Abstract
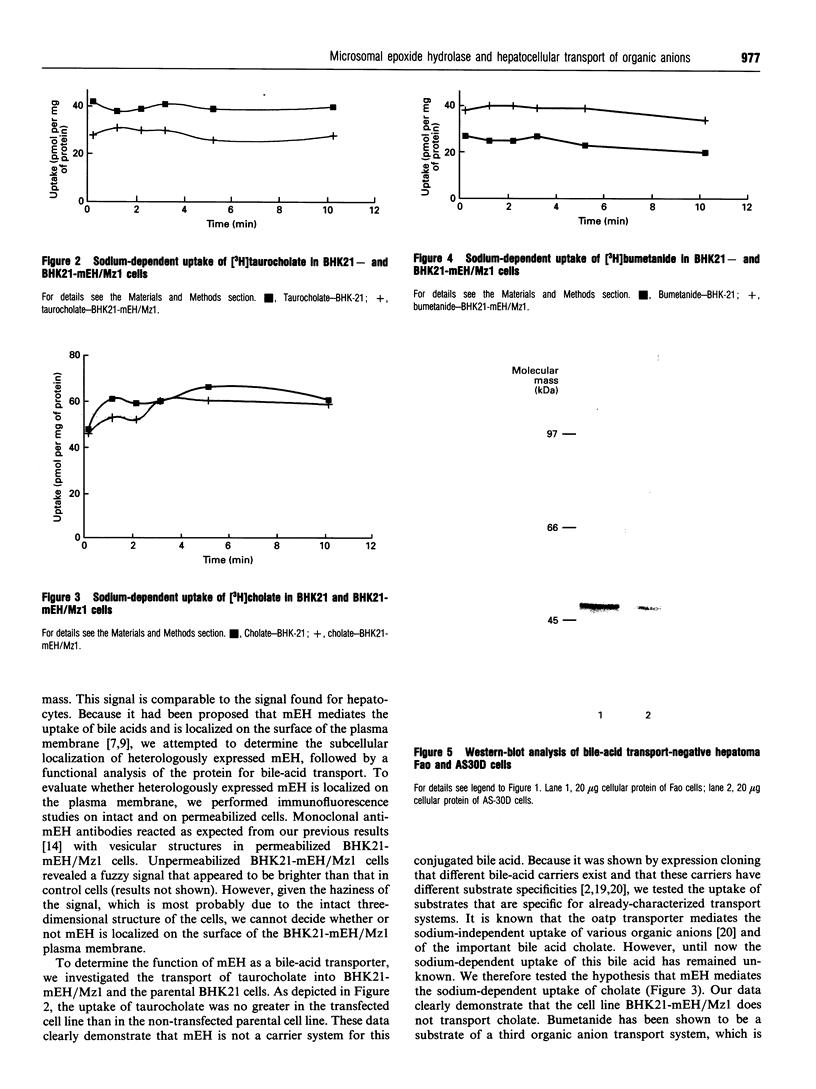
Recently two different bile-acid carriers for the hepatocellular sodium-dependent uptake of taurocholate have been described. The first transport system was isolated and characterized by functional expression cloning in Xenopus laevis oocytes. The corresponding cDNA clone, named Ntcp for Na+/taurocholate co-transporting polypeptide, codes for a protein of 362 amino acids and shows no similarity to previously known sequences. The transport function of this carrier system is well documented by expression in Xenopus laevis oocytes and by transient and stably transfected cell lines. In addition, several lines of evidence implied that the well-known xenobiotic-metabolizing enzyme microsomal epoxide hydrolase (mEH, EC 3.3.2.3) is also able to mediate sinusoidal uptake of taurocholate. Furthermore, it was claimed that the same enzyme also mediates the uptake of the conjugated bile acid into the smooth endoplasmic reticulum (ER). No direct proof of the transport function of mEH by its heterologous expression has yet been published. In the present work we used a stable transfected cell line that expressed high levels of heterologous mEH for uptake studies of various bile acids and the loop diuretic bumetanide. The uptake of the conjugated bile acid taurocholate, of the non-conjugated bile acid cholate and of the organic anion bumetanide was measured in the transfected as well as in the non-transfected parental cell line. These organic anions represent the main substrates of the known transport systems for organic anions in the rat liver. The results show that the microsomal epoxide hydrolase is unable to transport taurocholate, cholate or bumetanide. Furthermore, Western-blot analysis revealed the expression of mEH in hepatoma tumor cell lines, which show no transport activity for these organic anions. These results show that it is unlikely that mEH can mediate the transport of these substrates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alves C., von Dippe P., Amoui M., Levy D. Bile acid transport into hepatocyte smooth endoplasmic reticulum vesicles is mediated by microsomal epoxide hydrolase, a membrane protein exhibiting two distinct topological orientations. J Biol Chem. 1993 Sep 25;268(27):20148–20155. [PubMed] [Google Scholar]
- Ananthanarayanan M., Ng O. C., Boyer J. L., Suchy F. J. Characterization of cloned rat liver Na(+)-bile acid cotransporter using peptide and fusion protein antibodies. Am J Physiol. 1994 Oct;267(4 Pt 1):G637–G643. doi: 10.1152/ajpgi.1994.267.4.G637. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M., von Dippe P., Levy D. Identification of the hepatocyte Na+-dependent bile acid transport protein using monoclonal antibodies. J Biol Chem. 1988 Jun 15;263(17):8338–8343. [PubMed] [Google Scholar]
