Abstract
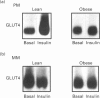
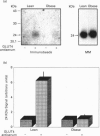
Cardiac ventricular tissue of lean and genetically obese (fa/fa) Zucker rats was used to study the expression, subcellular distribution and insulin-induced recruitment of the glucose transporter GLUT4 and to elucidate possible molecular alterations of the translocation process. Hearts were removed from basal and insulin-treated (20 min) lean and obese Zucker rats, and processed for subcellular fractionation and Western blotting of proteins. In obese rats, the total GLUT4 content in a crude membrane fraction was reduced to 75 +/- 8% (P = 0.019) of lean controls. In contrast, GLUT4 abundance in plasma membranes was not significantly different between lean and obese rats with a concomitant decrease (47 +/- 3%) in the microsomal fraction of obese animals. In plasma membranes of lean animals insulin was found to increase the GLUT4 abundance to 294 +/- 43% of control with a significantly (P = 0.009) reduced effect in the obese group (139 +/- 10% of control). In these animals insulin failed to recruit GLUT4 from the microsomal fraction, whereas the hormone induced a significant decrease (41 +/- 4%) of microsomal GLUT4 in lean controls. In GLUT4-enriched membrane vesicles, obtained from cardiac microsomes of lean rats, a 24 kDa GTP-binding protein could be detected, whereas no significant labelling of this species was observed in GLUT4 vesicles prepared from obese animals. In addition to the translocation of GLUT4, insulin was found to promote the movement of the small GTP-binding protein rab4A from the cytosol (decrease to 61 +/- 13% of control) to the plasma membrane (increase to 177 +/- 19% of control) in lean rats with no effect of the hormone on rab4A redistribution in the obese group. In conclusion, cardiac glucose uptake of insulin-resistant obese Zucker rats is subject to multiple cellular abnormalities involving a reduced expression, altered redistribution and defective recruitment of GLUT4. We show here an association of the latter defect with alterations at the level of small GTP-binding proteins possibly leading to an impaired trafficking of GLUT4 in the insulin-resistant state.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini G., Hohman R., Charron M. J., Lodish H. F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991 Mar 5;266(7):4037–4040. [PubMed] [Google Scholar]
- Banks E. A., Brozinick J. T., Jr, Yaspelkis B. B., 3rd, Kang H. Y., Ivy J. L. Muscle glucose transport, GLUT-4 content, and degree of exercise training in obese Zucker rats. Am J Physiol. 1992 Nov;263(5 Pt 1):E1010–E1015. doi: 10.1152/ajpendo.1992.263.5.E1015. [DOI] [PubMed] [Google Scholar]
- Bray G. A. The Zucker-fatty rat: a review. Fed Proc. 1977 Feb;36(2):148–153. [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Cormont M., Tanti J. F., Zahraoui A., Van Obberghen E., Le Marchand-Brustel Y. Rab4 is phosphorylated by the insulin-activated extracellular-signal-regulated kinase ERK1. Eur J Biochem. 1994 Feb 1;219(3):1081–1085. doi: 10.1111/j.1432-1033.1994.tb18591.x. [DOI] [PubMed] [Google Scholar]
- Cormont M., Tanti J. F., Zahraoui A., Van Obberghen E., Tavitian A., Le Marchand-Brustel Y. Insulin and okadaic acid induce Rab4 redistribution in adipocytes. J Biol Chem. 1993 Sep 15;268(26):19491–19497. [PubMed] [Google Scholar]
- Czech M. P., Buxton J. M. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993 May 5;268(13):9187–9190. [PubMed] [Google Scholar]
- Eckel J., Wirdeier A., Herberg L., Reinauer H. Insulin resistance in the heart: studies on isolated cardiocytes of genetically obese Zucker rats. Endocrinology. 1985 Apr;116(4):1529–1534. doi: 10.1210/endo-116-4-1529. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Sherman W. M., Reed M. J., Elton C. W., Dohm G. L. Exercise training increases glucose transporter protein GLUT-4 in skeletal muscle of obese Zucker (fa/fa) rats. FEBS Lett. 1990 Jul 30;268(1):13–16. doi: 10.1016/0014-5793(90)80960-q. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., de Venté J. E., Peterson R. G., Dohm G. L. Altered expression of muscle glucose transporter GLUT-4 in diabetic fatty Zucker rats (ZDF/Drt-fa). Am J Physiol. 1991 Dec;261(6 Pt 1):E782–E788. doi: 10.1152/ajpendo.1991.261.6.E782. [DOI] [PubMed] [Google Scholar]
- Galante P., Maerker E., Scholz R., Rett K., Herberg L., Mosthaf L., Häring H. U. Insulin-induced translocation of GLUT 4 in skeletal muscle of insulin-resistant Zucker rats. Diabetologia. 1994 Jan;37(1):3–9. doi: 10.1007/BF00428770. [DOI] [PubMed] [Google Scholar]
- Goud B., McCaffrey M. Small GTP-binding proteins and their role in transport. Curr Opin Cell Biol. 1991 Aug;3(4):626–633. doi: 10.1016/0955-0674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Handberg A., Kayser L., Høyer P. E., Voldstedlund M., Hansen H. P., Vinten J. Metformin ameliorates diabetes but does not normalize the decreased GLUT 4 content in skeletal muscle of obese (fa/fa) Zucker rats. Diabetologia. 1993 Jun;36(6):481–486. doi: 10.1007/BF02743261. [DOI] [PubMed] [Google Scholar]
