Abstract
Congenital syphilis (CS) is on the rise in the United States and is a growing public health concern. CS is an infection with Treponema pallidum in an infant or fetus, acquired via transplacental transmission when a pregnant woman has untreated or inadequately treated syphilis. Pregnant women with untreated syphilis are more likely to experience pregnancies complicated by stillbirth, prematurity, low birth weight, and early infant death, while their children can develop clinical manifestations of CS such as hepatosplenomegaly, bone abnormalities, developmental delays, and hearing loss. One of the ways CS can be prevented is by identifying and treating infected women during pregnancy with a benzathine penicillin G regimen that is both appropriate for the maternal stage of syphilis and initiated at least 30 days prior to delivery. In this article we discuss many of the challenges faced by both public health and healthcare systems with regards to this preventable infection, summarize missed opportunities for CS prevention, and provide practical solutions for future CS prevention strategies.
Keywords: congenital syphilis, sexually transmitted infections, pregnancy, prenatal care
Introduction
For the past 7 years, congenital syphilis (CS) has been rising yearly in the United States. CS, an ancient and devastating disease, can be acquired in utero at any stage of maternal syphilis and at any gestational age during pregnancy from a woman with untreated or inadequately treated syphilis.1,2 Pregnant women with untreated syphilis are more likely to have poorer pregnancy outcomes, including stillbirth, early infant death, prematurity, and low birth weight, while their infants may have clinical manifestations of CS.3
CS can be prevented by identifying and treating infected women before pregnancy or by adequately treating maternal infection during pregnancy with a benzathine penicillin G regimen that is both appropriate for the maternal stage of syphilis and initiated at least 30 days before delivery. Adequate treatment during pregnancy is 98% efficacious in preventing CS.2,4 Because CS is preventable, its resurgence points to missed opportunities for intervention—areas where both public health and health care systems can do more. In this report, we summarize missed opportunities for CS prevention and provide potential solutions to reverse this trend.
Scope of the problem
Since 2012, when CS reached a relative low in the United States with 334 reported cases, cases of CS have increased annually. In 2019, 1,870 cases were reported in the United States—an almost fivefold increase in 7 years. Increases in CS closely mirror increases in syphilis among women of reproductive age (15–44 years) (Fig. 1).5 Syphilis among women has increased steadily since 2013 with cases increasing 33.6%—from 21,116 to 28,216 cases—during 2018–2019 alone. Of the 28,216 cases in 2019, the majority (84.7%) were among women of reproductive age.5
FIG. 1.
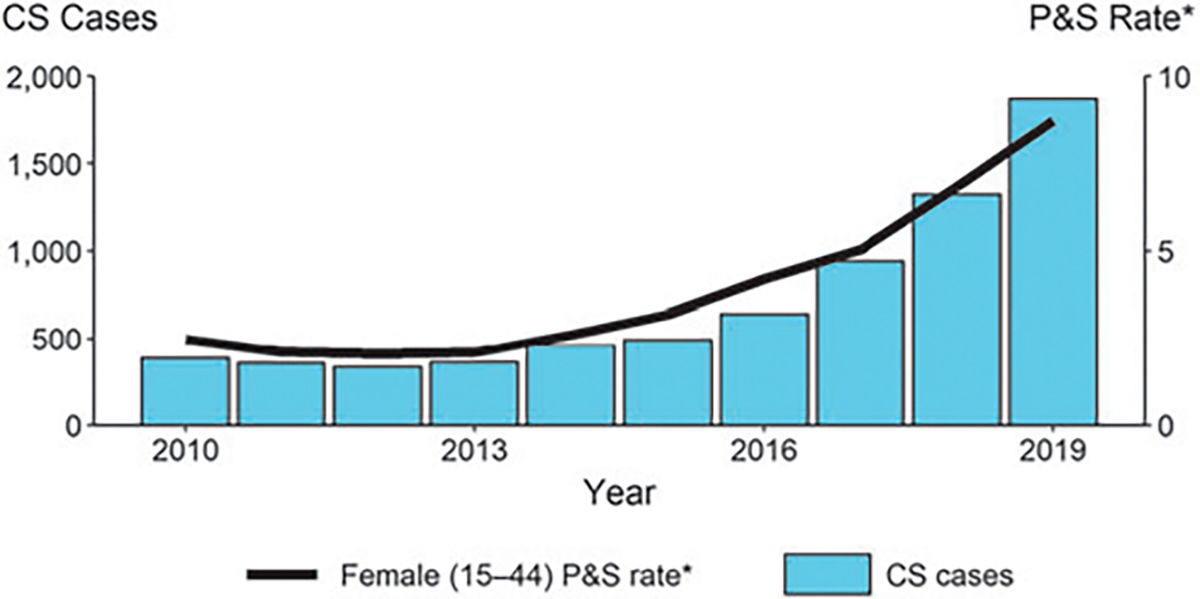
CS—reported cases by year of birth and rates of reported cases of P&S syphilis among females aged 15–44 years, United States, 2010–2019.5 *Per 100,000. CS, congenital syphilis; P&S, primary and secondary syphilis.
