Abstract
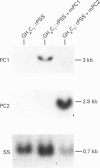
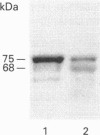
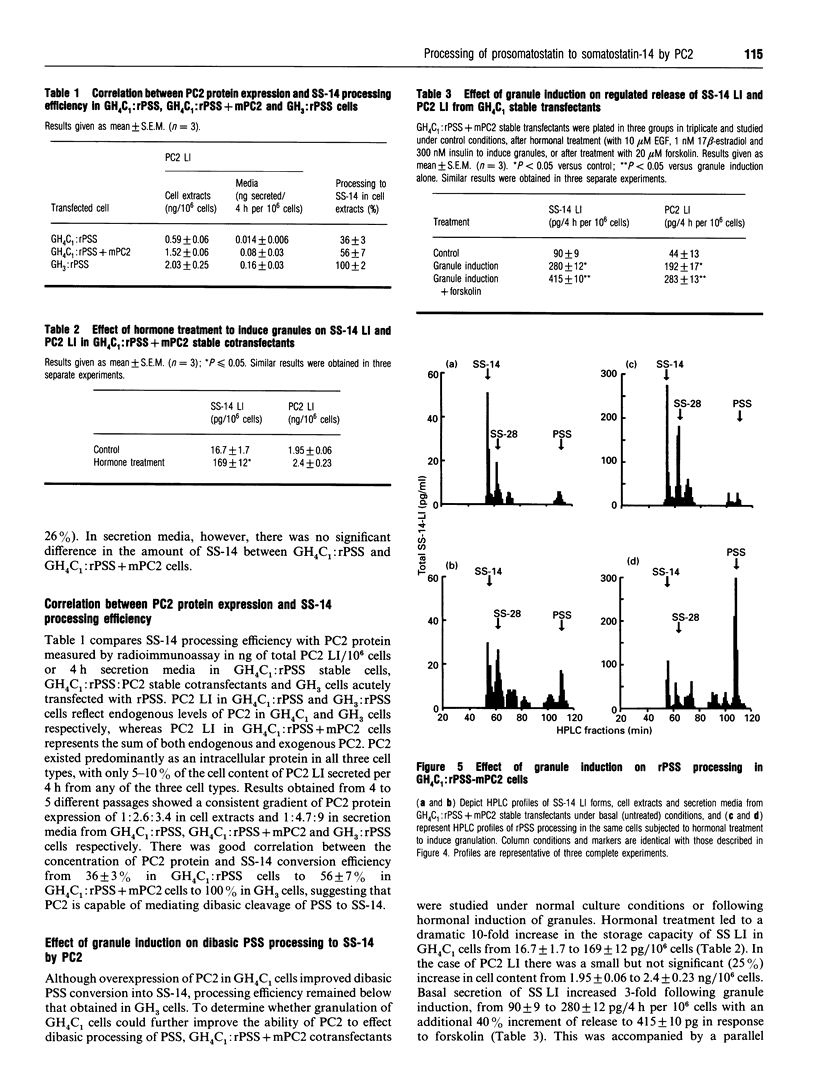
The role of PC2 in prosomatostatin (PSS) processing was investigated in GH3/GH4C1 pituitary cells. These cells are sparsely granulated, express different amounts of PC2 and no PC1. We described heterologous processing of rat PSS (rPSS) co-expressed with PC2 in stably transfected cells, correlate PC2 protein levels under different conditions of transfection with efficiency of PSS processing to somatostatin-14 (SS-14), determine the effect of modulating cell granularity on enzyme expression and PSS processing, and compare the relative potency of PC2 with that of PC1, PSS and cleavage products were monitored by HPLC and radioimmunoassay of SS-like immunoreactivity (SSLI). Radioimmunoassay analysis of N-terminal PC2-like immunoreactivity (PC2 LI) in GH4C1:rPSS, GH4C1:rPSS + PC2 and GH3:rPSS transfectants showed a gradient of PC2 protein of 1:2.6:3.4 in cell extracts and 1:4.7:9 in secretion media from these cells respectively. The concentration of PC2 protein correlated with SS-14 conversion efficiency was 36 +/- 3% in GH4C1:rPSS cells, 56 +/- 7% in GH4C1:rPSS-PC2 cells and 100% in GH3:rPSS cells. Treatment of GH4C1:rPSS + PC2 cells with epidermal growth factor, insulin, and beta-estradiol to induce granules, significantly increased basal and forskolin-stimulated co-release of SS LI and PC2 LI, but had no influence on SS-14 processing efficiency. Hormone treatment led to a small increase in the ratio of mature PC2 (68 kDa) to proPC2 (75 kDa) forms. PC1 stably transfected in GH4C1 cells produced significantly greater SS-14 conversion (62% in cells, 66% in media) compared with PC2 transfectants (53% in cells, 47% in media) These results provide the first proof that PC2 can effect dibasic processing of mammalian PSS, and, along with PC1, qualifies as an authentic SS-14 convertase. The activity of PC2 requires the milieu of the secretory cell but not the secretory granule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón C., Lincoln B., Rhodes C. J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J Biol Chem. 1993 Feb 25;268(6):4276–4280. [PubMed] [Google Scholar]
- Argos P., Taylor W. L., Minth C. D., Dixon J. E. Nucleotide and amino acid sequence comparisons of preprosomatostatins. J Biol Chem. 1983 Jul 25;258(14):8788–8793. [PubMed] [Google Scholar]
- Baskin D. G., Ensinck J. W. Somatostatin in epithelial cells of intestinal mucosa is present primarily as somatostatin 28. Peptides. 1984 May-Jun;5(3):615–621. doi: 10.1016/0196-9781(84)90092-5. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Paquet L., Boudreault A., Lazure C., Chrétien M., Seidah N. G. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993 Sep 15;294(Pt 3):735–743. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R., Ling N., Esch F. A new prosomatostatin-derived peptide reveals a pattern for prohormone cleavage at monobasic sites. Science. 1987 Nov 20;238(4830):1126–1129. doi: 10.1126/science.2891188. [DOI] [PubMed] [Google Scholar]
