Abstract
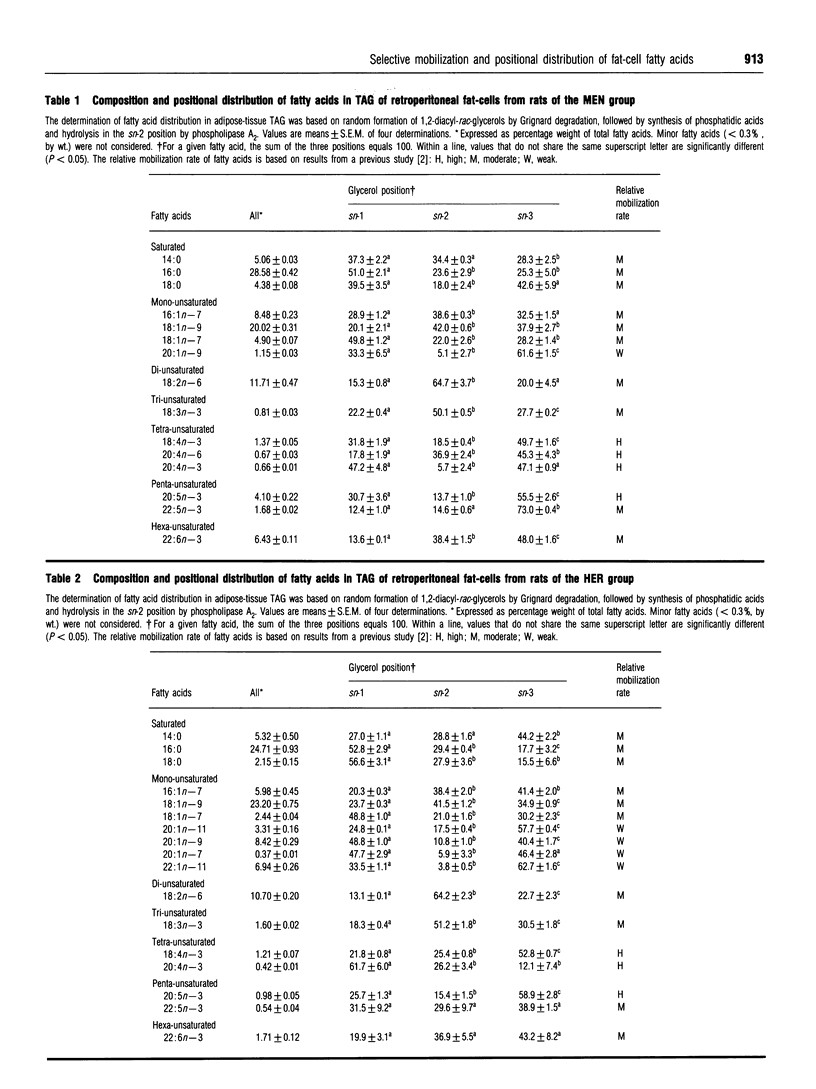
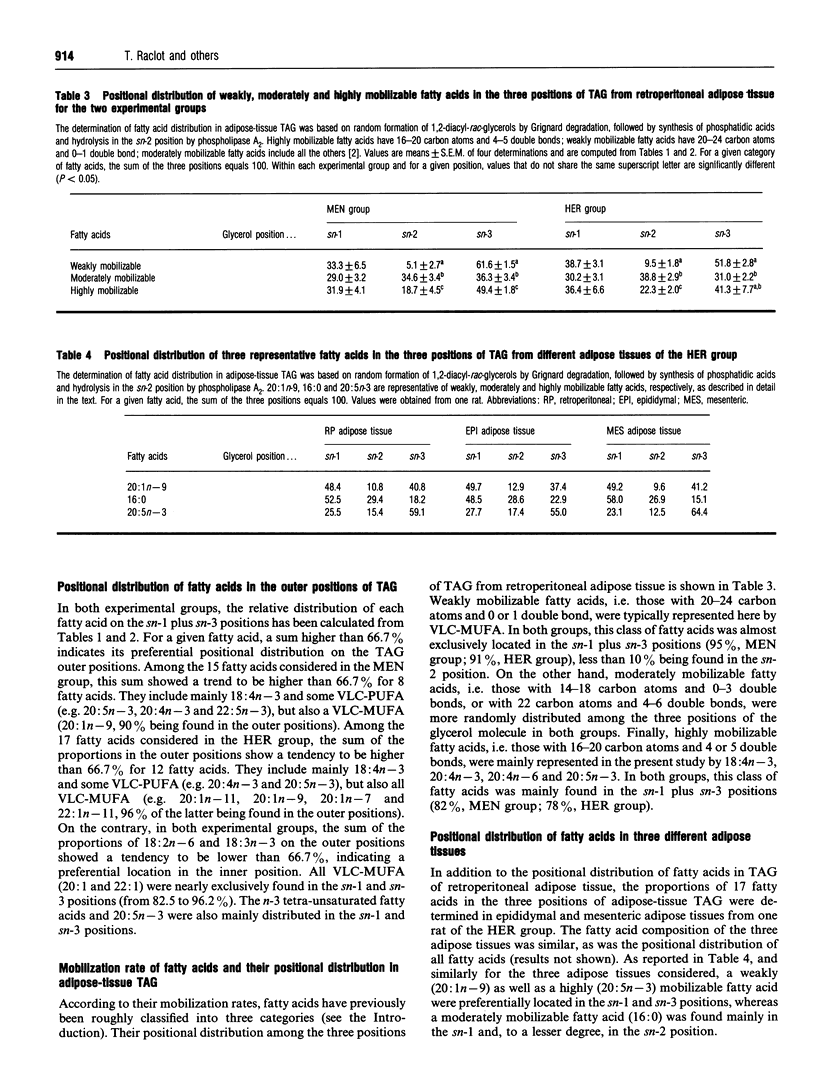
Fatty acids have been shown to be selectively mobilized from rat white fat-cells, whatever the dietary manipulations. For convenience, fatty acids have been classified as being highly, weakly and moderately mobilizable. The aim of this study was to examine whether the selective mobilization of fatty acids can be explained, even partly, by their positional distribution in adipose-tissue triacylglycerols (TAG) via the known specificity of hormone-sensitive lipase for the sn-1 and sn-3 positions. Adipose tissue was dietarily manipulated in order to obtain a wide spectrum of fatty acids, including large amounts of either very-long-chain polyunsaturated fatty acids (VLC-PUFA) or very-long-chain monounsaturated fatty acids (VLC-MUFA). The determination of fatty acid distribution in adipose tissue TAG was based on random formation of 1,2-diacyl-rac-glycerols by Grignard degradation, followed by synthesis of phosphatidic acids and hydrolysis in the sn-2 position by phospholipase A2. Regardless of the fatty acid composition and location of fat depots, highly (e.g. 18:4n-3 and some of the VLC-PUFA) and weakly (e.g. VLC-MUFA) mobilizable fatty acids were located mainly in the outer (sn-1 and sn-3) positions of the glycerol moiety (79.5% and 92.5% on average, respectively). Other fatty acids, which are rather moderately mobilizable, were more randomly distributed. We conclude that the selective mobilization of white-fat-cell fatty acids is not based on their positional distribution in TAG.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCKERHOFF H. A STEREOSPECIFIC ANALYSIS OF TRIGLYCERIDES. J Lipid Res. 1965 Jan;6:10–15. [PubMed] [Google Scholar]
- Belzung F., Raclot T., Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol. 1993 Jun;264(6 Pt 2):R1111–R1118. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- Body D. R. The lipid composition of adipose tissue. Prog Lipid Res. 1988;27(1):39–60. doi: 10.1016/0163-7827(88)90004-5. [DOI] [PubMed] [Google Scholar]
- Bottino N. R., Vandenburg G. A., Reiser R. Resistance of certain long-chain polyunsaturated fatty acids of marine oils to pancreatic lipase hydrolysis. Lipids. 1967 Nov;2(6):489–493. doi: 10.1007/BF02533177. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H., Ackman R. G. Positional distribution of isomers of monoenoic fatty acids in animal glycerolipids. J Lipid Res. 1967 Nov;8(6):661–666. [PubMed] [Google Scholar]
