Abstract
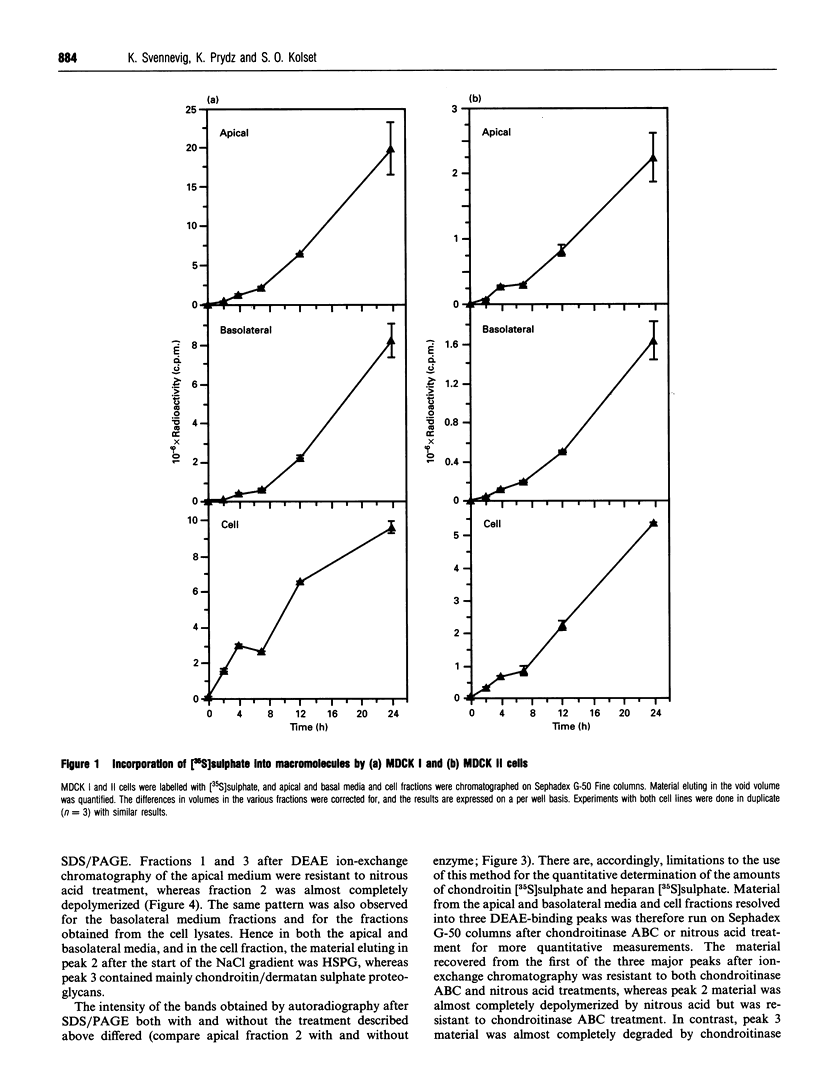
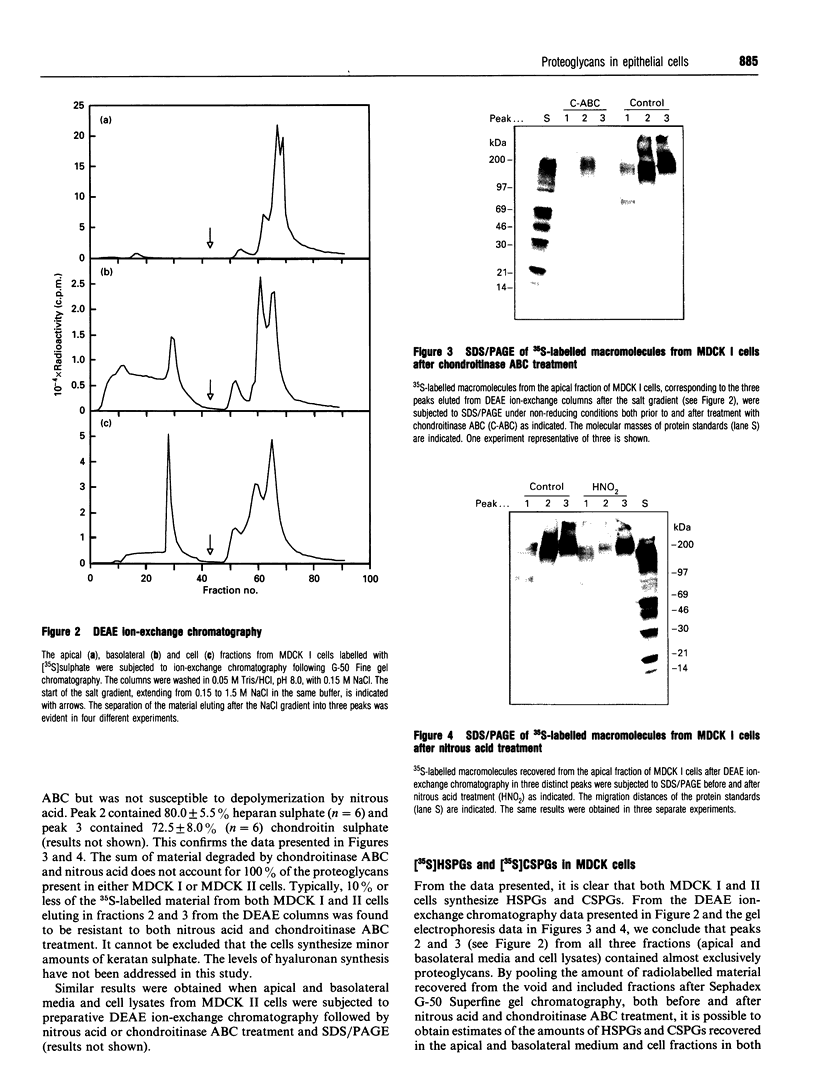
Madin-Darby canine kidney (MDCK) cells were cultured on polycarbonate filters to study the synthesis and sorting of proteoglycans in polarized epithelial cells. Two strains of MDCK cells were used. MDCK I cells resemble distal tubule epithelial cells, and MDCK II cells share some characteristics with proximal tubule cells. Both strains were grown to confluency and labelled with [35S]sulphate for 24 h. The apical and basolateral media and the cell fractions were harvested and analysed by DEAE ion-exchange chromatography. A large portion of the [35S]sulphate-labelled macromolecules bound strongly to the ion-exchange columns, and could be eluted in three distinct peaks. The latest eluting peak was demonstrated to contain almost exclusively chondroitin sulphate, whereas peak 2 contained mostly heparan sulphate, demonstrated by using chondroitinase ABC and nitrous acid (pH 1.5) respectively to depolymerize the [35S]glycosaminoglycan chains. Peak 1 contained negligible amounts of proteoglycans. Large differences could be observed in proteoglycan sorting in MDCK I and II cells. Strain I secreted approx. 