RESUME
Introduction: Le diagnostic microscopique de mésothéliome pleural malin est réalisé généralement sur des biopsies pleurales faites sous thoracoscopie. Néanmoins, la cytologie pleurale peut contribuer à un diagnostic précoce en utilisant des procédures moins invasives. Objectif : Evaluer la valeur diagnostique des différents critères cytologiques diagnostiques consensuels dans la mise en évidence du mésothéliome pleural malin et dans la différenciation entre adénocarcinome, mésothéliome et hyperplasie mésothéliale réactionnelle en prenant comme gold standard le diagnostic retenu sur les prélèvements tissulaires Méthodes: Tous les patients explorés pour un épanchement pleural qui ont eu une cytologie pleurale et une biopsie pleural associée, et ce durant la période allant de janvier 2015 à février 2020 ont été inclus. Toutes les cytologies et les biopsies ont été relues par 2 pathologistes (MM, RY). Les critères cytologiques consignés ont été les critères diagnostiques des mésothéliomes pleuraux malins selon les recommandations internationales pour le diagnostic cytologique du mésothéliome pleural malin epithélioide et mixte. En prenant comme référence, le diagnostic fait sur les prélèvements tissulaires, les sensibilités, spécificités, valeurs prédictives positives et négatives ont été calculées. Résultats: 189 biopsies ainsi que leurs cytologies pleurales correspondantes ont été incluses et relues. Les diagnostics retenus sur les prélèvements biopsiques étaient les suivants : adénocarcinomes dans 100 cas, lymphomes dans 30 cas, carcinomes à petites cellules dans 20 cas, carcinomes epidermoïdes dans 5 cas, 7 mésothéliomes pleuraux malins et 27 remaniements inflammatoires. Après relecture des cytologies pleurales, le diagnostic a été modifié dans 21/189 cytologies. Les critères cytologiques les plus sensibles étaient: les blebs cytoplasmiques, l’hypercellularité, les morules. Les critères cytologiques les plus spécifiques étaient l’absence de granulations extra-cellulaires d’acide hyaluronique dans les cytologies réactionnelles, l’absence de fenêtres inter-cellulaires dans les amas cellulaires des cytologies malignes non mésothéliomateuses. La valeur prédictive positive la plus élevée était attribuée aux corps extra-cellulaires de granulations d’acide hyaluronique et la valeur prédictive négative la plus élevée était attribuée aux blebs cytoplasmiques dans les cytologies pleurales réactionnelles et les cytologies malignes non mésothéliomateuses. Conclusion: Ces résultats mettent en évidence le fait qu’aucun signe cytologique n’est pathognomonique du diagnostic de mésothéliome pleural malin d’où la nécessité de techniques immunocytochimiques dans les cas équivoques.
ABSTRACT
INTRODUCTION: The diagnosis of malignant pleural mesothelioma (MPM) depends on microscopic examination performed on pleural biopsies taken under thoracoscopy. However, it has recently been established that cytology presents a significant diagnostic contribution enabling an earlier diagnosis with a minimally invasive procedure. AIM: To assess the diagnostic value of consensual cytological features of MPM in the differentiation between adenocarcinoma, mesothelioma and reactive mesothelial cells in pleural liquid. METHODS: All available retrospective records from the computerized pathology database system and pathology reports were searched for malignant pleural effusion cytology specimens, over a 5-year period from January 2015 to February 2020. The cytological criteria based on the international Guidelines for cytopathologic diagnosis of epithelioid and mixed type of MPM were assessed. Malignant mesothelial cells, MNML and RL were considered as the gold standard. RESULTS: 189 pleural biopsies with their corresponding cytology specimens were available for review. Among the reviewed cytologies, the diagnoses of 21/189 pleural cytologies were modified. The highest sensitivities were attributed to cytoplasmic blebbing, hypercellularity and cell ball clusters. The most specific feature was the absence of extracellular granular hyaluronic acid cores in reactive cytology and the absence of intercellular openings in NMML cell clusters. Extracellular granular hyaluronic acid cores had the highest positive predictive value and the highest negative predictive value was attributed to the cytoplasmic blebbing in both reactive cytology and NMML. CONCLUSION: These results highlight the fact that no sign is pathognomonic of the diagnosis of MPM pointing out the necessity of immunocytochemical techniques in equivocal cases.