- Brockerhoff H., Hoyle R. J., Hwang P. C. Incorporation of fatty acids of marine origin into triglycerides and phospholipids of mammals. Biochim Biophys Acta. 1967 Dec 5;144(3):541–548. doi: 10.1016/0005-2760(67)90043-4. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H., Hoyle R. J., Hwang P. C., Litchfield C. Positional distribution of fatty acids in depot triglycerides of aquatic animals. Lipids. 1968 Jan;3(1):24–29. doi: 10.1007/BF02530964. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H., Hoyle R. J., Wolmark N. Positional distribution of fatty acids in triglycerides of animal depot fats. Biochim Biophys Acta. 1966 Feb 1;116(1):67–72. doi: 10.1016/0005-2760(66)90092-0. [DOI] [PubMed] [Google Scholar]
- Christie W. W., Moore J. H. A comparison of the structures of triglycerides from various pig tissues. Biochim Biophys Acta. 1970 Jun 9;210(1):46–56. doi: 10.1016/0005-2760(70)90060-3. [DOI] [PubMed] [Google Scholar]
- Christie W. W., Moore J. H. A semimicro method for the stereospecific analysis of triglycerides. Biochim Biophys Acta. 1969 Apr 29;176(3):445–452. doi: 10.1016/0005-2760(69)90211-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fredrikson G., Belfrage P. Positional specificity of hormone-sensitive lipase from rat adipose tissue. J Biol Chem. 1983 Dec 10;258(23):14253–14256. [PubMed] [Google Scholar]
- Fredrikson G., Tornqvist H., Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta. 1986 Apr 15;876(2):288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- Gavino V. C., Gavino G. R. Adipose hormone-sensitive lipase preferentially releases polyunsaturated fatty acids from triglycerides. Lipids. 1992 Dec;27(12):950–954. doi: 10.1007/BF02535570. [DOI] [PubMed] [Google Scholar]
- Heimermann W. H., Holman R. T., Gordon D. T., Kowalyshyn D. E., Jensen R. G. Effect of double bond position in octadecenoates upon hydrolysis by pancreatic lipase. Lipids. 1973 Jan;8(1):45–47. doi: 10.1007/BF02533239. [DOI] [PubMed] [Google Scholar]
