chung Y;2022 [9] | COVID-19: 10/62 (16.13%) | — | COVID-19: 8 (8–9) (n = 57) | — | COVID-19: 9 (9–10) (n = 57) | — |
APGAR score<6 COVID-19: 6/62 (9.7%) |
— | COVID-19: 0(0) | — |
| Abedzadeh-Kalahroudi M;2021 [11] | COVID-19: 9/26 (34.6%) | — | — | — | — | — |
APGAR score<7 COVID-19: 2/26 (7.7%) |
— | COVID-19: 2/26 (7.7%) | — |
| Al-Matary A;2021 [19] | COVID-19: 29/200 (14.5%) | — |
APGAR score > 5 COVID-19: 182/200 (91.0%) |
— |
APGAR score > 5 COVID-19: 195/200 (97.5%) |
— | — | — | COVID-19: stillbirth 4/204 (2.0%) | — |
| Antoun L;2020 [12] | — | — | — | — | — | — |
APGAR score<7 COVID-19: 1/20 (0.5%) |
— | — | — |
| Knight M;2020 [13] | — | — | — | — | — | — | — | — |
COVID-19: stillbirth 3/427 (1%) Neonatal death 2/427 (1%) |
— |
* indicates statistical difference with p value less than 0.05; # indicates statistical difference with p value less than 0.01
APGAR scores
Twelve studies documented this outcome, six of which recorded 1 min/5min APGAR scores, and five documented APGAR scores lower than 7. Among the six studies that recorded 1 min/5min APGAR scores, two [14, 15] studies found that neonates born to infected mothers had lower APGAR scores than those in the control group, with one study [15] showing significant statistical significance. Among the five studies that documented low APGAR scores, two [8, 20] found significant statistical differences between the neonates born to the COVID-19 infected group and those in the control group, while the remaining three studies only recorded the probability of APGAR scores being lower than 7 (ranging from 0.5 to 9.7%). Relevant data are presented in Table 3-3.
Neonatal mortality/stillbirth
Among the included studies, ten documented neonatal mortality/stillbirth rates, with three [6, 14, 20] studies indicating higher rates of neonatal mortality/stillbirth in the infected group compared to the control group. Only one study [6] found a statistical difference in mortality rates between neonates born to infected and non-infected mothers (p < 0.001), and four studies recorded the percentage of neonatal deaths/stillbirths (ranging from 0 to 7.7%). Relevant data are presented in Table 3-3.
Secondary outcome measures: pregnancy complications
All 15 included studies documented pregnancy complications, with 13 studies describing GDM and 12 studies documenting preeclampsia. A retrospective cohort study from India [20] showed that COVID-19 infection could lead to an increased incidence of obstetric complications in hospitalized pregnant women. The most common pregnancy complications were GDM and preeclampsia. In the study by Antoun L [12], the highest proportion of women had gestational diabetes (17.3%). Studies by Gurol-Urganci I [6], Al Hashmi I [8], and Antoun L [12] indicated that women diagnosed with COVID-19 had a higher incidence of preeclampsia compared to those not infected with COVID-19. This suggests that pregnant women infected with COVID-19 may experience certain pregnancy complications at a higher rate than those not infected.
Discussion
In this study, our primary aim was to assess the impact of COVID-19 on pregnancy outcomes in women of reproductive age. After establishing the inclusion criteria, we analyzed 15 studies employing various methodologies. These studies documented outcomes such as preterm birth and mode of delivery, while 12 studies described low birth weight and APGAR scores. The most common pregnancy outcomes were increased rates of preterm birth and cesarean delivery, while low birth weight and low APGAR scores were the most common neonatal outcomes. A synthesis of data across different outcome measures revealed that pregnant women diagnosed with COVID-19 had higher rates of preterm birth, cesarean delivery, and low birth weight in neonates compared to those not infected with COVID-19.