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a rare and highly aggressive tumor accounting for 0.2% of all cancers and has a dismal prognosis with limited therapeutic options. It often presents with pleural effusion as first clinical symptom, even if it is not specific (1). Commonly, the diagnosis of MPM mainly depends on microscopic examination performed on pleural biopsies taken under thoracoscopy, as has been the gold standard for decades (2). However, it has recently been established that cytology presents a significant contribution to the diagnosis of MPM providing an opportunity to enable an earlier diagnosis with a minimally invasive procedure, especially for oldest patients or those with comorbidities who cannot undergo pleural biopsy. Cytology allows a significant time saving for the therapeutic management (3). Indeed, cytological examination of effusion specimens is based on cytomorphological criteria already elucidated through the literature. The reliability of cytology in the diagnosis of MPM remains controversial due to the multiple cytomorphological similarities between malignant and reactive mesothelial cells on one hand, and to the noteworthy overlap existing between malignant mesothelioma and adenocarcinoma cells on the other hand (4, 5). Our aim was to assess the diagnostic value of consensual cytological features of MPM in the differentiation between adenocarcinoma, mesothelioma and reactive mesothelial cells in pleural liquid by comparing the diagnosis made on cytological specimen to the diagnosis made on tissue. The latter is considered as the gold standard.
METHODS
This was a retrospective and descriptive study evaluating the diagnostic value of the cytological diagnosis of MPM without immunocytochemical study.
All available retrospective records from the computerized pathology database system and pathology reports were searched for malignant pleural effusion cytology specimens, over a 5-year period from January 2015 to February 2020.
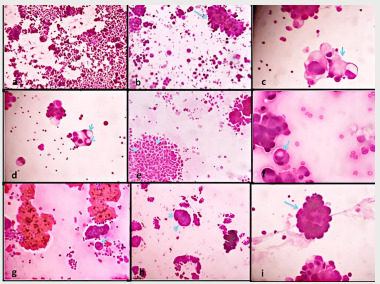
- Inclusion criteria: All pleural biopsies (either malignant or benign) which were associated to pleural cytologies with the complete records mentioned were included. - Exclusion criteria: Cases with no concomitant pleural biopsies and cytologies were excluded. - Non inclusion criteria: Peritoneal cytologies weren’t included. No immunocytochemical sections were reviewed in this study. - Laboratory technique: Cytological material was routinely stained with papanicolaou stain. - Microscopic criteria assessed: The cytological criteria that were assessed were those which were most often described in the MPM based on the international Guidelines for cytopathologic diagnosis of epithelioid and mixed type malignant mesothelioma. Complementary statement from the International Mesothelioma Interest Group, also endorsed by the International Academy of Cytology and the Papanicolaou Society of Cytopathology (5). These criteria were as follow: 1) the abundance of neoplastic cells single or clusters, 2) cell ball or papillary cell groups with scalloped borders, 3) optically dense cytoplasm with lacy peripheral vacuoles or blebs, 4) intracytoplasmic vacuoles overlapping the nuclei appearing as “punched out holes”, 5) intercellular clear spaces or «windows» within the clusters, 6) «cell in cell» engulfment of mesothelial cells, also called cellular cannibalism, 7) cytomegaly with multinucleation, 8) the presence of a prominent nucleoli, 9) acidophilic extracellular matrix bodies also known as collagen cores, 10) the monomorphic aspect of the clusters as well as atypical and mitotic figures. The presence or absence of these features was recorded in each case. The different characteristics are represented in figure 1.
Figure 1. a) cytologic smear characterized by abundant neoplastic cells in a mesothelioma (HEx250), b) papillary cell groups with scalloped borders in an adenocarcinoma (arrow) (HEx250), c) tumor cell with peripheral vacuoles or blebs in RL (arrow) (HEx400), d) intracytoplasmic vacuoles overlapping the nuclei appearing as “punched out holes” in an adenocarcinoma (arrow) (HEx400), e) intercellular clear spaces or «windows» within the clusters (arrows) in a mesothelioma (HEx250), f) «cell in cell» engulfment of mesothelial cells (arrow) in a mesothelioma (HEx400), g) tumour cell with multinucleation (arrow) in an adenocarcinoma (HEx400), h) acidophilic extracellular matrix bodies also known as collagen cores around papillary cell groups in a RL (arrow) (HEx250), i) monomorphic aspect of tumor clusters (arrow) in a mesothelioma (HEx400).