- Henriksen E. J., Bourey R. E., Rodnick K. J., Koranyi L., Permutt M. A., Holloszy J. O. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990 Oct;259(4 Pt 1):E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- Henriksen E. J., Rodnick K. J., Mondon C. E., James D. E., Holloszy J. O. Effect of denervation or unweighting on GLUT-4 protein in rat soleus muscle. J Appl Physiol (1985) 1991 May;70(5):2322–2327. doi: 10.1152/jappl.1991.70.5.2322. [DOI] [PubMed] [Google Scholar]
- Kandror K. V., Pilch P. F. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror K., Pilch P. F. Identification and isolation of glycoproteins that translocate to the cell surface from GLUT4-enriched vesicles in an insulin-dependent fashion. J Biol Chem. 1994 Jan 7;269(1):138–142. [PubMed] [Google Scholar]
- Kemmer F. W., Berger M., Herberg L., Gries F. A., Wirdeier A., Becker K. Glucose metabolism in perfused skeletal muscle. Demonstration of insulin resistance in the obese Zucker rat. Biochem J. 1979 Mar 15;178(3):733–741. doi: 10.1042/bj1780733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M., Wells J. A., Stephens J. M., Elton C. W., Friedman J. E., Tapscott E. B., Pekala P. H., Dohm G. L. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J. 1990 Sep 1;270(2):397–400. doi: 10.1042/bj2700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. A., Horton E. D., Hirshman M. F., Horton E. S. Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J Clin Invest. 1992 Oct;90(4):1568–1575. doi: 10.1172/JCI116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P. J., Cartee G. D., Gulve E. A., Holloszy J. O. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys Res Commun. 1990 Oct 30;172(2):728–736. doi: 10.1016/0006-291x(90)90735-6. [DOI] [PubMed] [Google Scholar]
- Kolter T., Uphues I., Wichelhaus A., Reinauer H., Eckel J. Contraction-induced translocation of the glucose transporter Glut4 in isolated ventricular cardiomyocytes. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1207–1214. doi: 10.1016/0006-291x(92)92333-s. [DOI] [PubMed] [Google Scholar]
- Koranyi L., James D., Mueckler M., Permutt M. A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest. 1990 Mar;85(3):962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Li G., Stahl P. D. Post-translational processing and membrane association of the two early endosome-associated rab GTP-binding proteins (rab4 and rab5). Arch Biochem Biophys. 1993 Aug 1;304(2):471–478. doi: 10.1006/abbi.1993.1377. [DOI] [PubMed] [Google Scholar]
- Ricort J. M., Tanti J. F., Cormont M., Van Obberghen E., Le Marchand-Brustel Y. Parallel changes in Glut 4 and Rab4 movements in two insulin-resistant states. FEBS Lett. 1994 Jun 20;347(1):42–44. doi: 10.1016/0014-5793(94)00510-9. [DOI] [PubMed] [Google Scholar]
- Russ M., Wichelhaus A., Uphues I., Kolter T., Eckel J. Photoaffinity labelling of cardiac membrane GTP-binding proteins in response to insulin. Eur J Biochem. 1994 Jan 15;219(1-2):325–330. doi: 10.1111/j.1432-1033.1994.tb19944.x. [DOI] [PubMed] [Google Scholar]
- Satoh S., Nishimura H., Clark A. E., Kozka I. J., Vannucci S. J., Simpson I. A., Quon M. J., Cushman S. W., Holman G. D. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993 Aug 25;268(24):17820–17829. [PubMed] [Google Scholar]
- Sherman W. M., Katz A. L., Cutler C. L., Withers R. T., Ivy J. L. Glucose transport: locus of muscle insulin resistance in obese Zucker rats. Am J Physiol. 1988 Sep;255(3 Pt 1):E374–E382. doi: 10.1152/ajpendo.1988.255.3.E374. [DOI] [PubMed] [Google Scholar]
- Slieker L. J., Roberts E. F., Shaw W. N., Johnson W. T. Effect of streptozocin-induced diabetes on insulin-receptor tyrosine kinase activity in obese Zucker rats. Diabetes. 1990 May;39(5):619–625. doi: 10.2337/diab.39.5.619. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., James D. E., Lienhard G. E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strout H. V., Vicario P. P., Biswas C., Saperstein R., Brady E. J., Pilch P. F., Berger J. Vanadate treatment of streptozotocin diabetic rats restores expression of the insulin-responsive glucose transporter in skeletal muscle. Endocrinology. 1990 May;126(5):2728–2732. doi: 10.1210/endo-126-5-2728. [DOI] [PubMed] [Google Scholar]
- Thoidis G., Kotliar N., Pilch P. F. Immunological analysis of GLUT4-enriched vesicles. Identification of novel proteins regulated by insulin and diabetes. J Biol Chem. 1993 Jun 5;268(16):11691–11696. [PubMed] [Google Scholar]
- Uphues I., Kolter T., Goud B., Eckel J. Insulin-induced translocation of the glucose transporter GLUT4 in cardiac muscle: studies on the role of small-molecular-mass GTP-binding proteins. Biochem J. 1994 Jul 1;301(Pt 1):177–182. doi: 10.1042/bj3010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B., Mühlbacher C., Carrascosa J., Obermaier-Kusser B., Seffer E., Mushack J., Pongratz D., Häring H. U. Subcellular distribution of GLUT 4 in the skeletal muscle of lean type 2 (non-insulin-dependent) diabetic patients in the basal state. Diabetologia. 1992 May;35(5):456–463. doi: 10.1007/BF02342444. [DOI] [PubMed] [Google Scholar]
- Zaninetti D., Crettaz M., Jeanrenaud B. Dysregulation of glucose transport in hearts of genetically obese (fa/fa) rats. Diabetologia. 1983 Dec;25(6):525–529. doi: 10.1007/BF00284464. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Huber L. A., Mâle P., Goud B., Mellman I. Reversible phosphorylation--dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992 Dec;11(12):4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]