This research indicates that pregnant women infected with COVID-19 have higher rates of preterm birth and cesarean delivery than those uninfected. As suggested by Piekos S N et al. [21]., the risk of preterm birth increases post-COVID-19 infection, irrespective of the severity of the infection. The global study on pregnancy and neonatal outcomes of COVID-19 (PAN-COVID) and a large cohort study by the American Academy of Pediatrics (AAP) [22] also suggest that COVID-19 infection increases the risk of preterm birth but does not elevate the risk of stillbirth, early neonatal death, or small for gestational age infants. A meta-analysis by Dubey P et al. [23]. indicated that preterm births and other adverse pregnancy outcomes are common among COVID-19 patients, with a higher probability of preterm birth compared to the control group and the general population. Most adverse pregnancy outcomes occurred in the early gestational period (25–35 weeks) and were associated with early detection of cases, suggesting that pregnant women should follow enhanced practices to avoid early pregnancy infection. However, if pregnant women do contract COVID-19, early detection and hospitalization are crucial to prevent adverse outcomes. Seven studies recorded reasons for cesarean delivery, most of which were due to indications of COVID-19 infection in the mother or fetus. Regarding the indications for elective cesarean section (CS) in COVID-19 patients, a review indicated sparse detailed reporting on COVID-19 as an indication for elective CS, mentioning reasons such as maternal condition deterioration, the need for antiviral treatment/infection control procedures, reducing abdominal pressure to improve breathing, and reducing the possibility of neonatal transmission, with the first two being primary reasons. However, in some cases, concern for neonatal infection was the sole reason for the increased CS rate, despite skepticism from some scholars. A review study [2] found only 2.7% (8/292) of neonates from vaginal deliveries tested positive, compared to 5.3% (20/374) from cesarean deliveries. Regarding maternal mortality, a systematic review and meta-analysis by Wang H et al. [24]. showed no statistically significant increase in the risk of death among COVID-19 infected pregnant women compared to uninfected ones. The low maternal mortality risk in our included studies may be associated with timely detection of COVID-19 infections and higher standards of medical treatment. The included studies show that neonates born to mothers infected with COVID-19 have a higher probability of low birth weight and lower APGAR scores. Khan D S A et al.‘s [25] research also found statistically significant differences in birth weight and APGAR scores at birth between high-risk and low-risk pregnancy groups during the COVID-19 pandemic, with symptomatic mothers’ neonates more likely to have lower birth weights. Our findings align with most studies reporting neonatal outcomes of COVID-19 pregnancies.
We counted the severity of the disease among the women included in the study, and the results showed that most of them were asymptomatic or suffered from mild to moderate symptoms. The most common symptoms reported by pregnant women with COVID-19 were dry cough, fever, fatigue, shortness of breath and dyspnea. Severe infections were found in only a small number of women, but those with severe infections showed some differences in pregnancy outcomes compared to asymptomatic and or mild to moderate infections. Chung Y [9] et al. showed that the frequency of preterm birth (≤ 37 weeks) was higher in pregnant women with severe COVID-19 than in those with mild COVID-19. Similar to this result, Saima Faraz et al. [10]. found that the newborns born to such mothers may be premature and have low birth weights but have similar mortality to those born to mothers with mild to moderate COVID-19, besides, pregnant women with severe COVID-19 have high mortality, peripartum complications, increased hospital stay, and are more likely to undergo Cesarean section delivery because of COVID-19 progression than pregnant patients with less severe forms of COVID-19. The newborns born to such mothers may be premature and have low birth weights but have similar mortality to those born to mothers with mild to moderate COVID-19. A study conducted in the United States [29]. also found that complications such as preterm labour were common in mothers with severe COVID-19. According to the NIH criteria [17], when evaluating outcomes according to maternal illness severity, preterm delivery was significantly associated with increasing severity of maternal COVID-19 illness. Although pregnancy and neonatal outcomes were not significantly different, the need for ICU for pregnant women with COVID-19 was significantly higher compared with those without COVID-19 [16]. Therefore, women with severe infections are at higher risk of preterm labor, ICU admission, and require greater attention from healthcare professionals.