- Statistical analysis: The sensitivity and specificity of each cytological criteria in favor of the diagnosis of MPM were defined and estimated as described below: True positive cases (TP) were cases of MPM in which the cytological criteria of mesothelioma were present and the biopsy concluded to a malignant mesothelioma. True negative cases (TN) were non-mesotheliomatous malignant lesions (NMML) or reactive lesions (RL) without diagnostic cytological criteria of MPM and with a concordant final diagnosis on biopsy. False positive cases (FP) were NMML or RL with diagnostic cytological criteria of MPM and with benign features on biopsy. False negative cases (FN) were cases of MPM that didn’t present diagnostic cytological criteria but were diagnosed as mesotheliomas on biopsy Sensitivity, specificity, positive and negative predictive values were calculated as follow: - % Sensitivity = [True Positive / (True Positive + False Negative)] x 100%. - % Specificity = [True Negative/ (True Negative + False Positive)] x 100%. - % Positive predictive value (VPP) = [True Positive / (True Positive + False Positive)]. - % Negative predictive value (VPN) = [True Negative / (True Negative + False Negative)]. Specificity, PPV and NPV were calculated taking into account respectively RL and NMML.
RESULTS
A total of 2186 pleural biopsies were received during the period of study. 189 (9%) cases with the corresponding pleural biopsy and cytology were listed and were available for review. The diagnoses performed and reviewed on biopsies consisted of adenocarcinomas in 100 cases, lymphomas in 30 cases, small cell carcinomas in 20 cases, squamous cell carcinomas in 5 cases, 7 MPM and 27 inflammatory processes. All the slides of cytologies were reviewed. Among them, the diagnoses of 21/189 pleural cytologies were modified. 18 cases that were initially classified as suspect cytology were reviewed as reactive cytologies. Three cases initially diagnosed as metastatic tumoral cytologies were reviewed as MPM. The other 168 cases were diagnosed as NMML in 155 cases, MPM in 4 cases and reactive cytologies in 9 cases. The 155 NMML diagnosed on cytologies consisted of adenocarcinomas in 100 cases, lymphomas in 30 cases, small cell carcinomas in 20 cases and squamous cell carcinomas in 5 cases when compared to biopsies.
The concordance between the diagnoses performed on cytologies and biopsies accounted for 100%. The sensitivities of the cytological findings that were considered as diagnostic of MPM were respectively as follow: hypercellularity (75%), clustering (75%), vacuolization in the perinuclear area (75%), multinucleation (75%) and protrusions through the cell membrane (100%). Papillary clustering of tumor cells, cellular cannibalism, macronucleoli, intercellular opening or “windows” created by a less cellular cohesiveness, the presence of extracellular acidophilic cores and the monomorphic appearance of tumor aggregates had a sensitivity reaching 50% respectively for each of those features.
Low cellularity and the absence of some cytological criteria such as cellular cannibalism, macronucleoli, intercellular windows, extracellular acidophilic bodies, mitoses and atypia presented a specificity reaching respectively 72%, 87%, 43%, 96%, 85%, 89% and 97% considering the NMML and 90%, 90%, 93%, 96%, 100%, 90% and 100% considering the RL.
The specificity related to the absence of blebbing, intracytoplasmic vacuoles, multinucleated cells as well as the polymorphic aspect of tumor cells in NMML was respectively estimated to 63%, 53%, 72% and 53% with a NPV respectively of 100%, 98%, 99% and 97%. For RL, the specificity of those same findings reached respectively 46%, 76%, 66% and 80% with a NPV of 100%, 95%, 95% and 92%. Data concerning PPV and NPV are listed in table 1. The highest PPV considering respectively NMML and RL were attributed to intercellular spaces and atypia. The highest NPV considering respectively NMML and RL were attributed respectively to low cellularity and mitoses.
Table 1 : Predictive values of different cytologic criteria in malignant non mesotheliomatous lesions (MNML) and reactive lesions (RL) .