The adverse outcomes of untreated syphilis in pregnant women make this increase in syphilis among women even more concerning. Among the 1,870 reported infants with CS in 2019, 128 (6.8%) were stillborn or died during early infancy. Another 712 (38.1%) had signs or symptoms of CS, including long bone changes, pseudoparalysis, enlarged liver or spleen (hepatosplenomegaly), jaundice due to syphilitic hepatitis, rash, copious nasal discharge (snuffles), and other characteristic presentations of syphilis infection. Of note, 31.1% of CS cases reported in 2019 were born premature (<37 weeks gestational age), which has important long-term implications for child health outcomes and economic impact for society (CDC, unpublished national case report data).
Traditional approaches
Because CS is preventable with adequate and timely treatment of syphilis in pregnancy, the Centers for Disease Control and Prevention (CDC), the U.S. Preventive Services Task Force (USPSTF), and the American College of Obstetricians and Gynecologists (ACOG) recommend syphilis screening in pregnancy.2,6,7 Per CDC guidance, all pregnant women should be tested for syphilis at their first prenatal visit. Repeat screening for pregnant women at high risk for syphilis acquisition should occur at 28 weeks and at delivery to detect infections or reinfections that occurred during pregnancy. For the purposes of screening, maternal risk factors for syphilis acquisition during pregnancy include the following: sex with multiple partners, sex in conjunction with drug use, or transactional sex; late entry to prenatal care (first visit during the second trimester or later) or no prenatal care; methamphetamine or heroin use; incarceration of the woman or her partner; unstable housing or homelessness; or living in a geographic area where there is a high prevalence of syphilis.8
Screening at delivery for high-risk individuals is especially important because many infants diagnosed with CS appear asymptomatic at birth, but without treatment are likely to develop symptoms after hospital discharge. If not treated before 3 months of age, infants with CS may go on to develop late manifestations that are characterized by more permanent findings such as bone abnormalities, cranial nerve deafness, interstitial keratitis, and intellectual disabilities.1,9,10 Health care providers should confirm that maternal syphilis testing was performed at least once during pregnancy before a mother or infant is discharged from the hospital to identify those who may otherwise have been missed. In addition, due to the high association between CS and stillbirth, all women who deliver a stillborn infant should be tested for syphilis and if positive, treated to prevent future adverse outcomes.2
In most states, these recommendations are echoed by laws directing providers to test pregnant women for syphilis. A 2016 article describing U.S. state laws related to prenatal syphilis screening found that 45 states had at least one law that requires syphilis testing of pregnant women. Most states (84.3%, n = 43) require screening at the first prenatal visit. Only one-third (n = 17) require another screening during the third trimester; 12 of these states require third trimester screening in all pregnancies, while 5 states require third trimester screening only if the patient is considered at increased risk for syphilis. An even smaller proportion of U.S. states (15.7%, n = 8) require screening at delivery; three of these states require screening of all women at delivery, and five states require screening at delivery only if the woman is considered at increased risk for syphilis. Only seven states have screening requirements that align with, or exceed, the CDC recommendations for syphilis screening among pregnant women.11 CDC maintains a webpage that includes additional information on this analysis, including the text of the laws underlying the categorization on states, which it updates annually to account for changes in law (https://www.cdc.gov/std/treatment/syphilis-screenings.htm).