All 15 included studies documented pregnancy complications, with gestational diabetes mellitus (GDM) and preeclampsia being the most common. During the pandemic, the healthcare system faced increasing service demands and pressures, affecting service delivery and care quality. With the unprecedented escalation in psychological distress and anxiety arising from the pandemic, as well as the difficulty in accessing medical care, may result in pregnant women are susceptible to complications [26]. This susceptibility is corroborated by a range of prevalence rates for GDM, varying between 7.8% and 11.5%, and for preeclampsia at 3.4% during the pandemic, as indicated by data collected from various institutions in France and China [30, 31]. Futhermore, a heightened prevalence of GDM, reaching 38.9%, in contrast to pre-pandemic rates was reported by a single-center investigation [32]. The lifestyle changes induced by the pandemic, such as increased sedentary behavior and escalated maternal stress due to lifestyle alterations, may contribute to elevated rates of GDM during this period. These changes are often accompanied by increased gestational weight gain, further exacerbating the risk of developing GDM. Conde-Agudelo and Romero’s meta-analysis revealed a 62% increased risk of preeclampsia with SARS-CoV-2 infection during pregnancy [33]. While the association between preeclampsia and COVID-19 is clear, it remains uncertain if this connection is mutually causal. The plausible connection between COVID-19 and preeclampsia lies in the virus’s mechanism of action, particularly its interaction with the ACE2 receptor and TMPRSS2 co-receptor. In the placenta, decreased ACE2 expression may induce placental oxidative stress and the release of anti-angiogenic factors like soluble sFlt-1, while diminishing proangiogenic factors, thus potentially contributing to the onset of PE or PE-like symptoms [34]. Placental changes appear more frequent in women during the acute phase of COVID-19 infection [35]. Healthcare providers should recognize that even asymptomatic SARS-CoV-2 infection in pregnant women poses a risk for the later development of preeclampsia. On the other hand, potential interruptions in healthcare access and prenatal screenings due to the pandemic that result in inadequate early detection of complications. Additionally, the main factors causing high gestational diabetes mellitus and high preeclampsia in COVID-19 women may also be the inflammatory response, immune imbalance and viral damage to the vascular endothelium. Regarding pharmacological interventions for pregnant women infected with COVID-19, research by Giesbers S et al. [27]. found that corticosteroids, particularly betamethasone, are beneficial when patients are admitted and require oxygen support. The Royal College of Obstetricians and Gynaecologists (RCOG) and the WHO state that using corticosteroids is unlikely to cause harm, with prednisolone preferred over dexamethasone due to extensive metabolism in the placenta, leading to minimal fetal transfer. Group R C et al. [28]. found that the FDA categorized remdesivir and cort.
Current understanding of pregnancy combined with SARS-CoV-2 infection remains limited. Some studies have small sample sizes, potentially leading to bias in the conclusions. Based on existing data, the clinical characteristics of pregnant women with COVID-19 are similar to those of non-pregnant women. Moreover, changes in the current pandemic, such as continuously emerging variants of the virus and vaccination rates in the population, which may affect reported morbidity and presentations, were not considered. Additionally, regional differences in the reported rates of adverse outcomes may exist. Further clinical research is needed to assess whether the higher rates of adverse outcomes in pregnant women with COVID-19 complications are due to the complications themselves or if SARS-CoV-2 infection exacerbates the condition of these patients.
Conclusion
This study, based on a cumulative evaluation and analysis of research data from various countries, indicates that pregnant women diagnosed with COVID-19 might have higher rates of preterm birth, cesarean delivery, and low birth weight in newborns compared to those not infected with COVID-19. These findings underscore the importance of exploring the factors contributing to the development of gestational diabetes mellitus and preeclampsia in women affected by COVID-19. It is conceivable that the elevated incidence of these complications could be attributed to several factors, including the physiological stress induced by the viral infection, inflammatory responses, immune imbalance, endothelial damage, and potential interruptions in healthcare access and prenatal screenings due to the pandemic that result in inadequate early detection of complications. Further research into the specific mechanisms driving these associations is crucial for developing targeted preventive strategies and optimizing clinical management protocols for pregnant women with COVID-19. Based on the experience of treating viral infections in pregnant women, adverse reactions related to fetal infection may occur even without intrauterine transmission. Therefore, symptomatic interventions should be carefully conducted under the guidance of clinicians and pharmacists to reduce the risk of adverse delivery outcomes and protect maternal and infant health.