|
Criteria |
PPV in MNML |
NPV in MNML |
NPV in RL |
PPV in RL |
|---|---|---|---|---|
|
Hypercellularity |
6%, CI95%: [-0.02, 0.14] |
- |
- |
50% |
|
Clusters |
2%, CI95%: [-0.06,0.1] |
- |
- |
21% |
|
Intracytoplasmic vacuoles |
4%, CI95%: [-0.04, 0.12] |
- |
- |
30% |
|
Multinucleation |
6%, CI95%: [-0.02, 0.14] |
- |
- |
23% |
|
Blebbing |
6%, CI95%: [-0.02, 0.14] |
- |
- |
20% |
|
Papillary structures |
11%, CI95%: [0.03, 0.19] |
- |
- |
40% |
|
Cellular cannibalism |
9%, CI95%: [0.01, 0.17] |
98%, CI95%: [0.9, 1.06] |
93%, CI95%: [0.75, 1.11] |
40% |
|
Prominent nucleoli |
2%, CI95%: [-0.06, 0.1] |
97%, CI95%: [0.89, 1.05] |
93%, CI95%: [0.75, 1.11] |
50% |
|
Intercellular windows |
25%, CI95%: [0.17, 0.33] |
98%, CI95%: [0.9, 1.06] |
93%, CI95%: [0.75, 1.11] |
66% |
|
Monomorphic tumor clusters |
2.7%, CI95%: [-0.77, 0.1] |
- |
- |
25% |
|
Extracellular acidophilic bodies |
8%, CI95%: [0, 0.16] |
98%, CI95%: [0.9, 1.06] |
93%, CI95%: [0.75, 1.11] |
100%, CI95%: [0.82, 1.18] |
|
Atypia |
20%, CI95%: [0.12, 0.28] |
98%, CI95%: [0.9, 1.06] |
90%, CI95%: [0.72, 1.08] |
100%, CI95%: [0.82, 1.18] |
|
Low cellularity |
- |
99%, CI95%: [0.91, 1.07] |
96%, CI95%: [0.78, 1.14] |
- |
|
Mitoses |
- |
97%, CI95%: [0.89, 1.05] |
98%, CI95%: [0.8, 1.16] |
- |
DISCUSSION
In this study, we aimed to assess the diagnostic value of the cytologic criteria indicative of MPM according to the International Academy of Cytology and the Papanicolaou Society of Cytopathology. The sensitivities, the specificities, the PPV and NPV were assessed considering the malignant mesothelial nature, the reactive nature and the malignant non mesothelial nature of the desquamative cells. The highest sensitivity was attributed to the protrusions through the cell membrane (100%). The highest specificity was reported for atypia (97%) considering NMML and the absence of atypia considering reactive cells (100%). The highest PPV considering respectively NMML and RL were attributed to intercellular spaces and atypia. The highest NPV considering respectively NMML and RL were attributed to low cellularity and mitoses. Hjerpe, et al. and Siddiqui et, al. reported that hypercellular effusion samples are very suspicious of malignancy (5, 9). In the current study, we observed, in accordance to the literature, a hypercellularity with a sensitivity of 75% and 72% respectively in MPM and NMML. Reactive effusions are mainly sparsely cellular. This criterion is even more in favor of malignancy in the presence of cell clusters and especially papillary structures, which can be observed in both MPM and adenocarcinoma (4, 8). Michael et al reported that clusters exhibit a greater diversity in shape and size in MPM than in adenocarcinoma (10). In reactive mesothelial hyperplasia, papillae are reported to be simpler with a monolayer cell arrangement (7). According to Cakir, et al. (11), cytologic and nuclear monotony of these cell populations is more in favor of MPM than adenocarcinoma in which they are more polymorphic. In our study, we found that balls or papillary aggregates have a sensitivity up to 75% in MPM whereas it is much lower in NMML (30%). This criterion is interestingly specific in benign mesothelial hyperplasia since it is lacking in most cases with a specificity of 90%. Besides, those cell clusters tend to be uniform in MPM cases with a sensitivity of 50% unlike in both NMML and reactive mesothelial proliferation cases, hence, displaying a specificity up to 80%.
Intercellular spaces or “windows” have been repeatedly cited in several studies as one of the cytomorphological hallmark of MPM but this feature could be found in both MPM and reactive mesothelial cells, much less in adenocarcinoma (5, 6, 8). In our study, we noted that this feature was almost totally absent in cases of adenocarcinoma and reactive cytologies with a quite high specificity (96%). Cakir, et al. (11) also described that cell-in-cell engulfments are most commonly seen in MPM effusion samples (57,5%), lesser in adenocarcinoma (32,5%) and rarely noted in reactive mesothelial proliferation (3,3%). This fact made this feature useful in distinguishing especially MPM from reactive mesothelial proliferation. Our results were consistent with those above-mentioned findings delineated by Cakir, et al. (11), with a specificity of 90%.
The extracellular granular hyaluronic acid cores are reported to be diagnostic in MPM because adenocarcinoma secretes mucins (2, 12). The sensitivity and specificity of this sign reached respectively 50% and 100% in our study. However, this sign has been reported in reactive cells by whitaker, et al (8).
