Abstract
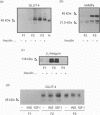
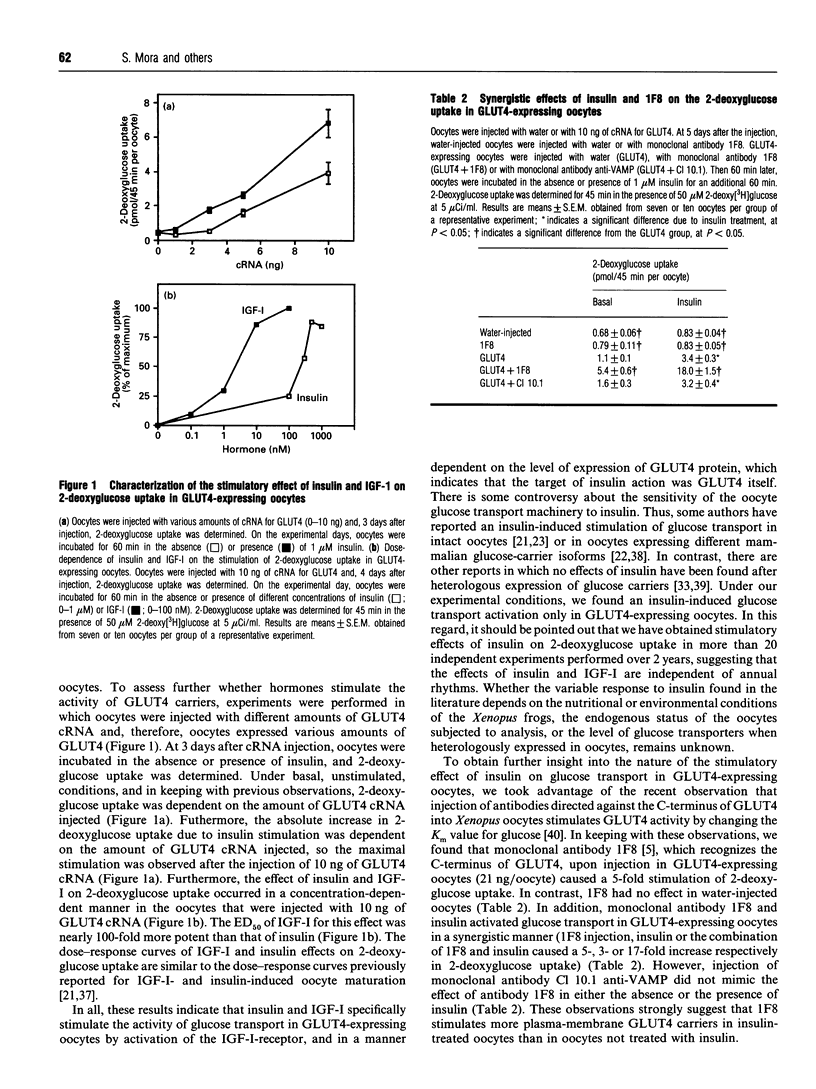
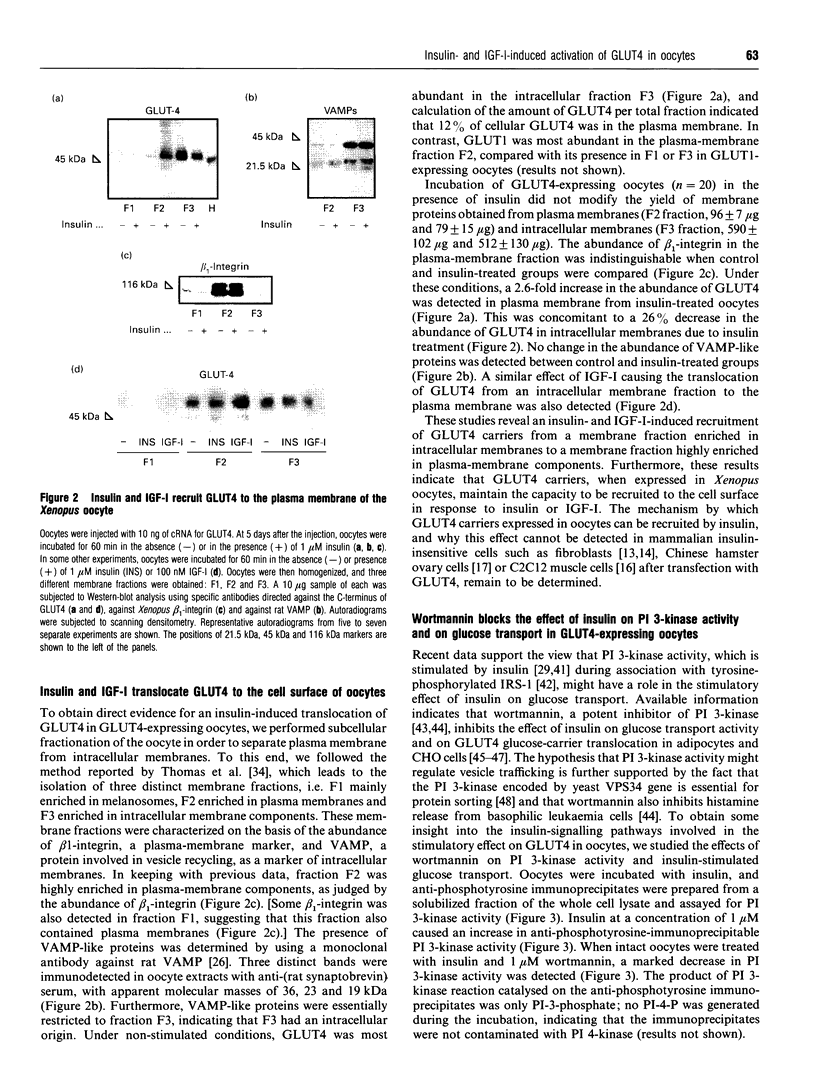
1. The heterologous expression of glucose transporters GLUT4 and GLUT1 in Xenopus oocytes has been shown to cause a differential targeting of these glucose-carrier isoforms to cellular membranes and a distinct induction of glucose transport activity. In this study we have evaluated the effect of insulin and insulin-like growth factor I (IGF-I) on glucose uptake and glucose transporter distribution in Xenopus oocytes expressing mammalian GLUT4 and GLUT1 glucose carriers. 2. Insulin and IGF-I stimulated 2-deoxyglucose uptake in GLUT4-expressing oocytes, but not in GLUT1-expressing oocytes or in water-injected oocytes. The stimulatory effect of insulin and IGF-I on 2-deoxyglucose uptake in GLUT4-expressing oocytes occurred via activation of the IGF-I receptor. 3. Subcellular-fractionation studies indicated that insulin and IGF-I stimulated translocation of GLUT4 to the cell surface of the oocyte. 4. Incubation of intact oocytes with insulin stimulated phosphatidylinositol 3-kinase activity, an effect that was blocked by the additional presence of wortmannin. Furthermore, wortmannin totally abolished the insulin-induced stimulation of 2-deoxyglucose uptake in GLUT4-expressing oocytes. 5. In this study, both the insulin-induced GLUT4 carrier translocation and GLUT4-dependent insulin-stimulated glucose transport have been reconstituted in the Xenopus oocyte. These observations, together with the fact that wortmannin, as found in adipocytes, inhibits insulin-stimulated glucose transport in oocytes, suggest that the heterologous expression of GLUT4 in oocytes is a useful experimental model by which to study the cell biology of insulin-induced GLUT4 translocation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Takata K., Katagiri H., Tsukuda K., Lin J. L., Ishihara H., Inukai K., Hirano H., Yazaki Y., Oka Y. Domains responsible for the differential targeting of glucose transporter isoforms. J Biol Chem. 1992 Sep 25;267(27):19636–19641. [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran J., Werner A., Chillarón J., Nunes V., Biber J., Testar X., Zorzano A., Estivill X., Murer H., Palacín M. Expression cloning of a human renal cDNA that induces high affinity transport of L-cystine shared with dibasic amino acids in Xenopus oocytes. J Biol Chem. 1993 Jul 15;268(20):14842–14849. [PubMed] [Google Scholar]
- Bornemann A., Ploug T., Schmalbruch H. Subcellular localization of GLUT4 in nonstimulated and insulin-stimulated soleus muscle of rat. Diabetes. 1992 Feb;41(2):215–221. doi: 10.2337/diab.41.2.215. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camps M., Castelló A., Muñoz P., Monfar M., Testar X., Palacín M., Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J. 1992 Mar 15;282(Pt 3):765–772. doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang L. M., Myers M. G., Jr, Seidner G. A., Birnbaum M. J., White M. F., Kahn C. R. Insulin receptor substrate 1 mediates insulin and insulin-like growth factor I-stimulated maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5172–5175. doi: 10.1073/pnas.90.11.