Abstract
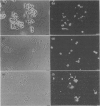
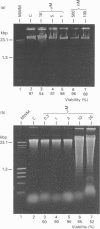
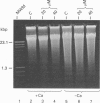
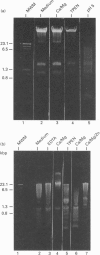
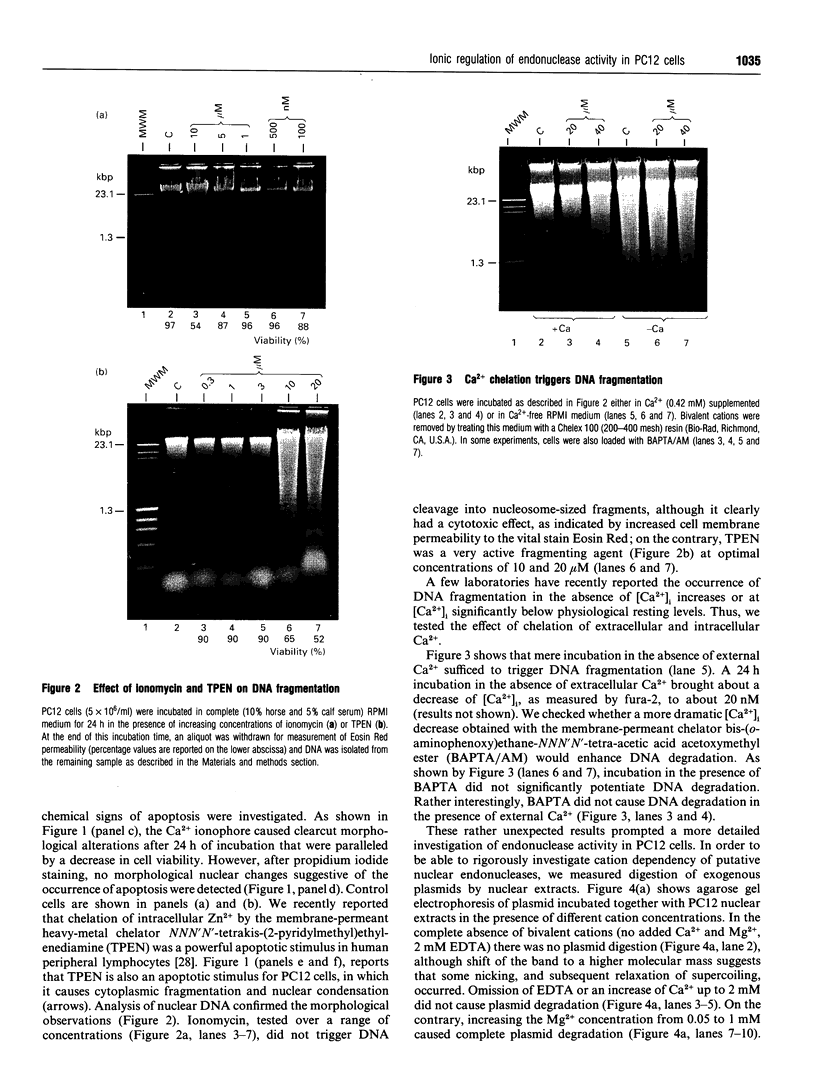
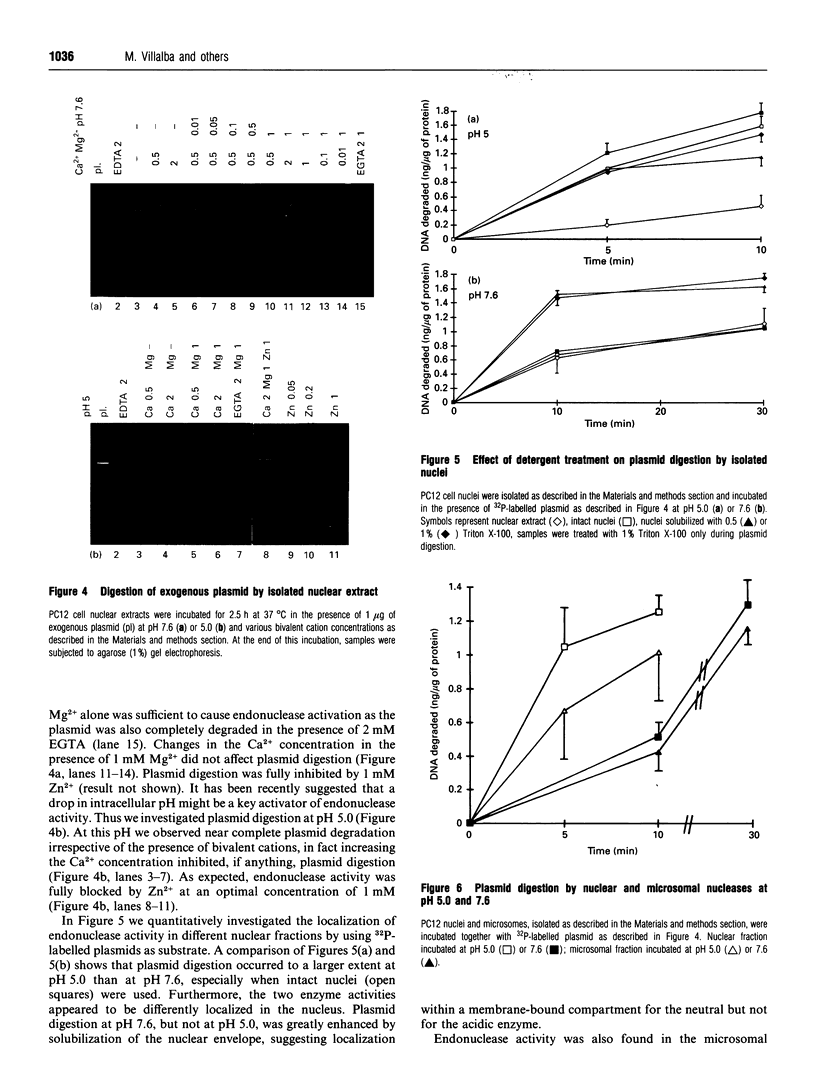
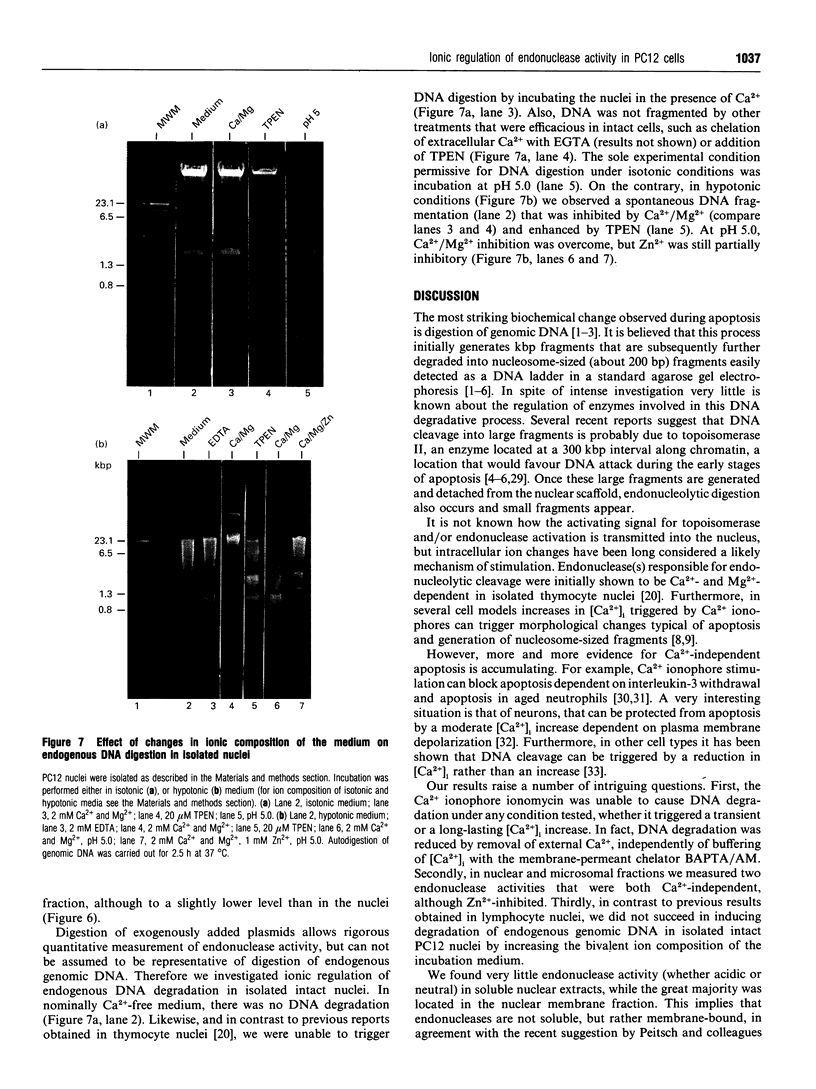
We have investigated the Ca2+ dependency of DNA degradation into nucleosome-sized fragments in intact chromaffin-like PC12 cells and PC12 nuclear fractions. In intact cells we were unable to trigger DNA fragmentation by inducing either transient or sustained elevations of cytoplasmic Ca2+ ([Ca2+]i) with the Ca2+ ionophore ionomycin. On the contrary, DNA fragmentation was induced in intact cells by the intracellular Zn2+ chelator NNN'N'-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN). To characterize further PC12 cell endonuclease activity, we then investigated digestion by purified PC12 cell fractions of exogenously added plasmids. In nuclear fractions two endonuclease activities were identified: an acidic (pH 5.0) endonuclease activity that was fully Ca2+- and Mg(2+)-independent; and a neutral (pH 7.6) endonuclease activity that was Ca(2+)-independent but Mg(2+)-dependent. Both endonuclease activities were inhibited by Zn2+. Nuclear membrane permeabilization greatly enhanced plasmid digestion at pH 7.6, but not at pH 5.0. This suggests that neutral endonuclease was located in a membrane-bound compartment, whereas acidic endonuclease was freely accessible to the substrate even in the presence of an intact nuclear membrane. In intact nuclei, digestion of genomic DNA could not be triggered by increasing the bivalent cation composition of the medium. On the contrary, in hypotonic medium we observed a large spontaneous nucleolytic DNA degradation that was increased by Zn2+ chelation. However, an acidic pH shift was a potent stimulus for DNA fragmentation in isotonic as well as hypotonic medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry M. A., Eastman A. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys. 1993 Jan;300(1):440–450. doi: 10.1006/abbi.1993.1060. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Greene L. A. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991 Oct;115(2):461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron-Leslie L. A., Evans R. B., Cidlowski J. A. Bcl-2 inhibits glucocorticoid-induced apoptosis but only partially blocks calcium ionophore or cycloheximide-regulated apoptosis in S49 cells. FASEB J. 1994 Jun;8(9):639–645. doi: 10.1096/fasebj.8.9.8005391. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Di Virgilio F., Milani D., Leon A., Meldolesi J., Pozzan T. Voltage-dependent activation and inactivation of calcium channels in PC12 cells. Correlation with neurotransmitter release. J Biol Chem. 1987 Jul 5;262(19):9189–9195. [PubMed] [Google Scholar]
- Filipski J., Leblanc J., Youdale T., Sikorska M., Walker P. R. Periodicity of DNA folding in higher order chromatin structures. EMBO J. 1990 Apr;9(4):1319–1327. doi: 10.1002/j.1460-2075.1990.tb08241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J. L., Johnson E. M., Jr Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci. 1992 Dec;15(12):501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Jiang S., Chow S. C., Nicotera P., Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca(2+)-ATPase inhibitor thapsigargin. Exp Cell Res. 1994 May;212(1):84–92. doi: 10.1006/excr.1994.1121. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., McDougall C. A., Harmon B. V., Halliday J. W. Calcium chelators induce apoptosis--evidence that raised intracellular ionised calcium is not essential for apoptosis. Biochim Biophys Acta. 1994 Sep 8;1223(2):247–254. doi: 10.1016/0167-4889(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Chow S. C., Orrenius S., Jondal M. NK cell-induced cytotoxicity is dependent on a Ca2+ increase in the target. FASEB J. 1990 Jun;4(9):2661–2664. doi: 10.1096/fasebj.4.9.2347464. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S. Signal transduction pathways to apoptosis. Trends Cell Biol. 1994 Oct;4(10):370–375. doi: 10.1016/0962-8924(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Mesner P. W., Winters T. R., Green S. H. Nerve growth factor withdrawal-induced cell death in neuronal PC12 cells resembles that in sympathetic neurons. J Cell Biol. 1992 Dec;119(6):1669–1680. doi: 10.1083/jcb.119.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy A., Laochumroonvorapong P., Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994 Oct 1;180(4):1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia M., Pizzo P., Sandoná D., Zanovello P., Rizzuto R., Di Virgilio F. Mitochondrial DNA is not fragmented during apoptosis. J Biol Chem. 1992 Jun 5;267(16):10939–10941. [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Oberhammer F. A., Hochegger K., Fröschl G., Tiefenbacher R., Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994 Aug;126(4):827–837. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Sun X. M., Cohen G. M. Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J Biol Chem. 1994 May 27;269(21):14857–14860. [PubMed] [Google Scholar]
- Treves S., Trentini P. L., Ascanelli M., Bucci G., Di Virgilio F. Apoptosis is dependent on intracellular zinc and independent of intracellular calcium in lymphocytes. Exp Cell Res. 1994 Apr;211(2):339–343. doi: 10.1006/excr.1994.1096. [DOI] [PubMed] [Google Scholar]
- Whyte M. K., Hardwick S. J., Meagher L. C., Savill J. S., Haslett C. Transient elevations of cytosolic free calcium retard subsequent apoptosis in neutrophils in vitro. J Clin Invest. 1993 Jul;92(1):446–455. doi: 10.1172/JCI116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B., Cedervall B., Jiang S., Nicotera P., Orrenius S. Involvement of Ca2+ in the formation of high molecular weight DNA fragments in thymocyte apoptosis. Biochem Biophys Res Commun. 1994 Jul 15;202(1):120–127. doi: 10.1006/bbrc.1994.1901. [DOI] [PubMed] [Google Scholar]