- Bloomquist B. T., Eipper B. A., Mains R. E. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991 Dec;5(12):2014–2024. doi: 10.1210/mend-5-12-2014. [DOI] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou A. S., Kent G., Rabbani S. N., Seidah N. G., Patel Y. C. Heterologous processing of prosomatostatin in constitutive and regulated secretory pathways. Putative role of the endoproteases furin, PC1, and PC2. J Biol Chem. 1993 Mar 15;268(8):6041–6049. [PubMed] [Google Scholar]
- Guest P. C., Arden S. D., Bennett D. L., Clark A., Rutherford N. G., Hutton J. C. The post-translational processing and intracellular sorting of PC2 in the islets of Langerhans. J Biol Chem. 1992 Nov 5;267(31):22401–22406. [PubMed] [Google Scholar]
- Halban P. A., Irminger J. C. Sorting and processing of secretory proteins. Biochem J. 1994 Apr 1;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Huang X. F., Arvan P. Formation of the insulin-containing secretory granule core occurs within immature beta-granules. J Biol Chem. 1994 Aug 19;269(33):20838–20844. [PubMed] [Google Scholar]
- Irminger J. C., Vollenweider F. M., Neerman-Arbez M., Halban P. A. Human proinsulin conversion in the regulated and the constitutive pathways of transfected AtT20 cells. J Biol Chem. 1994 Jan 21;269(3):1756–1762. [PubMed] [Google Scholar]
- Janovick J. A., Jennes L., Conn P. M. GH3 cells transfected with gonadotropin-releasing hormone (GnRH) receptor complementary deoxyribonucleic acid release secretogranin-II through a constitutive pathway after GnRH analog-regulated synthesis: evidence that secretory proteins do not contain a sequence that obligates processing through a secretory granule or by regulated secretion. Endocrinology. 1995 Jan;136(1):202–208. doi: 10.1210/endo.136.1.7828532. [DOI] [PubMed] [Google Scholar]
- Lusson J., Vieau D., Hamelin J., Day R., Chrétien M., Seidah N. G. cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6691–6695. doi: 10.1073/pnas.90.14.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin R. B., Noe B. D., Spiess J. Identification of a somatostatin-14-generating propeptide converting enzyme as a member of the kex2/furin/PC family. Endocrinology. 1991 Oct;129(4):2263–2265. doi: 10.1210/endo-129-4-2263. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M., Ramla D., Seidah N. G., Chrétien M. Developmental expression of the prohormone convertases PC1 and PC2 in mouse pancreatic islets. Endocrinology. 1994 Oct;135(4):1651–1660. doi: 10.1210/endo.135.4.7925129. [DOI] [PubMed] [Google Scholar]
- Martens G. J., Braks J. A., Eib D. W., Zhou Y., Lindberg I. The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5784–5787. doi: 10.1073/pnas.91.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. K., Greiner F., Bauer G. E., Elde R. P., Noe B. D. Separate cell types that express two different forms of somatostatin in anglerfish islets can be immunohistochemically differentiated. J Histochem Cytochem. 1987 Feb;35(2):155–162. doi: 10.1177/35.2.2878951. [DOI] [PubMed] [Google Scholar]