Author contributions
NH: Concept and design, methodology, supervision, writing-review & editing, funding acquisition. KX: data extraction & curation, writing-original draft preparation, data analysis, language polish. WS: writing-original draft preparation, Tables production, data analysis. SY: Tables production, methodology, writing-review & editing, supervision. TL: language polish.
Funding
This study was supported by the " High-quality Papers Development Fund” and the “Evaluation of the efficacy and safety of Azulfidine in the treatment of novel coronavirus infections based on evidence-based pharmacology” (Grant No. AAJBCF202302080001) from Shandong Provincial Hospital, Shandong, China.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellos I, Pandita A, Panza R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:194–204. 10.1016/j.ejogrb.2020.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papapanou M, Papaioannou M, Petta A, Routsi E, Farmaki M, Vlahos N, Siristatidis C. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: an overview of systematic reviews. Int J Environ Res Public Health 2021, 18(2). [DOI] [PMC free article] [PubMed]
- 3.Beijing Center for Disease Control and Prevention. Recently, influenza is still at a high epidemic level, and influenza B virus is the main epidemic strain [https://news.cctv.com/2024/02/01/ARTIyXIpiOrchAC2dWstIFuc240201.shtml]
- 4.Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecology: Official J Int Soc Ultrasound Obstet Gynecol. 2020;56(1):15–27. 10.1002/uog.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Girolamo R, Khalil A, Alameddine S, D’Angelo E, Galliani C, Matarrelli B, Buca D, Liberati M, Rizzo G, D’Antonio F. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021;3(6):100468. 10.1016/j.ajogmf.2021.100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurol-Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, Harris T, Hawdon J, Morris E, Muller P et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. American journal of obstetrics and gynecology: 2021, 225(5):522.e521-522.e511. [DOI] [PMC free article] [PubMed]
- 7.Oncel MY, Akın IM, Kanburoglu MK, Tayman C, Coskun S, Narter F, Er I, Oncan TG, Memisoglu A, Cetinkaya M, et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish neonatal society. Eur J Pediatrics. 2021;180(3):733–42. 10.1007/s00431-020-03767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Hashmi I, Khalaf A, Seshan V, Alsabti H, Al Omari O, Yehia D, Baqer M, Al Khadhuri J. Maternal and neonatal outcomes of healthy pregnant women with COVID-19 Versus High-risk pregnant women: a Multi-center Case-Control Comparison Study. Clin Nurs Res. 2022;31(4):702–12. 10.1177/10547738211064027 [DOI] [PubMed] [Google Scholar]
- 9.Chung Y, Kim EJ, Kim HS, Park KH, Baek JH, Kim J, Lee JY, Lee CS, Lim S, Kim SW, et al. Maternal and neonatal outcomes in pregnant women with Coronavirus Disease 2019 in Korea. J Korean Med Sci. 2022;37(41):e297. 10.3346/jkms.2022.37.e297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraz S, Aftab N, Ammar A, Al Mulai I, Paulose L, Fernandes S. An insight on the maternal-fetal outcomes of critically ill pregnant women during the Second Wave of COVID-19. Cureus. 2022;14(1):e20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abedzadeh-Kalahroudi M, Sehat M, Vahedpour Z, Talebian P, Haghighi A. Clinical and obstetric characteristics of pregnant women with Covid-19: a case series study on 26 patients. Taiwan J Obstet Gynecol. 2021;60(3):458–62. 10.1016/j.tjog.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoun L, Taweel NE, Ahmed I, Patni S, Honest H. Maternal COVID-19 infection, clinical characteristics, pregnancy, and neonatal outcome: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;252:559–62. 10.1016/j.ejogrb.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, O’Brien P, Quigley M, Brocklehurst P, Kurinczuk JJ. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ (Clinical Res ed). 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binte Masud S, Zebeen F, Alam DW, Hossian M, Zaman S, Begum RA, Nabi MH, Hawlader MDH. Adverse birth outcomes among pregnant women with and without COVID-19: a comparative study from Bangladesh. J Prev Med Public Health = Yebang Uihakhoe Chi. 2021;54(6):422–30. 10.3961/jpmph.21.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alipour Z, Samadi P, Eskandari N, Ghaedrahmati M, Vahedian M, Khalajinia Z, Mastanijahroodi A. Relationship between coronavirus disease 2019 in pregnancy and maternal and fetal outcomes: retrospective analytical cohort study. Midwifery. 2021;102:103128. 10.1016/j.midw.2021.103128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirjani R, Hosseini R, Soori T, Rabiei M, Hosseini L, Abiri A, Moini A, Shizarpour A, Razani G, Sepidarkish M. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med 2020, 27(7). [DOI] [PMC free article] [PubMed]