Missed opportunities
To better understand why pregnant women with syphilis fall through the cracks, a 2018 study explored opportunities to prevent CS using national level data. Based on the mother’s prenatal care, testing, and treatment history, each CS case was assigned to one of four mutually exclusive missed opportunities. The missed opportunities include: lack of timely prenatal care (28.2%), no timely syphilis testing despite receipt of timely prenatal care (8.9%), lack of adequate maternal treatment despite timely diagnoses of syphilis (30.7%), and late identification of seroconversion during pregnancy (11.2%).12 The underlying causes of these missed opportunities will be explored in the next section.
Underlying causes
Gaps in prenatal care
Despite CDC, USPSTF, and ACOG recommendations to screen for syphilis in pregnancy, and varying degrees of legislation reinforcing these guidelines, provider adherence to recommendations varies greatly. An analysis using the 2013 MarketScan claims database to evaluate syphilis screening practices among commercially insured women who delivered an infant, revealed that only 85% (288,324/338,854) of these women had a syphilis test performed at least once during the prenatal period. Syphilis testing rates were ~80% during the first trimester, 20% in the third trimester, and 8% in the week of delivery, with some women screened multiple times. Pregnant women in the South were more likely to receive a syphilis test compared to pregnant women in other regions, especially the Northeast (87% vs. 79%).13
For pregnancies that end in stillbirth, lack of syphilis testing is especially distressing. Another study using the 2013 MarketScan claims database found that approximately one-third of women with stillbirth cases did not receive syphilis testing either prenatally or after the stillbirth delivery.14 Similarly, a recent study using medical chart review found that among mothers with confirmed stillbirth cases, only half (51.4%) had any syphilis testing conducted during that pregnancy. Of those, only slightly more than half (54.3%) were tested before their stillbirth delivery and 42.9% were tested for syphilis only after delivery.15
The 2018 study exploring missed opportunities for prevention of CS on a national level found that the most common missed opportunity for CS prevention is a lack of adequate maternal treatment despite timely diagnoses of syphilis (31%).12 Looking across two state and local jurisdiction investigations conducted since 2000 reveals that 14.7%–25.0% of women who delivered a CS baby were inadequately treated in pregnancy and 8.0%–26.2% did not receive any treatment at all.16,17
Access to care
Although CS is easily prevented by the timely diagnosis and treatment of a pregnant woman with syphilis, prevention hinges on access to health care with sufficient time for diagnosis and treatment before delivery. Lack of timely prenatal care, the second most common missed opportunity, affected 28.2% of all CS births in 2018.12 For many, the causes underlying their diagnosis are complicated by socioeconomic systems that span multiple sectors. Often, they are interconnected (e.g., poverty, incarceration, homelessness, and substance use) and compounded by health care system access issues, creating multiple barriers that effectively block access to needed prenatal care services.18
At the behest of states seeing rising cases of CS, CDC conducted a series of rapid ethnographic assessments (REAs) to provide context into structural, behavioral, and health care systems access issues contributing to local increases in CS using qualitative interviews with key stakeholders. These REAs identified interwoven factors stretching across multiple sectors in the community that complicate addressing CS on the individual and societal level.
For some women, obstacles begin before pregnancy. A consistent refrain across the REAs is inadequate sexual health education, often accompanied by social norms discouraging open discussions about sexuality. In these cases, unplanned pregnancy is stigmatized while the tools needed to prevent pregnancy are withheld. Stigma and its accompanying shame lead some to try to hide a pregnancy, resulting in late entry into prenatal care or no receipt of services until delivery. Here, the need to access sexual health services is outweighed by fears that service-seeking and receipt will not be confidential, borne by concerns of being seen accessing prenatal services and having word spread throughout the community or to parents, family, or friends19 (PS Loosier, personal communication).
Pregnant women who do seek care may also experience difficulty accessing the health care system, especially if they are reliant on public insurance.20 For some, access to appropriate health services is delayed by the steps required to obtain public health insurance. This is especially true in states which have not expanded Medicaid beyond traditional categorical eligibility. For example, a REA in Caddo Parish, Louisiana, revealed what some affected participants called a “Catch 22.”19 (Note: The state was not participating in Medicaid expansion at the time). Although Louisiana extended Medicaid eligibility to pregnant women through a state family planning waiver, delays in Medicaid enrollment often led to women seeking prenatal care after 20 weeks gestational age; as a result of this delayed entry to care, many women were deemed “high risk” and therefore required referral to maternal fetal medicine. Even for those who have or acquire Medicaid earlier in pregnancy, other systemic barriers thwart efforts to enter into prenatal care. For example, even if someone used a prenatal care provider for a previous birth, that provider might not be able to accept them for a subsequent birth if their practice quota of Medicaid patients is met or if their annual window for accepting Medicaid patients has closed (PS Loosier, personal communication).