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. F., Young P. W., Yonezawa K., Kasuga M., Holman G. D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994 Jun 15;300(Pt 3):631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Fischbarg J., Cheung M., Czegledy F., Li J., Iserovich P., Kuang K., Hubbard J., Garner M., Rosen O. M., Golde D. W. Evidence that facilitative glucose transporters may fold as beta-barrels. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11658–11662. doi: 10.1073/pnas.90.24.11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. E., Dudek R. W., Whitehead D. S., Downes D. L., Frisell W. R., Caro J. F., Dohm G. L. Immunolocalization of glucose transporter GLUT4 within human skeletal muscle. Diabetes. 1991 Jan;40(1):150–154. doi: 10.2337/diab.40.1.150. [DOI] [PubMed] [Google Scholar]
- Garcia J. C., Strube M., Leingang K., Keller K., Mueckler M. M. Amino acid substitutions at tryptophan 388 and tryptophan 412 of the HepG2 (Glut1) glucose transporter inhibit transport activity and targeting to the plasma membrane in Xenopus oocytes. J Biol Chem. 1992 Apr 15;267(11):7770–7776. [PubMed] [Google Scholar]
- Gawantka V., Ellinger-Ziegelbauer H., Hausen P. Beta 1-integrin is a maternal protein that is inserted into all newly formed plasma membranes during early Xenopus embryogenesis. Development. 1992 Jun;115(2):595–605. doi: 10.1242/dev.115.2.595. [DOI] [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Gould G. W., Derechin V., James D. E., Tordjman K., Ahern S., Gibbs E. M., Lienhard G. E., Mueckler M. Insulin-stimulated translocation of the HepG2/erythrocyte-type glucose transporter expressed in 3T3-L1 adipocytes. J Biol Chem. 1989 Feb 5;264(4):2180–2184. [PubMed] [Google Scholar]
- Gould G. W., Lienhard G. E. Expression of a functional glucose transporter in Xenopus oocytes. Biochemistry. 1989 Nov 28;28(24):9447–9452. doi: 10.1021/bi00450a030. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Thomas H. M., Jess T. J., Bell G. I. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991 May 28;30(21):5139–5145. doi: 10.1021/bi00235a004. [DOI] [PubMed] [Google Scholar]
- Gumà A., Mora C., Santalucía T., Viñals F., Testar X., Palacín M., Zorzano A. System A transport activity is stimulated in skeletal muscle in response to diabetes. FEBS Lett. 1992 Sep 21;310(1):51–54. doi: 10.1016/0014-5793(92)81144-b. [DOI] [PubMed] [Google Scholar]
- Haney P. M., Slot J. W., Piper R. C., James D. E., Mueckler M. Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J Cell Biol. 1991 Aug;114(4):689–699. doi: 10.1083/jcb.114.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. W., Ruiz M., Birnbaum M. J. Isoform-specific subcellular targeting of glucose transporters in mouse fibroblasts. J Cell Biol. 1992 Feb;116(3):785–797. doi: 10.1083/jcb.116.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F., Ito K., Todaka M., Hayashi H., Kamohara S., Ishii K., Okada T., Hazeki O., Ui M., Ebina Y. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun. 1993 Sep 15;195(2):762–768. doi: 10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- Keller K., Strube M., Mueckler M. Functional expression of the human HepG2 and rat adipocyte glucose transporters in Xenopus oocytes. Comparison of kinetic parameters. J Biol Chem. 1989 Nov 15;264(32):18884–18889. [PubMed] [Google Scholar]
