Abstract
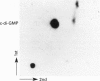
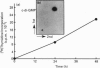
1. The effect of the novel, naturally occurring nucleotide 3'-5' cyclic diguanylic acid (c-di-GMP) on the lymphoblastoid Molt 4 cell line was studied. When exposed to this guanine nucleotide. Molt 4 cells exhibited a marked increase in [3H]thymidine incorporation, up to 200-fold at 50 microM c-di-GMP. Correspondingly, the DNA content of the treated cells was 9-fold higher than untreated cells. Stimulation of [3H]thymidine incorporation into the cells was time- and concentration-dependent. This effect was specific and was not observed with GMP or cyclic GMP, nor with the unhydrolysable GTP analogues, guanosine 5'-[gamma-thio]triphosphate and guanosine 5'-[beta gamma-imido]-triphosphate. C-di-GMP entrance into the cells was experimentally verified and occurred without using any means of cell permeabilization. SDS/PAGE analysis of cells exposed to [32P]c-di-GMP, followed by autoradiography, revealed the labelling of three low-molecular-mass proteins at 18-27 kDa. The labelling is highly specific to c-di-GMP and its extent was not affected by other guanine nucleotides. 2. One of the c-di-GMP-binding proteins was found to be the p21ras protein, by immunoprecipitation with the anti-Ras monoclonal antibody Y13-259. The effects described appear to be unique for c-di-GMP and, taken together, raise the possibility that an irreversible binding of this guanine nucleotide to the growth-promoting p21ras protein results in a fixed active conformation of this protein affecting DNA synthesis. Strikingly, although at 48 h of growth markedly high DNA levels were found in Molt 4 cells treated with c-di-GMP, this guanine nucleotide had no effect on cell replication during this period. Thus Molt 4 cells exposed to c-di-GMP enter the S phase uncoordinated with their overall replication rate.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amikam D., Benziman M. Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol. 1989 Dec;171(12):6649–6655. doi: 10.1128/jb.171.12.6649-6655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor Y., Mayer R., Benziman M., Delmer D. Evidence for a cyclic diguanylic acid-dependent cellulose synthase in plants. Plant Cell. 1991 Sep;3(9):989–995. doi: 10.1105/tpc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Smith G. L. Effects of withdrawal of a mitogenic stimulus on progression of fibroblasts into S phase: differences between serum and purified multiplication-stimulating activity. J Cell Physiol. 1977 Jun;91(3):441–448. doi: 10.1002/jcp.1040910314. [DOI] [PubMed] [Google Scholar]
- Buchou T., Charollais R. H., Mester J. Involvement of serum factor(s) adsorbed to the dish in the response of cycloheximide-pretreated BP-A31 cells to serum pulses. Exp Cell Res. 1988 Feb;174(2):411–420. doi: 10.1016/0014-4827(88)90311-4. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Field J., Broek D., Kataoka T., Wigler M. Guanine nucleotide activation of, and competition between, RAS proteins from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2128–2133. doi: 10.1128/mcb.7.6.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. D., Downward J., Rayter S., Warne P., Tutt A. L., Glennie M., Cantrell D. A. CD2 antigen mediated activation of the guanine nucleotide binding proteins p21ras in human T lymphocytes. J Immunol. 1991 Jun 1;146(11):3709–3712. [PubMed] [Google Scholar]
- Graves J. D., Lucas S. C., Alexander D. R., Cantrell D. A. Guanine nucleotide regulation of inositol phospholipid hydrolysis and CD3-antigen phosphorylation in permeabilized T lymphocytes. Biochem J. 1990 Jan 15;265(2):407–413. doi: 10.1042/bj2650407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. E., Buchou T., Mester J. Serum-induced G0/G1 transition in chemically transformed 3T3 cells. Independence of protein synthesis, stable "memory", and enhancement by cycloheximide pretreatment. Exp Cell Res. 1987 Mar;169(1):95–104. doi: 10.1016/0014-4827(87)90228-x. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Koyasu S., McConkey D. J., Clayton L. K., Abraham S., Yandava B., Katagiri T., Moingeon P., Yamamoto T., Reinherz E. L. Phosphorylation of multiple CD3 zeta tyrosine residues leads to formation of pp21 in vitro and in vivo. Structural changes upon T cell receptor stimulation. J Biol Chem. 1992 Feb 15;267(5):3375–3381. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Lacal J. C., Reep B. R., Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci U S A. 1989 May;86(9):3131–3134. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., King R. J. GTP analogues cause preferential translocation of an 18 kDa cytosolic G-protein to the membrane fraction in the ZR-75-1 human breast-cancer cell line. Biochem J. 1990 Oct 1;271(1):223–229. doi: 10.1042/bj2710223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R., Ullrich S., Kahn R. A., Wollheim C. B. Redistribution of ADP-ribosylation factor during stimulation of permeabilized cells with GTP analogues. Biochem J. 1991 May 1;275(Pt 3):639–644. doi: 10.1042/bj2750639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P., Mayer R., Weinhouse H., Amikam D., Huggirat Y., Benziman M., de Vroom E., Fidder A., de Paus P., Sliedregt L. A. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J Biol Chem. 1990 Nov 5;265(31):18933–18943. [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Kaziro Y. Function of Ras as a molecular switch in signal transduction. J Biol Chem. 1992 Dec 5;267(34):24149–24152. [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Kaziro Y. Induction of neurite formation in PC12 cells by microinjection of proto-oncogenic Ha-ras protein preincubated with guanosine-5'-O-(3-thiotriphosphate). Mol Cell Biol. 1987 Dec;7(12):4553–4556. doi: 10.1128/mcb.7.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Therrien S., Naccache P. H. Guanine nucleotide-induced polymerization of actin in electropermeabilized human neutrophils. J Cell Biol. 1989 Sep;109(3):1125–1132. doi: 10.1083/jcb.109.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]