Having access to insurance and subsequent access to an appropriate health care provider may address some—but not all—of these barriers. Economic concerns and attenuated social support networks often necessitate that women place other survival priorities ahead of sexual health and pregnancy concerns. Women working hourly wage jobs may not have the flexibility to take off the time needed to schedule and attend prenatal care visits. When they do, many rely upon overburdened safety net provider systems characterized by long patient backlogs creating scheduling and day-of delays. As one participant in Caddo Parish noted, women seeking prenatal care from one of the largest providers of safety-net care in the area should “pack a lunch” and expect to wait for most of the day before being seen. Other frequently reported barriers included lack of transportation and an inability to find or afford childcare for other children while they seek prenatal care (PS Loosier, personal communication).
Patient-level concerns
Mothers of CS infants may be particularly vulnerable and exist at the intersection of multiple determinants that place them at high risk for negative outcomes. In a qualitative analysis of 24 interviews and maternal records observations in Indiana, six mothers were homeless and three unstably housed at the time of syphilis diagnosis. Eight had a history of incarceration.18
An REA focused on CS in Maricopa County, Arizona, found that aggressive enforcement of local immigration policies had created a climate of distrust between pregnant women at risk of syphilis and local institutions, including medical services.21 Because of this, many avoided contact with health care providers out of fear that they would come to the attention of immigration officials. Public health officials in this and other locales have also suggested that substance use during pregnancy may cause some women to avoid prenatal care out of concern for legal consequences or fear that their other children will be taken away22 (PS Loosier, personal communication).
These issues are mirrored among women with syphilis more broadly, where they are connected to other important social determinants of health, including race and ethnicity. Syphilis disproportionately affects women of color, with primary and secondary (P&S) syphilis rates significantly higher among American Indian/Alaska Native women (15.4 cases per 100,000 population) and black women (10.2 per 100,000) relative to white women (2.3 per 100,000).5 According to 2018 data, women with early syphilis who were interviewed by public health staff reported a variety of behavioral characteristics that complicate interactions with the health care system, including injection drug use (11.4%), methamphetamine use (18.9%), heroin use (7.3%), and sex with a person who injects drugs (12.6%).23
Complicated clinical considerations
Because of its numerous clinical presentations that can be confused with other diseases, syphilis is known as the “great imitator,”24 and has always been difficult to diagnose based on clinical examination. In fact, Sir William Osler, the father of modern medicine, is quoted as stating, “he who knows syphilis, knows medicine.”24 With the advent of penicillin, testing, and strong public health efforts, syphilis became much less common and physicians began seeing syphilis, and its myriad of clinical presentations, less frequently. In addition, many patients with syphilis are asymptomatic. Thus, laboratory testing is often essential and the basis for diagnosis of syphilis.