- Jandacek R. J., Whiteside J. A., Holcombe B. N., Volpenhein R. A., Taulbee J. D. The rapid hydrolysis and efficient absorption of triglycerides with octanoic acid in the 1 and 3 positions and long-chain fatty acid in the 2 position. Am J Clin Nutr. 1987 May;45(5):940–945. doi: 10.1093/ajcn/45.5.940. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., deJong F. A., Clark R. M. Determination of lipase specificity. Lipids. 1983 Mar;18(3):239–252. doi: 10.1007/BF02534556. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., deJong F. A., Lambert-Davis L. G., Hamosh M. Fatty acid and positional selectivities of gastric lipase from premature human infants: in vitro studies. Lipids. 1994 Jun;29(6):433–435. doi: 10.1007/BF02537313. [DOI] [PubMed] [Google Scholar]
- Leray C., Pelletier X., Hemmendinger S., Cazenave J. P. Thin-layer chromatography of human platelet phospholipids with fatty acid analysis. J Chromatogr. 1987 Sep 25;420(2):411–416. doi: 10.1016/0378-4347(87)80198-6. [DOI] [PubMed] [Google Scholar]
- Leray C., Raclot T., Groscolas R. Positional distribution of n-3 fatty acids in triacylglycerols from rat adipose tissue during fish oil feeding. Lipids. 1993 Apr;28(4):279–284. doi: 10.1007/BF02536311. [DOI] [PubMed] [Google Scholar]
- Lin D. S., Conner W. E. Are the n-3 fatty acids from dietary fish oil deposited in the triglyceride stores of adipose tissue? Am J Clin Nutr. 1990 Apr;51(4):535–539. doi: 10.1093/ajcn/51.4.535. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Myher J. J., Kuksis A. Stereospecific analysis of triacylglycerols via racemic phosphatidylcholines and phospholipase C. Can J Biochem. 1979 Feb;57(2):117–124. doi: 10.1139/o79-015. [DOI] [PubMed] [Google Scholar]
- Raclot T., Groscolas R. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J Lipid Res. 1993 Sep;34(9):1515–1526. [PubMed] [Google Scholar]
- Soma M. R., Mims M. P., Chari M. V., Rees D., Morrisett J. D. Triglyceride metabolism in 3T3-L1 cells. An in vivo 13C NMR study. J Biol Chem. 1992 Jun 5;267(16):11168–11175. [PubMed] [Google Scholar]
- Tornqvist H., Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem. 1976 Feb 10;251(3):813–819. [PubMed] [Google Scholar]
- Yurkowski M., Brockerhoff H. Fatty acid distribution of triglycerides determined by deacylation with methyl magnesium bromide. Biochim Biophys Acta. 1966 Aug 3;125(1):55–59. doi: 10.1016/0005-2760(66)90143-3. [DOI] [PubMed] [Google Scholar]