67% of the proteoglycans to the apical side and 17% to the basolateral side. The cell fraction contained 17% of the proteoglycans after 24 h of labelling. In contrast, 19% of the proteoglycans were sorted to the apical side of MDCK II cells and 61% to the basolateral side, whereas the cell fraction contained 20%. Furthermore, the level of [35S]proteoglycan biosynthesis (apical and basolateral media and cell fraction total) was higher in MDCK I cells than in strain II. Based on the amount of material degraded by chondroitinase ABC and nitrous acid respectively, and the total amounts of [35S]proteoglycans recovered from the cells, it was calculated that the MDCK I strain synthesized approx. 56% chondroitin sulphate and 44% heparan sulphate. In contrast, the MDCK II strain synthesized 69% heparan sulphate and 31% chondroitin sulphate. To further identify the [35S]proteoglycans synthesized by MDCK I and II cells, antibodies against perlecan, versican and syndecan were used. The antibody against mouse syndecan did not cross-react with any of the proteoglycans produced in MDCK I or II cells. Both MDCK I and II cells expressed perlecan; 57-61% could be recovered from the basolateral fractions and 18-34% from the apical medium. Versican was also found in both MDCK I and II cells. Compared with perlecan, a larger percentage of versican (43-53%) was found in the cell fractions.