The laboratory evaluation itself, however, is not simple. There are no commercially available direct detection tests for Treponema pallidum, the organism that causes syphilis. Testing typically involves the use of two types of antibody tests, where one is used for screening and the other as a confirmatory test if the first is reactive. The two test types include a nontreponemal test (i.e., Rapid Plasma Reagin [RPR] or Venereal Disease Research Laboratory [VDRL]) and a treponemal test (i.e., T. pallidum particle agglutination assay [TP-PA], fluorescent treponemal antibody absorbed test [FTA-ABS], microhemagglutination assay for T. pallidum antibodies [MHA-TP] and various immunoassays).2 The need for two different types of antibody tests can be confusing to many health care professionals, particularly when discordant results emerge.25
To complicate matters more, treponemal antibodies typically remain present for life, so a positive treponemal test can indicate a current active infection or past treated infection. Thus, treatment decisions can require a lengthy investigation into the patient’s treatment history that can be facilitated by the health department which maintains records of past positive syphilis serology. After a patient is treated for syphilis, nontreponemal titers must be followed at regular intervals to determine treatment response. Traditionally, adequate treatment response is defined as a fourfold decline in the nontreponemal titer within 6–12 months after initiation of therapy.2
Pregnancy can complicate an already-complicated laboratory evaluation for syphilis. Pregnancy can alter immune status, impacting antibody levels. It has been reported that a higher proportion of false-positive treponemal tests occur in pregnancy.26 Conversely, false-negative nontreponemal test results due to the prozone reaction (when the antibody titer is so high that it interferes with the proper formation of the antigen-antibody lattice network necessary for the nontreponemal test to return positive results) may occur more often in pregnant women.27
Furthermore, if syphilis is diagnosed during pregnancy and treatment is initiated, following titers in pregnancy to evaluate treatment response is complicated. Since pregnancy typically lasts 9 months, depending on when treatment was initiated, the titer may not have time to decline. Providers may have to follow titers more closely especially for women who are at high risk for reinfection or live in an area where the prevalence of syphilis is high. Providers can also consult with an expert for individualized care.
Innovative Solutions
The issues surrounding the increase in CS are complex, with an interplay between health care systems, provider care, policy, societal factors, and individual risk. Effective interventions will require a strong understanding of the various aspects of the problem and the population facing it; innovation to drive new solutions; and collaboration across sectors to tackle obstacles that impede receipt of high-quality care and contribute to missed opportunities. Despite the complexity of CS, there are many programs and partners who can contribute to solutions.
Prenatal care providers
It is critical that all pregnant women receive timely prenatal care and timely syphilis screening. Prenatal care providers need to test everyone at their first prenatal care visit and retest at 28 weeks and delivery if they are considered high risk for syphilis or live in high-prevalence areas for syphilis. All pregnant women who receive a syphilis diagnosis should receive prompt and appropriate treatment. Providers must also ensure that the local health department has been notified.
Multistep serologic screening can make differentiating between new, untreated infection and past, treated infection difficult, but partnering with local Sexually Transmitted Disease (STD) clinicians or health departments can be helpful. They can also help update providers about trends in local syphilis rates, especially among women of reproductive age.
CDC offers a number of resources to help clinicians navigate these concerns: Screening and treatment recommendations for syphilis in pregnancy are outlined in CDC’s 2015 STD Treatment Guidelines and are available at Syphilis in Pregnancy—2015 STD Treatment Guidelines (cdc.gov). In addition, the National Network of STD Clinical Prevention Training Centers (NNPTC) and National Network of STD Prevention Training Centers (nnptc.org), composed of eight regional and two national training centers throughout the United States, offers providers training on the clinical management of STIs. The NNPTC also provides clinical consultations for health care providers through their website within three business days, initiated upon submission of a clinical consultation request (www.STDCCN.org). For clinicians desiring on-demand learning opportunities, the free National STD Curriculum (www.std.uw.edu) addresses the epidemiology, pathogenesis, clinical manifestations, diagnosis, management, and prevention of STIs, including CS, and offers free continuing medical education credit, continuing nursing education/continuing education (CE) contact hours, and pharmacology CE for advanced practice nurses.
To expand syphilis screening during pregnancy to those at risk for poor follow-up, prenatal care providers could consider looking for opportunities to screen for syphilis outside of the clinic setting, such as during emergency department (ED) or inpatient consults. Prenatal care providers should also ensure that mothers are not discharged from the hospital without documentation of appropriate syphilis screening during pregnancy.
Finally, patient education about the risks of infection or reinfection of syphilis and other STIs in pregnancy, can be integrated into prenatal care counseling in the context of health care provision. Educating pregnant patients about the value of condom use and partner treatment to prevent STI acquisition and reinfection provides the patient with the tools to make informed decisions to protect her health. However, providers should also acknowledge that many of the major drivers of STIs in pregnancy occur beyond the level of individual behavior. The establishment of rapport, use of nonjudgmental language (verbal and nonverbal), and respect for the individual remain essential components of behavioral counseling methods.28
Other health care providers
Since lack of prenatal care is a consistent issue for the mothers of infants diagnosed with CS, partnerships with nontraditional health care sites have the potential to reach pregnant women who might seek care at these other sites. EDs and urgent care centers are uniquely positioned to screen for syphilis among uninsured and underinsured at-risk populations as they often serve as a primary point of care for these populations.29–31 Partnering with EDs to implement syphilis screening may help identify syphilis in pregnant women who would otherwise fall through the cracks.
