Abstract
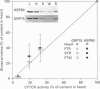
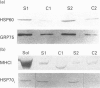
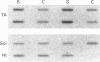
Molecular chaperones and cytosolic stress proteins are actively involved in the stabilization, import and refolding of precursor proteins into mitochondria. The purpose of the present study was to evaluate the relationship between mitochondrial content under steady-state conditions, and during the induction of organelle biogenesis, with the expression of stress proteins and mitochondrial chaperonins. A comparison of steady-state levels of mitochondrial enzyme activity [cytochrome c oxidase (CYTOX)] with chaperonin levels [the heat-shock protein HSP60, the glucose-regulated protein GRP75 (mtHSP70)] in striated muscles possessing a wide range of oxidative capacities revealed a proportional expression between the two. This relationship was disrupted by chronic contractile activity brought about by 10 days of 10 Hz stimulation of the tibialis anterior (TA) muscle, which induced 2.4-fold increases in CYTOX activity, but 3.2- and 9.3-fold increases in HSP60 and GRP75 respectively. The inducible stress protein HSP70i was detected at low levels in control TA muscle, and was increased 9.6-fold by chronic contractile activity, to values comparable with those found in the unstressed soleus muscle. This increase occurred in the absence of changes in type I MHC levels, indicating independent regulation of these genes. Despite the increases in HSP60 and HSP70i proteins, contractile activity did not alter their respective mRNA levels, illustrating post-transcriptional mechanisms of gene regulation during contractile activity. In contrast, the mRNA levels encoding the co-chaperonin CPN10 were increased 3.3-fold by contractile activity. Thus, the expression of individual mitochondrial chaperonins is independently regulated and uncoordinated. The extent of the induction of these stress proteins and chaperonins by contractile activity exceeded that of membrane enzymes (e.g. CYTOX). It remains to be determined whether this marked induction of proteins comprising part of the protein import machinery is beneficial for the translocation of enzyme precursors into the mitochondria during conditions of accelerated biogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agsteribbe E., Huckriede A., Veenhuis M., Ruiters M. H., Niezen-Koning K. E., Skjeldal O. H., Skullerud K., Gupta R. S., Hallberg R., van Diggelen O. P. A fatal, systemic mitochondrial disease with decreased mitochondrial enzyme activities, abnormal ultrastructure of the mitochondria and deficiency of heat shock protein 60. Biochem Biophys Res Commun. 1993 May 28;193(1):146–154. doi: 10.1006/bbrc.1993.1602. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hiranuma K., Hirata K., Abe T., Hirano T., Matsuno K., Hirano H., Suzuki K., Higashi K. Induction of mitochondrial chaperonin, hsp60, by cadmium in human hepatoma cells. Biochem Biophys Res Commun. 1993 Jul 15;194(1):531–536. doi: 10.1006/bbrc.1993.1852. [DOI] [PubMed] [Google Scholar]
- Hood D. A., Balaban A., Connor M. K., Craig E. E., Nishio M. L., Rezvani M., Takahashi M. Mitochondrial biogenesis in striated muscle. Can J Appl Physiol. 1994 Mar;19(1):12–48. doi: 10.1139/h94-002. [DOI] [PubMed] [Google Scholar]
- Hood D. A. Co-ordinate expression of cytochrome c oxidase subunit III and VIc mRNAs in rat tissues. Biochem J. 1990 Jul 15;269(2):503–506. doi: 10.1042/bj2690503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D. A., Kelton R., Nishio M. L. Mitochondrial adaptations to chronic muscle use: effect of iron deficiency. Comp Biochem Physiol Comp Physiol. 1992 Mar;101(3):597–605. doi: 10.1016/0300-9629(92)90514-q. [DOI] [PubMed] [Google Scholar]
- Hood D. A., Pette D. Chronic long-term electrostimulation creates a unique metabolic enzyme profile in rabbit fast-twitch muscle. FEBS Lett. 1989 Apr 24;247(2):471–474. doi: 10.1016/0014-5793(89)81393-6. [DOI] [PubMed] [Google Scholar]