Full text
PDF




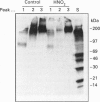
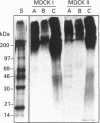
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brion C., Miller S. G., Moore H. P. Regulated and constitutive secretion. Differential effects of protein synthesis arrest on transport of glycosaminoglycan chains to the two secretory pathways. J Biol Chem. 1992 Jan 25;267(3):1477–1483. [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Crepaldi T., Pollack A. L., Prat M., Zborek A., Mostov K., Comoglio P. M. Targeting of the SF/HGF receptor to the basolateral domain of polarized epithelial cells. J Cell Biol. 1994 Apr;125(2):313–320. doi: 10.1083/jcb.125.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Teplow D. B., Selkoe D. J. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1564–1568. doi: 10.1073/pnas.91.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. C., Simons K., van Meer G. Two strains of the Madin-Darby canine kidney (MDCK) cell line have distinct glycosphingolipid compositions. EMBO J. 1986 Mar;5(3):483–489. doi: 10.1002/j.1460-2075.1986.tb04237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V., Cohen I. R., Grässel S., Murdoch A. D. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994 Sep 15;302(Pt 3):625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987 Dec;105(6 Pt 2):3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S. Biophysiology of glomerular filtration and proteinuria. Lab Invest. 1984 Jul;51(1):7–21. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Klein D. J., Oegema T. R., Jr, Fredeen T. S., van der Woude F., Kim Y., Brown D. M. Partial characterization of proteoglycans synthesized by human glomerular epithelial cells in culture. Arch Biochem Biophys. 1990 Mar;277(2):389–401. doi: 10.1016/0003-9861(90)90595-p. [DOI] [PubMed] [Google Scholar]
- Miettinen H. M., Edwards S. N., Jalkanen M. Analysis of transport and targeting of syndecan-1: effect of cytoplasmic tail deletions. Mol Biol Cell. 1994 Dec;5(12):1325–1339. doi: 10.1091/mbc.5.12.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S., Languino L. R., Ruoslahti E., Border W. A. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990 Aug;86(2):453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Isolation and characterization of the heparan sulfate proteoglycan of the bovine glomerular basement membrane. J Biol Chem. 1984 Oct 25;259(20):12749–12755. [PubMed] [Google Scholar]
- Parton R. G., Prydz K., Bomsel M., Simons K., Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989 Dec;109(6 Pt 2):3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. C., Scalera V., Simmons N. L. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta. 1981 Feb 18;673(1):26–36. [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Sandvig K., Prydz K., Ryd M., van Deurs B. Endocytosis and intracellular transport of the glycolipid-binding ligand Shiga toxin in polarized MDCK cells. J Cell Biol. 1991 May;113(3):553–562. doi: 10.1083/jcb.113.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger C. A., Zuk A., Zinkl G. M., Kendall D., Matlin K. S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci. 1994 Feb;107(Pt 2):527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Stevenson B. R., Anderson J. M., Braun I. D., Mooseker M. S. Phosphorylation of the tight-junction protein ZO-1 in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. Biochem J. 1989 Oct 15;263(2):597–599. doi: 10.1042/bj2630597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Sawada H., Farquhar M. G. Basement membrane heparan sulfate proteoglycans are concentrated in the laminae rarae and in podocytes of the rat renal glomerulus. Proc Natl Acad Sci U S A. 1985 May;82(10):3296–3300. doi: 10.1073/pnas.82.10.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Soroka C. J., MacKay K., Striker L., Striker G., Farquhar M. G. Basement membrane heparan sulfate proteoglycan is the main proteoglycan synthesized by glomerular epithelial cells in culture. Am J Pathol. 1989 Oct;135(4):637–646. [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., de Almeida J. B., Narula N., Holtzman E. J., Ercolani L., Ausiello D. A. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991 Sep;114(6):1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. J., Bayliss M. T., Harper K., Mason R. M., Davies M. Glomerular mesangial cells in vitro synthesize an aggregating proteoglycan immunologically related to versican. Biochem J. 1994 Aug 15;302(Pt 1):49–56. doi: 10.1042/bj3020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. J., Jenner L., Mason R. M., Davies M. Human glomerular epithelial cell proteoglycans. Arch Biochem Biophys. 1990 Apr;278(1):11–20. doi: 10.1016/0003-9861(90)90224-m. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Mason R. M., Davies M. Characterization of proteoglycans synthesized by human adult glomerular mesangial cells in culture. Biochem J. 1991 Jul 1;277(Pt 1):81–88. doi: 10.1042/bj2770081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S., Lehtonen E., Jalkanen M., Bernfield M., Saxén L. Epithelial-mesenchymal interactions regulate the stage-specific expression of a cell surface proteoglycan, syndecan, in the developing kidney. Dev Biol. 1989 Aug;134(2):382–391. doi: 10.1016/0012-1606(89)90110-3. [DOI] [PubMed] [Google Scholar]
- van Genderen I. L., van Meer G., Slot J. W., Geuze H. J., Voorhout W. F. Subcellular localization of Forssman glycolipid in epithelial MDCK cells by immuno-electronmicroscopy after freeze-substitution. J Cell Biol. 1991 Nov;115(4):1009–1019. doi: 10.1083/jcb.115.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Fuller S. D., Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985 Nov;4(11):2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]