Abstract
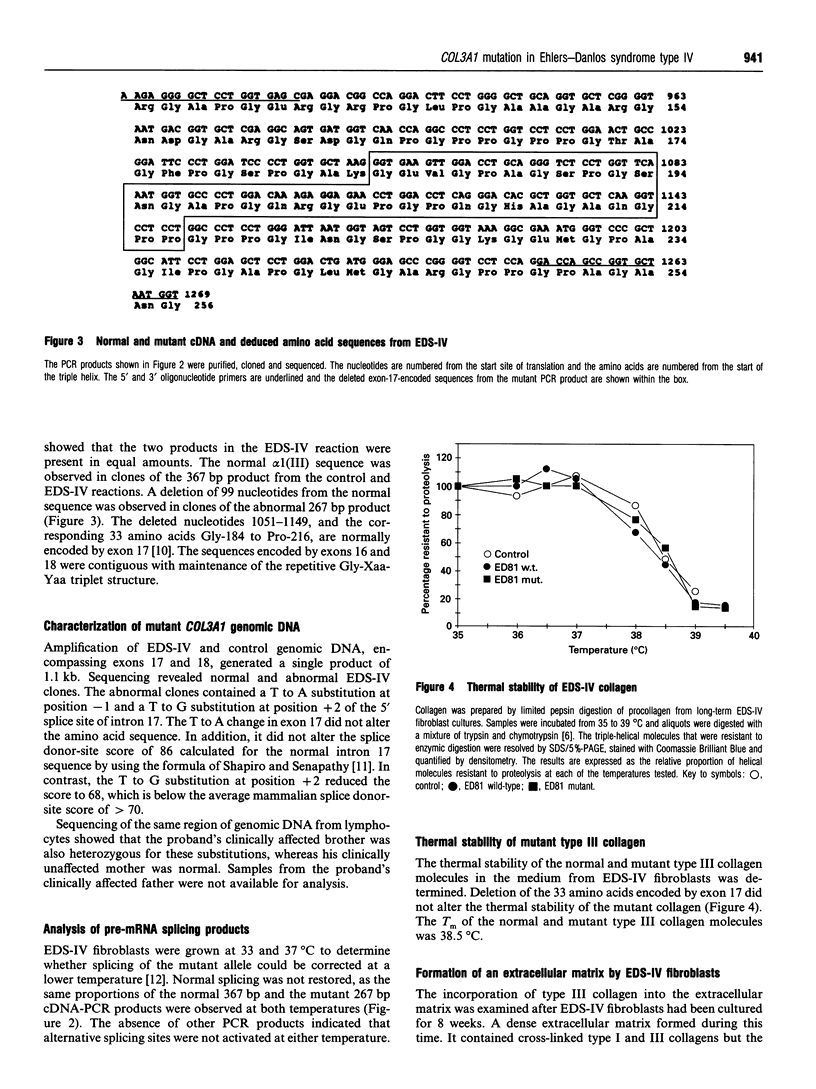
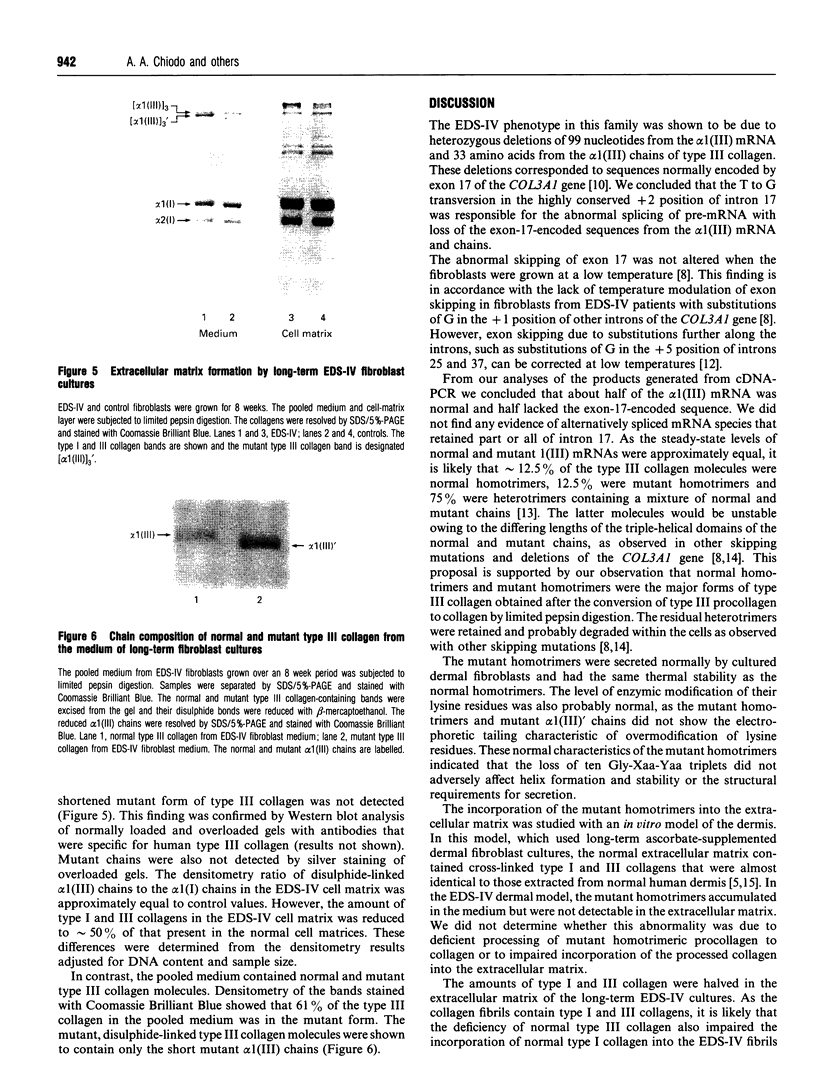
A novel heterozygous mutation of the COL3A1 gene that encodes the alpha 1(III) chains of type III collagen was identified in a family with the acrogeric form of Ehlers-Danlos syndrome type IV (EDS-IV). Cultured dermal fibroblasts produced normal and shortened alpha 1(III) chains. The triple helix of the latter chain was shortened owing to a 33 amino acid deletion of Gly-184 to Pro-216. The corresponding region of cDNA lacked 99 base pairs from nucleotides 1051 to 1149. The deletions corresponded exactly to the normal sequence encoded by exon 17 of the COL3A1 gene. The proband was heterozygous for a T to G transversion at position +2 of intron 17, which resulted in skipping of exon 17. The splicing defect was not corrected by growing the fibroblasts at 33 degrees C and no other splicing variants were identified at 33 or 37 degrees C. The affected brother had the same mutation but his unaffected mother did not. Heterotrimeric type III collagen molecules containing normal and mutant chains were retained within the cell. The mutant homotrimeric molecules were modified and secreted normally and were thermally stable. These normal characteristics of the mutant homotrimers suggested that the loss of ten Gly-Xaa-Yaa triplets (where Gly-Xaa-Yaa is a repetitive amino acid triplet structure in which Xaa and Yaa are other amino acids, proline and hydroxyproline being more common in the Yaa position) did not adversely affect the formation and stability of the triple helix or the structural requirements for secretion. However, the mutant homotrimers were not incorporated into the extracellular matrix of an in vitro model of EDS-IV dermis. The EDS-IV phenotype in this family was probably due to a deficiency in the amount of normal type III collagen available for formation of the heterotypic collagen fibrils of the extracellular matrix. Intracellular and extracellular quality-control mechanisms prevented the incorporation of heterotrimeric and homotrimeric mutant type III collagen molecules into the cross-linked extracellular matrix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Kontusaari S., Baldwin C. T., Kuivaniemi H., Prockop D. J. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989 Jun 1;260(2):509–516. doi: 10.1042/bj2600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Golub S. B. Deposition and selective degradation of structurally-abnormal type I collagen in a collagen matrix produced by osteogenesis imperfecta fibroblasts in vitro. Matrix Biol. 1994 Apr;14(3):251–262. doi: 10.1016/0945-053x(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson-Chanda V., Su M. W., Weil D., Chu M. L., Ramirez F. Cloning and analysis of the 5' portion of the human type-III procollagen gene (COL3A1). Gene. 1989 May 30;78(2):255–265. doi: 10.1016/0378-1119(89)90228-x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Holbrook K. A., Barsh G. S., Smith L. T., Bornstein P. Altered secretion of type III procollagen in a form of type IV Ehlers-Danlos syndrome. Biochemical studies in cultured fibroblasts. Lab Invest. 1981 Apr;44(4):336–341. [PubMed] [Google Scholar]
- Chan D., Lamande S. R., Cole W. G., Bateman J. F. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem J. 1990 Jul 1;269(1):175–181. doi: 10.1042/bj2690175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D., Taylor T. K., Cole W. G. Characterization of an arginine 789 to cysteine substitution in alpha 1 (II) collagen chains of a patient with spondyloepiphyseal dysplasia. J Biol Chem. 1993 Jul 15;268(20):15238–15245. [PubMed] [Google Scholar]
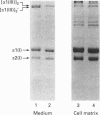
- Cole W. G., Chan D. Analysis of the heterogeneity of human collagens by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1981 Aug 1;197(2):377–383. doi: 10.1042/bj1970377. [DOI] [PMC free article] [PubMed] [Google Scholar]
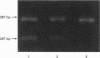
- Cole W. G., Chiodo A. A., Lamande S. R., Janeczko R., Ramirez F., Dahl H. H., Chan D., Bateman J. F. A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1990 Oct 5;265(28):17070–17077. [PubMed] [Google Scholar]
- Lamandé S. R., Chessler S. D., Golub S. B., Byers P. H., Chan D., Cole W. G., Sillence D. O., Bateman J. F. Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the pro alpha 1 (I) chain carboxyl-terminal propeptide which impair subunit assembly. J Biol Chem. 1995 Apr 14;270(15):8642–8649. doi: 10.1074/jbc.270.15.8642. [DOI] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope F. M., Nicholls A. C., Narcisi P., Temple A., Chia Y., Fryer P., De Paepe A., De Groote W. P., McEwan J. R., Compston D. A. Type III collagen mutations in Ehlers Danlos syndrome type IV and other related disorders. Clin Exp Dermatol. 1988 Sep;13(5):285–302. doi: 10.1111/j.1365-2230.1988.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga A., Gugler E., Gitzelmann R., Steinmann B. Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988 May 5;263(13):6226–6232. [PubMed] [Google Scholar]