Public health STD programs
Public health STD programs are crucial to prevent CS. STD programs use Disease Intervention Specialists (DIS), who can facilitate timely treatment of pregnant women and offer partner testing and treatment. DIS also verify workup and appropriate treatment of neonates. Using their in-depth knowledge of the communities served, they can help connect pregnant women to prenatal care and can reach out to nontraditional partners to provide additional resources for screening high-risk populations.
Statewide or local syphilis case review boards run by the health department in collaboration with hospitals and local community members can help identify areas of missed opportunities and support systemic changes to prevent CS.32 A concerted effort from public health officials is needed for the ongoing prioritization of pregnant women with syphilis and continued improvements to DIS training for CS.
Other nonhealth care-related partners
Health care systems and public health STD programs can partner with organizations, which provide services to pregnant women outside of traditional prenatal care clinics, such as jails, homeless shelters, and medication-assisted treatment programs for opioid use disorders to offer screenings for syphilis. Similarly, they can partner with organizations already engaged in maternal and child health, such as the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and Nurse-Family Partnerships (https://www.nursefamilypartnership.org/).
Policy and laws
Screening policies can potentially increase prenatal syphilis screening, and reduce CS rates, and may impact provider adherence. Jurisdictions seeking to improve provider adherence to recommended prenatal screenings may consider what role policies might play in their jurisdiction. Although CDC recommendations may affect provider practice, provider adherence to these recommendations is ultimately voluntary and dependent on provider knowledge of the recommendations. Alternatively, state laws may be highly indicative of the jurisdictions’ standards of care, and adherence is not voluntary.
As a result, state laws may be especially influential on provider practices related to CS prevention. Additional policies regarding access to prenatal care for Medicaid-insured women, Medicaid reimbursement, increased reimbursement for STI prevention counseling, and improved pregnancy prevention strategies with easier access to family planning services could be beneficial as well. Finally, policies that ensure culturally competent care for pregnant women who use illicit drugs, while alleviating concerns regarding legal consequences of substance use, could be an important area of focus.
Pharmacies
Ensuring consistent access to benzathine penicillin G is key. The costly nature and infrequent use in smaller clinical settings means that many clinicians cannot afford to keep it in-office and must refer out to the health department or have the patient take a prescription to a pharmacy and then return to the provider’s office, so the medication can be administered. Further delays may be introduced if the pharmacy does not keep benzathine penicillin G in stock and must order it. This added delay between diagnosis and treatment may contribute to a lack of timely treatment despite timely testing. One solution may be to develop a network of pharmacies that will always have benzathine penicillin G on-hand. Alternatively, partnering with health departments that can swiftly deliver needed medications to individual practices could be practical solution.
Laboratory
The need for innovation and development of new tests for syphilis is well documented.33 Biomedical research and innovative laboratory tests used to diagnose syphilis require access to biospecimens, which are difficult to obtain. To answer this need, CDC recently launched a syphilis serum repository to build a biospecimen bank.34 Any test developed should be faster than existing tests, have high sensitivity and specificity, and should be suitable for screening applications. Automation and electronic result reading and reporting are also desirable in laboratory settings. An ideal test would be a genetic direct detection test for T. pallidum in an easily accessible specimen such as urine, saliva, or blood.
Although such a novel test remains elusive, there have been notable improvements in the speed of existing serology tests: treponemal and nontreponemal tests in automated formats have entered the market in the last decade, which have the potential to improve turn-around time. In addition, there are now two rapid tests for qualitative treponemal antibody detection on the U.S. market; one of them is a dual HIV-syphilis test. This test is currently under evaluation in pregnant women in a concerted world-wide effort orchestrated by the World Health Organization.35 The development of a rapid, dual treponemal/nontreponemal test is taking place in international settings and could be particularly useful for syphilis testing during pregnancy.36
Conclusion
Issues surrounding CS are multifactorial and solutions are not simple. They touch on societal and individual levels and need to be addressed by multiple sectors. Health care providers should be familiar with testing requirements and be attentive to syphilis rates in their community. Community-based organizations and governmental programs serving pregnant women can link women to prenatal care and encourage syphilis testing. With rates of syphilis in women of reproductive age, pregnant women and their infants rising, health departments can raise awareness of the increasing risk of CS cases in the local area, and partner with providers and community-based organizations to increase linkage to care. Health departments can also continue to prioritize follow-up with pregnant women diagnosed with syphilis.