The presence of macronucleoli, cytoplasmic vacuolization and cytoplasmic blebbing have usually been favoring of MPM. Many studies through the literature indicated that macronucleoli were mainly seen in malignant effusions, whether in MPM or adenocarcinoma, whereas it’s a rare feature in reactive mesothelial cells ( 8, 13). Moreover, the sensitivity of macronucleoli in the study of Kaur, et al. (13) was about 41.9%. In the current study, this sign had a sensitivity of 50% and a specificity of 93%, in concordance with the literature. Cytoplasmic membrane protrusions or blebbing have been described as favoring a diagnosis of MPM in several studies (11, 13). In the current study, all the cases of MPM presented this feature with a sensitivity of 100%. However, this feature can be seen in cases of adenocarcinoma in the form of numerous microvacuoles unlike the large, well-defined vacuolation found in MPM (11, 14). In our study, the sensitivity of atypia reached 25%. This might be due to the fact that MPM are known to display rather mild atypia, which can be observed in reactive mesothelial proliferations (3, 6, 15). The major limitation of this study consists in the few number of malignant mesotheliomas included that can be explained by the rarity of these tumors. Besides, the results may be challenging to assimilate because of the multiple categories studied consisting in NMML, RL and MPM in addition to the numerous cytological signs assessed. The categories studied were necessary because the answer in the cytology report can’t be limited to a binary response consisting in benign or malignant. According to the International Standards, differentiating reactive lesions from malignant non mesotheliomatous ones and mesotheliomas is compulsory and challenging because of the unspecific signs. All these results point out the absence of a pathognomonic cytologic sign of malignant mesothelioma justifying the necessity of using immunocytochemical techniques (3, 8, 15, 16). Immunocytochemical markers are the same ones used in biopsies. According to the recommendations throughout the literature, at least four markers are needed, consisting in both positive and negative markers for mesothelial nature and for other differential diagnoses whether reactive cytologies or metastastic malignancies.
Antibodies choice depends on their sensitivity or specificity which should reach at least 80% to have a significant predictive value (4 , 6). Desmin and BAP-1 antibodies have been proposed and used to distinguish reactive cytologies from malignant ones since malignant mesothelial cells are known to lose desmin’s expression with a predictive negative value of 100% (5) and more recently BAP-1 expression (5, 13). Besides, calretinin, cytokeratin (CK) 5/6, WT1, EMA, and Mesothelin were the most commonly used mesothelial markers (5, 13, 16, 17). The major limitation of our study consists in the analysis of cytologic criteria without immunocytochemistry.
CONCLUSION
In this study, we assessed the sensitivity, specificity and the predictive values of the different cytomorphologic features useful in the cytologic diagnosis of MPM according to the literature recommendations. The highest sensitivities were attributed to cytoplasmic blebbing, hypercellularity and cell ball clusters. The most specific feature was the absence of extracellular granular hyaluronic acid cores in reactive cytology and the absence of intercellular openings in NMML cell clusters.
The highest positive predictive value was attributed to the extracellular granular hyaluronic acid cores and the highest negative predictive value was attributed to the cytoplasmic blebbing in both reactive cytology and NMML. These results highlight the fact that no single cytologic criteria can be diagnostic of mesothelioma and that this diagnosis may be suspected when many diagnostic signs are observed. The contributive role of cytology in the early diagnosis of MPM should be acknowledged, given its impact on the management of MPM, especially the earlier onset of treatment hence, a better expected prognosis for the patient.
However, cytology can be unsatisfactory in some equivocal cases, therefore requiring complementary tests such as immunocytochemistry in order to enhance the diagnostic accuracy of MPM.