- Molloy S. S., Thomas L., VanSlyke J. K., Stenberg P. E., Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994 Jan 1;13(1):18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Goodman R. H., Horovitch S. J., Habener J. F. Primary structure of the gene encoding rat preprosomatostatin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3337–3340. doi: 10.1073/pnas.81.11.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Murakami K., Nakayama K. Identification of an isoform with an extremely large Cys-rich region of PC6, a Kex2-like processing endoprotease. FEBS Lett. 1993 Jul 26;327(2):165–171. doi: 10.1016/0014-5793(93)80163-o. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Kim W. S., Torii S., Hosaka M., Nakagawa T., Ikemizu J., Baba T., Murakami K. Identification of the fourth member of the mammalian endoprotease family homologous to the yeast Kex2 protease. Its testis-specific expression. J Biol Chem. 1992 Mar 25;267(9):5897–5900. [PubMed] [Google Scholar]
- Neerman-Arbez M., Cirulli V., Halban P. A. Levels of the conversion endoproteases PC1 (PC3) and PC2 distinguish between insulin-producing pancreatic islet beta cells and non-beta cells. Biochem J. 1994 May 15;300(Pt 1):57–61. doi: 10.1042/bj3000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni E. A., Sevarino K. A., Jackson I. M. Processing of proTRH to its intermediate products occurs before the packing into secretory granules of transfected AtT20 cells. Endocrinology. 1993 Mar;132(3):1271–1277. doi: 10.1210/endo.132.3.8440188. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., O'Neil W. Peptides derived from cleavage of prosomatostatin at carboxyl- and amino-terminal segments. Characterization of tissue and secreted forms in the rat. J Biol Chem. 1988 Jan 15;263(2):745–751. [PubMed] [Google Scholar]
- Patel Y. C., Wheatley T., Ning C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology. 1981 Dec;109(6):1943–1949. doi: 10.1210/endo-109-6-1943. [DOI] [PubMed] [Google Scholar]
- Pradayrol L., Jörnvall H., Mutt V., Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett. 1980 Jan 1;109(1):55–58. doi: 10.1016/0014-5793(80)81310-x. [DOI] [PubMed] [Google Scholar]
- Rabbani S. N., Patel Y. C. Peptides derived by processing of rat prosomatostatin near the amino-terminus: characterization, tissue distribution, and release. Endocrinology. 1990 Apr;126(4):2054–2061. doi: 10.1210/endo-126-4-2054. [DOI] [PubMed] [Google Scholar]
- Rangaraju N. S., Xu J. F., Harris R. B. Pro-gonadotropin-releasing hormone protein is processed within hypothalamic neurosecretory granules. Neuroendocrinology. 1991 Jan;53(1):20–28. doi: 10.1159/000125692. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Rufaut N. W., Brennan S. O., Hakes D. J., Dixon J. E., Birch N. P. Purification and characterization of the candidate prohormone-processing enzyme SPC3 produced in a mouse L cell line. J Biol Chem. 1993 Sep 25;268(27):20291–20298. [PubMed] [Google Scholar]
- Scammell J. G., Burrage T. G., Dannies P. S. Hormonal induction of secretory granules in a pituitary tumor cell line. Endocrinology. 1986 Oct;119(4):1543–1548. doi: 10.1210/endo-119-4-1543. [DOI] [PubMed] [Google Scholar]
