Abstract
The cDNA coding for rat liver microsomal glutathione transferase was subcloned into the mammalian expression vector pCMV-5 and the construct was transfected into, and transiently expressed in, simian COS cells. This resulted in high expression (0.7% of the microsomal protein). The activity towards 1-chloro-2,4-dinitrobenzene in microsomes was 15-30 nmol/min per mg, which increased upon N-ethylmaleimide treatment to 60-200 nmol/min per mg. Control and antisense-vector-treated cells displayed very low activity (3-6 nmol/min per mg). A DNA fragment coding for rat microsomal glutathione transferase was generated by PCR, cloned into the bacterial expression vector pSP19T7LT and transformed into Escherichia coli strain BL21 (DE3) (which contained the plasmid pLys SL). Isopropyl beta-D-thiogalactopyranoside (IPTG; 1 mM) induced the expression of significant amounts of enzymically active protein (4 mg/l of culture as measured by Western blots). The recombinant protein was purified and characterized and found to be indistinguishable from the rat liver enzyme with regard to enzymic activity, molecular mass and N-terminal amino acid sequence. Human liver cDNA was used to obtain the coding region of human microsomal glutathione transferase by PCR. This PCR product was cloned into pSP19T7LT, which, upon induction with IPTG, yielded significant amounts (9 mg/l of culture) of active enzyme in BL21 (DE3) cells. Thus, for the first time, it is now possible to express both human and rat microsomal glutathione transferase in an enzymically active form in Escherichia coli.
Full text
PDF

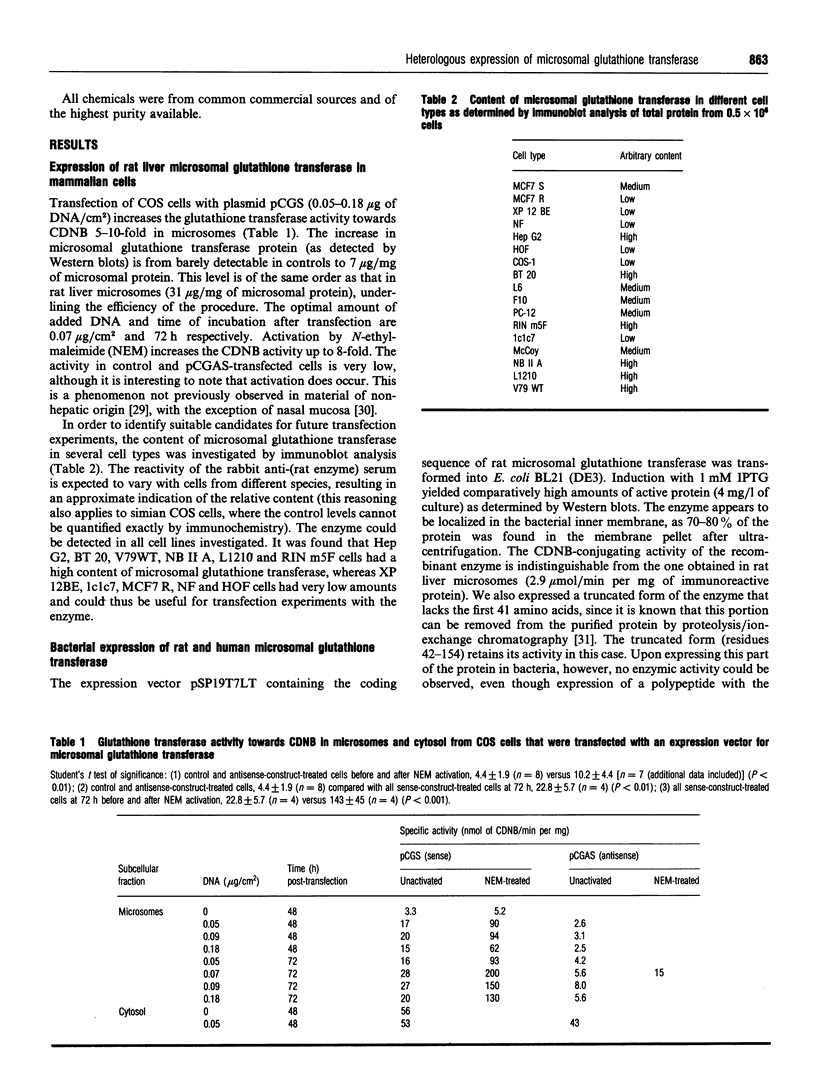
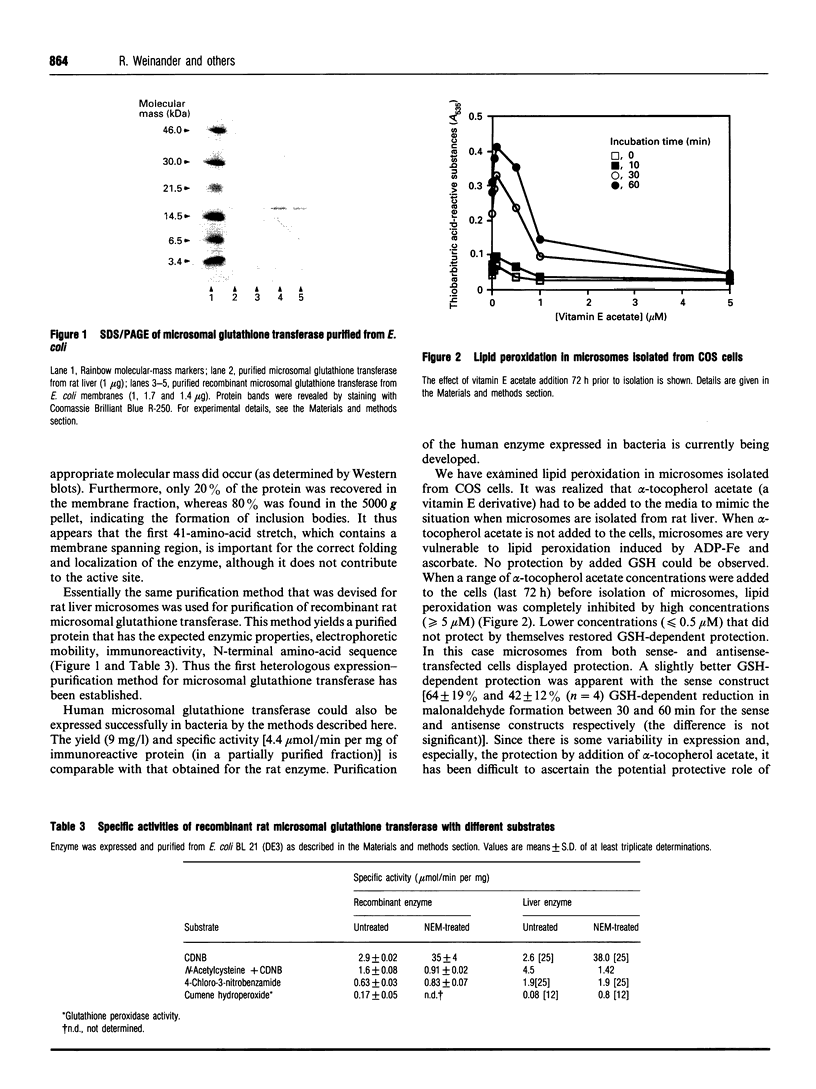


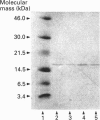
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson C., Söderström M., Mannervik B. Activation and inhibition of microsomal glutathione transferase from mouse liver. Biochem J. 1988 Feb 1;249(3):819–823. doi: 10.1042/bj2490819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C., Weinander R., Lundqvist G., DePierre J. W., Morgenstern R. Functional and structural membrane topology of rat liver microsomal glutathione transferase. Biochim Biophys Acta. 1994 Feb 16;1204(2):298–304. doi: 10.1016/0167-4838(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Armstrong R. N. Glutathione S-transferases: reaction mechanism, structure, and function. Chem Res Toxicol. 1991 Mar-Apr;4(2):131–140. doi: 10.1021/tx00020a001. [DOI] [PubMed] [Google Scholar]