References
- Le Stang N, Burke L, Blaizot G, Gibbs AR, Lebailly P, Clin B. Differential Diagnosis of Epithelioid Malignant Mesothelioma With Lung and Breast Pleural Metastasis: A Systematic Review Compared With a Standardized Panel of Antibodies—A New proposal that may influence pathologic practice. Arch Pathol Lab Med. 2020;144(4):446–456. doi: 10.5858/arpa.2018-0457-OA. [DOI] [PubMed] [Google Scholar]
- Rakha EA, Patil S, Abdulla K, Abdulkader M, Chaudry Z, Soomro IN. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn Cytopathol. 2010;38(12):874–879. doi: 10.1002/dc.21303. [DOI] [PubMed] [Google Scholar]
- Abd Own S, Hoijer J, Hillerdahl G, Dobra K, Hjerpe A. Effusion cytology of malignant mesothelioma enables earlier diagnosis and recognizes patients with better prognosis. Diagn Cytopathol. 2021 May;49(5):606–614. doi: 10.1002/dc.24395. [DOI] [PubMed] [Google Scholar]
- Chen L, Caldero SG, Gmitro S, Smith ML, De Petris G, Zarka MA. Small orangiphilic squamous-like cells: An underrecognized and useful morphological feature for the diagnosis of malignant mesothelioma in pleural effusion cytology. Cancer Cytopathol. 2014;122(1):70–75. doi: 10.1002/cncy.21345. [DOI] [PubMed] [Google Scholar]
- Hjerpe A, Abdown S, Dobra K. Cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma: ten years of clinical experience in relation to international guidelines. Arch Pathol Lab Med. 2018;142(8):893–901. doi: 10.5858/arpa.2018-0020-RA. [DOI] [PubMed] [Google Scholar]
- Husain AN, Colby TV, Oordonez NG, Allen TC, Attanoos RL, Beasley MB. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142(1):89–108. doi: 10.5858/arpa.2017-0124-RA. [DOI] [PubMed] [Google Scholar]
- Bruno R, Ali G, Poma AM, Proietti A, Libener R, Mariani N. Differential Diagnosis of Malignant Pleural Mesothelioma on Cytology: A Gene Expression Panel versus BRCA1-Associated Protein 1 and p16 Tests. J Mol Diagn. 2020;22(4):457–466. doi: 10.1016/j.jmoldx.2019.12.009. [DOI] [PubMed] [Google Scholar]
- Whitaker D. The cytology of malignant mesothelioma. Cytopathology. 2000;11(3):139–151. doi: 10.1046/j.1365-2303.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui MT, Schmitt F, Churg A. Proceedings of the American Society of Cytopathology companion session at the 2019 United States and Canadian Academy of Pathology Annual meeting, part 2: effusion cytology with focus on theranostics and diagnosis of malignant mesothelioma. J Am Soc Cytopathol. 2019;8(6):352–361. doi: 10.1016/j.jasc.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Michael CW, King JAC, Hester RB. Confocal laser scanning microscopy and three dimensional reconstruction of cell clusters in serous fluids. Diagn Cytopathol. 1997;17:272–279. doi: 10.1002/(sici)1097-0339(199710)17:4<272::aid-dc7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cakir E, Demirag F, Aydin M, Unsal E. Cytopathologic differential diagnosis of malignant mesothelioma, adenocarcinoma and reactive mesothelial cells: a logistic regression analysis. Diagn Cytopathol. 2009;37(1):4–10. doi: 10.1002/dc.20938. [DOI] [PubMed] [Google Scholar]
- Roboz J, Greaves J, Silides D, Chahinjan AP, Holland JF. Hyaluronic acid content of effusions as a diagnostic aid for malignant mesothelioma. Cancer Res. 1985;45(4):1850–1854. [PubMed] [Google Scholar]
- Kaur K, Patel T, Samanta S, Patra S, Trivedi P. Role of Cytology in the Current Guidelines for Malignant Mesothelioma: Largest Study from India. Acta Cytol. 2020;14:1–11. doi: 10.1159/000512011. [DOI] [PubMed] [Google Scholar]
- Paintal A, Raparia K, Nayar R. Cytomorphologic findings of malignant mesothelioma in FNA biopsies and touch preps of core biopsies. Diagn Cytopathol. 2016;44(1):14–19. doi: 10.1002/dc.23337. [DOI] [PubMed] [Google Scholar]
- Segal A, Sterrett GF, Frost FA, Shilkin KB, Olsen NJ, Musk A. A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: results of a 20-year audit. Pathology. 2013;45(1):44–48. doi: 10.1097/PAT.0b013e32835bc848. [DOI] [PubMed] [Google Scholar]
- Yahya ZM, Ali HH, Hussein HG. Evaluation of the sensitivity and specificity of immunohistochemical markers in the differential diagnosis of effusion cytology. Oman Med J. 2013;28(6):410–416. doi: 10.5001/omj.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondola S, Manners D, Nowak AK. Malignant pleural mesothelioma: an update on diagnosis and treatment options. Ther Adv Respir Dis. 2016;10(3):275–288. doi: 10.1177/1753465816628800. [DOI] [PMC free article] [PubMed] [Google Scholar]