The U.S. Department of Health and Human Services recently released a 5-year Sexually Transmitted Infections National Strategic Plan for addressing rising rates of STIs (https://www.hhs.gov/programs/topic-sites/sexually-transmitted-infections/plan-overview/index.html). It focuses, in part, on addressing the social determinants of health as factors that complicate access to prenatal care and other health services, and timely treatment of syphilis in pregnant women.
The Sexually Transmitted Infections National Strategic Plan was followed by a National Academies of Sciences, Engineering, and Medicine (NASEM) Committee report, Sexually Transmitted Infections: Adopting A Sexual Health Paradigm, that reviewed the current state of STIs in the United States and provided advice on future public health programs, policy, and research to address the STI epidemic (https://www.nationalacademies.org/our-work/prevention-and-control-of-sexually-transmitted-infections-in-the-united-states). The NASEM report emphasizes a sexual health approach to the future of STD work with an emphasis on destigmatizing STIs, improving sexual health services to women and expanding attention and resources to underserved populations while also acknowledging structural inequities as root causes of STI outcomes.
The whole of government approach recommended by both of these plans, combined with efforts at the local level, along with new and established partnerships, will hopefully address key obstacles to the prevention of CS; the expansiveness of the issue highlights the breadth of professions and diversity of people it touches. It may take a village to raise a child, but it will take a nation to prevent CS.
Funding Information
No funding was received for this article.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cooper JM, Sanchez PJ. Congenital syphilis. Semin Perinatol 2018;42:176–184. [DOI] [PubMed] [Google Scholar]
- 2.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and reports: Morbidity and mortality weekly report. Recommend Rep 2015;64(Rr-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: A systematic review and meta-analysis. Bull World Health Organ 2013;91:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel GD Jr,. Efficacy of treatment for syphilis in pregnancy. Obstet Gynecol 1999;93:5–8. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2019. US Department of Health and Human Services. Available at: https://www.cdc.gov/std/statistics/2019/default.htm Accessed April 14, 2021.
- 6.Assessment USPSTF. Screening for syphilis infection in pregnancy. U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2009;150:705–709. [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick SJ, Papile LA, Macones GA. (eds) Guidelines for perinatal care. American Academy of Pediatrics; 2017. Sep 17. [Google Scholar]
- 8.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. (In press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiumara NJ. Syphilis among mothers and children. Ann N Y Acad Sci 1988;549:187–192. [DOI] [PubMed] [Google Scholar]
- 10.Lago EG, Vaccari A, Fiori RM. Clinical features and follow-up of congenital syphilis. Sex Trans Dis 2013;40:85–94. [DOI] [PubMed] [Google Scholar]
- 11.Warren HP, Cramer R, Kidd S, Leichliter JS. State requirements for prenatal syphilis screening in the United States, 2016. Matern Child Health J 2018;22:1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimball A, Torrone E, Miele K, et al. Missed opportunities for prevention of congenital syphilis—United States, 2018. MMWR Morb Mortal Wkly Rep 2020;69:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neblett Fanfair R, Tao G, Owusu-Edusei K, Gift TL, Bernstein KT. Suboptimal prenatal syphilis testing among commercially insured women in the United States, 2013. Sex Trans Dis 2017;44:219–221. [DOI] [PubMed] [Google Scholar]