- 17.Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, Spong CY. Pregnancy outcomes among women with and without severe Acute Respiratory Syndrome Coronavirus 2 infection. JAMA Netw open. 2020;3(11):e2029256. 10.1001/jamanetworkopen.2020.29256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang R, Mei H, Zheng T, Fu Q, Zhang Y, Buka S, Yao X, Tang Z, Zhang X, Qiu L, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18(1):330. 10.1186/s12916-020-01798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Matary A, Almatari F, Al-Matary M, AlDhaefi A, Alqahtani MHS, Alhulaimi EA, AlOtaiby S, Almehiny K, John LS, Alanazi FS, et al. Clinical outcomes of maternal and neonate with COVID-19 infection - multicenter study in Saudi Arabia. J Infect Public Health. 2021;14(6):702–8. 10.1016/j.jiph.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta P, Kumar S, Sharma SS. SARS-CoV-2 prevalence and maternal-perinatal outcomes among pregnant women admitted for delivery: experience from COVID-19-dedicated maternity hospital in Jammu, Jammu and Kashmir (India). J Med Virol. 2021;93(9):5505–14. 10.1002/jmv.27074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piekos SN, Roper RT, Hwang YM, Sorensen T, Price ND, Hood L, Hadlock JJ. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Health. 2022;4(2):e95–104. 10.1016/S2589-7500(21)00250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, Playle R, Perry A, Bourne T, Lees CC. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecology: Official J Int Soc Ultrasound Obstet Gynecol. 2021;57(4):573–81. 10.1002/uog.23619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubey P, Reddy SY, Manuel S, Dwivedi AK. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490–501. 10.1016/j.ejogrb.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Li N, Sun C, Guo X, Su W, Song Q, Liang Q, Liang M, Ding X, Lowe S, et al. The association between pregnancy and COVID-19: a systematic review and meta-analysis. Am J Emerg Med. 2022;56:188–95. 10.1016/j.ajem.2022.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan DSA, Hamid LR, Ali A, Salam RA, Zuberi N, Lassi ZS, Das JK. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21(1):801. 10.1186/s12884-021-04250-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ (Clinical Res ed). 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giesbers S, Goh E, Kew T, Allotey J, Brizuela V, Kara E, Kunst H, Bonet M, Thangaratinam S. Treatment of COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;267:120–8. 10.1016/j.ejogrb.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E et al. Effect of Dexamethasone in Hospitalized patients with COVID-19 – preliminary Report. 2020:2020.2006.2022.20137273.
- 29.Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2(3):100134. 10.1016/j.ajogmf.2020.100134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayem G, Lecarpentier E, Deruelle P, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49(7):101826. 10.1016/j.jogoh.2020.101826. 10.1016/j.jogoh.2020.101826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111e. 1-111.e14. 10.1016/j.ajog.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirsky EL, Mastronardi AM, Paudel AM, Young ML, Zite NB, Maples JM. Comparison of the prevalence of gestational diabetes Pre-COVID-19 pandemic Versus during COVID-19 [A196]. Volume 139. Obstetrics & Gynecology; 2022. pp. S57–57.
- 33.Conde-Agudelo A, Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;226(1):68–89. 10.1016/j.ajog.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espino-Y-Sosa S, Martinez-Portilla RJ, Torres-Torres J et al. Novel ratio Soluble Fms-like tyrosine Kinase-1/Angiotensin-II (sFlt-1/ANGII) in pregnant women is Associated with critical illness in COVID-19.Viruses. 2021;13:1906. [DOI] [PMC free article] [PubMed]
- 35.Linehan L, O’Donoghue K, Dineen S, White J, Higgins JR, Fitzgerald B. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–6. 10.1016/j.placenta.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.