- Klip A., Marette A. Acute and chronic signals controlling glucose transport in skeletal muscle. J Cell Biochem. 1992 Jan;48(1):51–60. doi: 10.1002/jcb.240480109. [DOI] [PubMed] [Google Scholar]
- Kotliar N., Pilch P. F. Expression of the glucose transporter isoform GLUT 4 is insufficient to confer insulin-regulatable hexose uptake to cultured muscle cells. Mol Endocrinol. 1992 Mar;6(3):337–345. doi: 10.1210/mend.6.3.1584210. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall B. A., Murata H., Hresko R. C., Mueckler M. Domains that confer intracellular sequestration of the Glut4 glucose transporter in Xenopus oocytes. J Biol Chem. 1993 Dec 15;268(35):26193–26199. [PubMed] [Google Scholar]
- Merrall N. W., Plevin R. J., Stokoe D., Cohen P., Nebreda A. R., Gould G. W. Mitogen-activated protein kinase (MAP kinase), MAP kinase kinase and c-Mos stimulate glucose transport in Xenopus oocytes. Biochem J. 1993 Oct 15;295(Pt 2):351–355. doi: 10.1042/bj2950351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Jr, Sun X. J., Cheatham B., Jachna B. R., Glasheen E. M., Backer J. M., White M. F. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3'-kinase. Endocrinology. 1993 Apr;132(4):1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Kakita S., Takahashi I., Kawahara K., Tsukuda E., Sano T., Yamada K., Yoshida M., Kase H., Matsuda Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992 Feb 5;267(4):2157–2163. [PubMed] [Google Scholar]
- Okada T., Kawano Y., Sakakibara T., Hazeki O., Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994 Feb 4;269(5):3568–3573. [PubMed] [Google Scholar]
- Piper R. C., Hess L. J., James D. E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y., Asano T., Lin J. L., Tsukuda K., Katagiri H., Ishihara H., Yazaki Y., Oka Y. Two glucose transporter isoforms are sorted differentially and are expressed in distinct cellular compartments. Biochem J. 1992 Feb 1;281(Pt 3):829–834. doi: 10.1042/bj2810829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., James D. E., Lienhard G. E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. M., Takeda J., Gould G. W. Differential targeting of glucose transporter isoforms heterologously expressed in Xenopus oocytes. Biochem J. 1993 Mar 15;290(Pt 3):707–715. doi: 10.1042/bj2900707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Functional expression of mammalian glucose transporters in Xenopus laevis oocytes: evidence for cell-dependent insulin sensitivity. Mol Cell Biol. 1989 Oct;9(10):4187–4195. doi: 10.1128/mcb.9.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993 Dec 5;268(34):25846–25856. [PubMed] [Google Scholar]