- Hood D. A., Zak R., Pette D. Chronic stimulation of rat skeletal muscle induces coordinate increases in mitochondrial and nuclear mRNAs of cytochrome-c-oxidase subunits. Eur J Biochem. 1989 Feb 1;179(2):275–280. doi: 10.1111/j.1432-1033.1989.tb14551.x. [DOI] [PubMed] [Google Scholar]
- Höhfeld J., Hartl F. U. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol. 1994 Jul;126(2):305–315. doi: 10.1083/jcb.126.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore J. L., Timson B. F., Saunders D. K., Kraemer R. R., Klemm R. D., Ross C. R. Stress protein induction in skeletal muscle: comparison of laboratory models to naturally occurring hypertrophy. J Appl Physiol (1985) 1994 Feb;76(2):598–601. doi: 10.1152/jappl.1994.76.2.598. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Locke M., Noble E. G., Atkinson B. G. Exercising mammals synthesize stress proteins. Am J Physiol. 1990 Apr;258(4 Pt 1):C723–C729. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- Locke M., Noble E. G., Atkinson B. G. Inducible isoform of HSP70 is constitutively expressed in a muscle fiber type specific pattern. Am J Physiol. 1991 Nov;261(5 Pt 1):C774–C779. doi: 10.1152/ajpcell.1991.261.5.C774. [DOI] [PubMed] [Google Scholar]
- Matz J. M., Blake M. J., Saari J. T., Bode A. M. Dietary copper deficiency reduces heat shock protein expression in cardiovascular tissues. FASEB J. 1994 Jan;8(1):97–102. [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Nakai A., Morimoto R. I. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol Cell Biol. 1993 Apr;13(4):1983–1997. doi: 10.1128/mcb.13.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W., Pfanner N. Roles of molecular chaperones in protein targeting to mitochondria. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):355–362. doi: 10.1098/rstb.1993.0034. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Reichmann H., Hoppeler H., Mathieu-Costello O., von Bergen F., Pette D. Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch. 1985 May;404(1):1–9. doi: 10.1007/BF00581484. [DOI] [PubMed] [Google Scholar]
- Rospert S., Glick B. S., Jenö P., Schatz G., Todd M. J., Lorimer G. H., Viitanen P. V. Identification and functional analysis of chaperonin 10, the groES homolog from yeast mitochondria. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10967–10971. doi: 10.1073/pnas.90.23.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. T., Hoogenraad N. J., Høj P. B. Isolation of a cDNA clone specifying rat chaperonin 10, a stress-inducible mitochondrial matrix protein synthesised without a cleavable presequence. FEBS Lett. 1994 Jan 10;337(2):152–156. doi: 10.1016/0014-5793(94)80263-7. [DOI] [PubMed] [Google Scholar]
- Salo D. C., Donovan C. M., Davies K. J. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med. 1991;11(3):239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Schwartz J. C., Arrang J. M., Garbarg M., Pollard H., Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991 Jan;71(1):1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Hood D. A. Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle. J Appl Physiol (1985) 1993 Feb;74(2):934–941. doi: 10.1152/jappl.1993.74.2.934. [DOI] [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single-fiber study. Eur J Biochem. 1989 Dec 22;186(3):749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992 Oct;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Garcia-Moll M., Mellor J., Salmons S., Harlan W. Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins. J Biol Chem. 1987 Feb 25;262(6):2764–2767. [PubMed] [Google Scholar]
- Williams R. S., Salmons S., Newsholme E. A., Kaufman R. E., Mellor J. Regulation of nuclear and mitochondrial gene expression by contractile activity in skeletal muscle. J Biol Chem. 1986 Jan 5;261(1):376–380. [PubMed] [Google Scholar]