- 14.Patel CG, Huppert JS, Tao G. Provider adherence to syphilis testing recommendations for women delivering a stillbirth. Sex Trans Dis 2017;44:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho YA, Allen K, Tao G, et al. Provider adherence to syphilis testing guidelines among stillbirth cases. Sex Trans Dis 2020;47:686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SJ, Klinger EJ, O’Toole D, Schillinger JA. Missed opportunities for preventing congenital syphilis infection in New York City. Obstet Gynecol 2012;120:882–888. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MM, Mickey T, Browne K, Kenney K, England B, Blasini-Alcivar L. Opportunities for the prevention of congenital syphilis in Maricopa County, Arizona. Sex Trans Dis 2008;35:341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiOrio D, Kroeger K, Ross A. Social vulnerability in congenital syphilis case mothers: Qualitative assessment of cases in Indiana, 2014 to 2016. Sex Trans Dis 2018;45:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroeger KA, Sangaramoorthy T, Loosier PS, Schmidt R, Gruber D. Pathways to congenital syphilis prevention: A rapid qualitative assessment of barriers, and the public health response, in Caddo Parish, Louisiana. Sex Trans Dis 2018;45:442–446. [DOI] [PubMed] [Google Scholar]
- 20.Taylor YJ, Liu TL, Howell EA. Insurance differences in preventive care use and adverse birth outcomes among pregnant women in a medicaid nonexpansion state: A retrospective cohort study. J Womens Health (2002) 2020;29:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangaramoorthy T, Kroeger KA. Rapid Ethnographic Assessments: A Practical Approach and Toolkit For Collaborative Community Research, First Edition. Oxfordshire, UK; Routledge; 2020. [Google Scholar]
- 22.2020 STD Prevention Conference September 14–24, 2020. Sex Trans Dis 2020;47(9S Suppl 2):S1–S173. PW14. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Syphilis Supplement; 2018. US Department of Health and Human Services. Available at: https://www.cdc.gov/std/stats18/syphilis2018/default.htm Accessed May 4, 2021. [Google Scholar]
- 24.Silverman ME, Murray TJ, Bryan CS (eds.). The Quotable Osler. Philiadelphia, PA: American College of Physicians, 2008. [Google Scholar]
- 25.Mmeje O, Chow JM, Davidson L, Shieh J, Schapiro JM, Park IU. Discordant syphilis immunoassays in pregnancy: Perinatal outcomes and implications for clinical management. Clin Infect Dis 2015;61:1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park IU, Chow JM, Bolan G, Stanley M, Shieh J, Schapiro JM. Screening for syphilis with the treponemal immunoassay: Analysis of discordant serology results and implications for clinical management. J Infect Dis 2011;204:1297–1304. [DOI] [PubMed] [Google Scholar]
- 27.Liu LL, Lin LR, Tong ML, et al. Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin Infect Dis 2014;59:384–389. [DOI] [PubMed] [Google Scholar]
- 28.Barrow RY, Ahmed F, Bolan GA, Workowski KA. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Morb Mortal Wkly Rep 2020;68:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung PT, Wiler JL, Lowe RA, Ginde AA. National study of barriers to timely primary care and emergency department utilization among Medicaid beneficiaries. Ann Emerg Med 2012;60:4–10.e2. [DOI] [PubMed] [Google Scholar]
- 30.Kangovi S, Barg FK, Carter T, Long JA, Shannon R, Grande D. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Affairs (Project Hope) 2013;32:1196–1203. [DOI] [PubMed] [Google Scholar]
- 31.Pane GA, Farner MC, Salness KA. Health care access problems of medically indigent emergency department walk-in patients. Ann Emerg Med 1991;20:730–733. [DOI] [PubMed] [Google Scholar]
- 32.Rahman MM, Hoover A, Johnson C, Peterman TA. Preventing congenital syphilis-opportunities identified by congenital syphilis case review noards. Sex Trans Dis 2019;46:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC call to action: Let’s work together to stem the tide of rising syphilis in the United States. Washington, DC: U.S. Department of Health and Human Services, 2017. [Google Scholar]
- 34.Shukla M, Sun Y, McCormick J, et al. Development of a syphilis serum bank to support research, development, and evaluation of syphilis diagnostic tests in the United States. Diagn Microbiol Infect Dis 2020;96:114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Network ProSPeRo. Standardised protocol for a prospective cross-sectional multicentre clinic-based evaluation of two dual point-of-care tests for the screening of HIV and syphilis in men who have sex with men, sex workers and pregnant women. BMJ Open 2020;10:e044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marks M, Yin YP, Chen XS, et al. Metaanalysis of the performance of a combined treponemal and nontreponemal rapid diagnostic test for syphilis and yaws. Clin Infect Dis 2016;63:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]