- Blumrich M., Zeyen-Blumrich U., Pagels P., Petzinger E. Immortalization of rat hepatocytes by fusion with hepatoma cells. II. Studies on the transport and synthesis of bile acids in hepatocytoma (HPCT) cells. Eur J Cell Biol. 1994 Aug;64(2):339–347. [PubMed] [Google Scholar]
- Boyer J. L., Ng O. C., Ananthanarayanan M., Hofmann A. F., Schteingart C. D., Hagenbuch B., Stieger B., Meier P. J. Expression and characterization of a functional rat liver Na+ bile acid cotransport system in COS-7 cells. Am J Physiol. 1994 Mar;266(3 Pt 1):G382–G387. doi: 10.1152/ajpgi.1994.266.3.G382. [DOI] [PubMed] [Google Scholar]
- Craft J. A., Baird S., Lamont M., Burchell B. Membrane topology of epoxide hydrolase. Biochim Biophys Acta. 1990 Aug 28;1046(1):32–39. doi: 10.1016/0005-2760(90)90091-b. [DOI] [PubMed] [Google Scholar]
- Friedberg T., Becker R., Oesch F., Glatt H. Studies on the importance of microsomal epoxide hydrolase in the detoxification of arene oxides using the heterologous expression of the enzyme in mammalian cells. Carcinogenesis. 1994 Feb;15(2):171–175. doi: 10.1093/carcin/15.2.171. [DOI] [PubMed] [Google Scholar]
- Galteau M. M., Antoine B., Reggio H. Epoxide hydrolase is a marker for the smooth endoplasmic reticulum in rat liver. EMBO J. 1985 Nov;4(11):2793–2800. doi: 10.1002/j.1460-2075.1985.tb04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B., Lübbert H., Stieger B., Meier P. J. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990 Apr 5;265(10):5357–5360. [PubMed] [Google Scholar]
- Hagenbuch B., Stieger B., Foguet M., Lübbert H., Meier P. J. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honscha W., Oesch F., Friedberg T. Tissue-specific expression and differential inducibility of several microsomal epoxide hydrolase mRNAs which are formed by alternative splicing. Arch Biochem Biophys. 1991 Jun;287(2):380–385. doi: 10.1016/0003-9861(91)90493-3. [DOI] [PubMed] [Google Scholar]
- Honscha W., Ottallah M., Kistner A., Platte H., Petzinger E. A membrane-bound form of protein disulfide isomerase (PDI) and the hepatic uptake of organic anions. Biochim Biophys Acta. 1993 Dec 12;1153(2):175–183. doi: 10.1016/0005-2736(93)90403-m. [DOI] [PubMed] [Google Scholar]
- Honscha W., Schulz K., Müller D., Petzinger E. Two different mRNAs from rat liver code for the transport of bumetanide and taurocholate in Xenopus laevis oocytes. Eur J Pharmacol. 1993 Aug 15;246(3):227–232. doi: 10.1016/0922-4106(93)90035-8. [DOI] [PubMed] [Google Scholar]