- Barnes H. J., Arlotto M. P., Waterman M. R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N., Khen M., Lancet D. Glutathione S-transferases in rat olfactory epithelium: purification, molecular properties and odorant biotransformation. Biochem J. 1993 Jun 1;292(Pt 2):379–384. doi: 10.1042/bj2920379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- DeJong J. L., Mohandas T., Tu C. P. The gene for the microsomal glutathione S-transferase is on human chromosome 12. Genomics. 1990 Feb;6(2):379–382. doi: 10.1016/0888-7543(90)90580-n. [DOI] [PubMed] [Google Scholar]
- DeJong J. L., Morgenstern R., Jörnvall H., DePierre J. W., Tu C. P. Gene expression of rat and human microsomal glutathione S-transferases. J Biol Chem. 1988 Jun 15;263(17):8430–8436. [PubMed] [Google Scholar]
- Hargus S. J., Fitzsimmons M. E., Aniya Y., Anders M. W. Stereochemistry of the microsomal glutathione S-transferase catalyzed addition of glutathione to chlorotrifluoroethene. Biochemistry. 1991 Jan 22;30(3):717–721. doi: 10.1021/bi00217a020. [DOI] [PubMed] [Google Scholar]
- Inoue H., Nojima H., Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990 Nov 30;96(1):23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Kaiser R., Holmquist B., Hempel J., Vallee B. L., Jörnvall H. Class III human liver alcohol dehydrogenase: a novel structural type equidistantly related to the class I and class II enzymes. Biochemistry. 1988 Feb 23;27(4):1132–1140. doi: 10.1021/bi00404a009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundqvist G., Morgenstern R. Studies on the activation of rat liver microsomal glutathione transferase in isolated hepatocytes. Biochem Pharmacol. 1992 Jan 22;43(2):131–135. doi: 10.1016/0006-2952(92)90269-o. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- McLellan L. I., Wolf C. R., Hayes J. D. Human microsomal glutathione S-transferase. Its involvement in the conjugation of hexachlorobuta-1,3-diene with glutathione. Biochem J. 1989 Feb 15;258(1):87–93. doi: 10.1042/bj2580087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W., Jörnvall H. Microsomal glutathione transferase. Primary structure. J Biol Chem. 1985 Nov 15;260(26):13976–13983. [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W. Microsomal glutathione transferase. Purification in unactivated form and further characterization of the activation process, substrate specificity and amino acid composition. Eur J Biochem. 1983 Aug 15;134(3):591–597. doi: 10.1111/j.1432-1033.1983.tb07607.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Morgenstern R., Lundqvist G., Andersson G., Balk L., DePierre J. W. The distribution of microsomal glutathione transferase among different organelles, different organs, and different organisms. Biochem Pharmacol. 1984 Nov 15;33(22):3609–3614. doi: 10.1016/0006-2952(84)90145-x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Lundqvist G., Hancock V., DePierre J. W. Studies on the activity and activation of rat liver microsomal glutathione transferase, in particular with a substrate analogue series. J Biol Chem. 1988 May 15;263(14):6671–6675. [PubMed] [Google Scholar]
- Mosialou E., Andersson C., Lundqvist G., Andersson G., Bergman T., Jörnvall H., Morgenstern R. Human liver microsomal glutathione transferase. Substrate specificity and important protein sites. FEBS Lett. 1993 Jan 2;315(1):77–80. doi: 10.1016/0014-5793(93)81137-o. [DOI] [PubMed] [Google Scholar]
- Mosialou E., Ekström G., Adang A. E., Morgenstern R. Evidence that rat liver microsomal glutathione transferase is responsible for glutathione-dependent protection against lipid peroxidation. Biochem Pharmacol. 1993 Apr 22;45(8):1645–1651. doi: 10.1016/0006-2952(93)90305-g. [DOI] [PubMed] [Google Scholar]
- Mosialou E., Morgenstern R. Activity of rat liver microsomal glutathione transferase toward products of lipid peroxidation and studies of the effect of inhibitors on glutathione-dependent protection against lipid peroxidation. Arch Biochem Biophys. 1989 Nov 15;275(1):289–294. doi: 10.1016/0003-9861(89)90375-5. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]