- Schnabel E., Mains R. E., Farquhar M. G. Proteolytic processing of pro-ACTH/endorphin begins in the Golgi complex of pituitary corticotropes and AtT-20 cells. Mol Endocrinol. 1989 Aug;3(8):1223–1235. doi: 10.1210/mend-3-8-1223. [DOI] [PubMed] [Google Scholar]
- Schäfer M. K., Day R., Cullinan W. E., Chrétien M., Seidah N. G., Watson S. J. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis. J Neurosci. 1993 Mar;13(3):1258–1279. doi: 10.1523/JNEUROSCI.13-03-01258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M., Day R. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie. 1994;76(3-4):197–209. doi: 10.1016/0300-9084(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Chrétien M. The family of pro-hormone and pro-protein convertases. Biochem Soc Trans. 1993 Aug;21(3):685–691. doi: 10.1042/bst0210685. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Hamelin J., Gaspar A., Collard M. W., Chrétien M. Testicular expression of PC4 in the rat: molecular diversity of a novel germ cell-specific Kex2/subtilisin-like proprotein convertase. Mol Endocrinol. 1992 Oct;6(10):1559–1570. doi: 10.1210/mend.6.10.1448111. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Sevarino K. A., Felix R., Banks C. M., Low M. J., Montminy M. R., Mandel G., Goodman R. H. Cell-specific processing of preprosomatostatin in cultured neuroendocrine cells. J Biol Chem. 1987 Apr 15;262(11):4987–4993. [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Shen F. S., Seidah N. G., Lindberg I. Biosynthesis of the prohormone convertase PC2 in Chinese hamster ovary cells and in rat insulinoma cells. J Biol Chem. 1993 Nov 25;268(33):24910–24915. [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Stoller T. J., Shields D. Retrovirus-mediated expression of preprosomatostatin: posttranslational processing, intracellular storage, and secretion in GH3 pituitary cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2087–2095. doi: 10.1083/jcb.107.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller T. J., Shields D. The role of paired basic amino acids in mediating proteolytic cleavage of prosomatostatin. Analysis using site-directed mutagenesis. J Biol Chem. 1989 Apr 25;264(12):6922–6928. [PubMed] [Google Scholar]
- Vindrola O., Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992 Jul;6(7):1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992 Apr 25;267(12):8270–8274. [PubMed] [Google Scholar]
- Xu H., Shields D. Prohormone processing in the trans-Golgi network: endoproteolytic cleavage of prosomatostatin and formation of nascent secretory vesicles in permeabilized cells. J Cell Biol. 1993 Sep;122(6):1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Shields D. Prosomatostatin processing in permeabilized cells. Endoproteolytic cleavage is mediated by a vacuolar ATPase that generates an acidic pH in the trans-Golgi network. J Biol Chem. 1994 Sep 9;269(36):22875–22881. [PubMed] [Google Scholar]
- Zhou Y., Lindberg I. Purification and characterization of the prohormone convertase PC1(PC3). J Biol Chem. 1993 Mar 15;268(8):5615–5623. [PubMed] [Google Scholar]