- Jacquemin E., Hagenbuch B., Stieger B., Wolkoff A. W., Meier P. J. Expression of the hepatocellular chloride-dependent sulfobromophthalein uptake system in Xenopus laevis oocytes. J Clin Invest. 1991 Dec;88(6):2146–2149. doi: 10.1172/JCI115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroker R., Anwer M. S., Hegner D. The lack of active bile acid transport in AS-30 D ascites hepatoma cells. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jul;303(3):299–301. doi: 10.1007/BF00498058. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick G. A., Hagenbuch B., Stieger B., Wolkoff A. W., Meier P. J. Functional characterization of the basolateral rat liver organic anion transporting polypeptide. Hepatology. 1994 Aug;20(2):411–416. [PubMed] [Google Scholar]
- Petzinger E., Müller N., Föllmann W., Deutscher J., Kinne R. K. Uptake of bumetanide into isolated rat hepatocytes and primary liver cell cultures. Am J Physiol. 1989 Jan;256(1 Pt 1):G78–G86. doi: 10.1152/ajpgi.1989.256.1.G78. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Beck T. W., Kasper C. B. Complementary DNA and amino acid sequence of rat liver microsomal, xenobiotic epoxide hydrolase. Arch Biochem Biophys. 1986 Jul;248(1):121–129. doi: 10.1016/0003-9861(86)90408-x. [DOI] [PubMed] [Google Scholar]
- Schwarz L. R., Burr R., Schwenk M., Pfaff E., Greim H. Uptake of taurocholic acid into isolated rat-liver cells. Eur J Biochem. 1975 Jul 15;55(3):617–623. doi: 10.1111/j.1432-1033.1975.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Stasiecki P., Oesch F., Bruder G., Jarasch E. D., Franke W. W. Distribution of enzymes involved in metabolism of polycyclic aromatic hydrocarbons among rat liver endomembranes and plasma membranes. Eur J Cell Biol. 1980 Apr;21(1):79–92. [PubMed] [Google Scholar]
- Stieger B., Hagenbuch B., Landmann L., Höchli M., Schroeder A., Meier P. J. In situ localization of the hepatocytic Na+/Taurocholate cotransporting polypeptide in rat liver. Gastroenterology. 1994 Dec;107(6):1781–1787. doi: 10.1016/0016-5085(94)90821-4. [DOI] [PubMed] [Google Scholar]
- Von Dippe P., Amoui M., Alves C., Levy D. Na(+)-dependent bile acid transport by hepatocytes is mediated by a protein similar to microsomal epoxide hydrolase. Am J Physiol. 1993 Mar;264(3 Pt 1):G528–G534. doi: 10.1152/ajpgi.1993.264.3.G528. [DOI] [PubMed] [Google Scholar]
- Waechter F., Bentley P., Germann M., Oesch F., Stäubli W. Immuno-electron-microscopic studies on the subcellular distribution of rat liver epoxide hydrolase and the effect of phenobarbitone and 2-acetamidofluorene treatment. Biochem J. 1982 Mar 15;202(3):677–686. doi: 10.1042/bj2020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dippe P., Levy D. Reconstitution of the immunopurified 49-kDa sodium-dependent bile acid transport protein derived from hepatocyte sinusoidal plasma membranes. J Biol Chem. 1990 Sep 5;265(25):14812–14816. [PubMed] [Google Scholar]




