Abstract
Hydralazine is an antihypertensive drug that elicits andti-nuclear antibodies in patients as an adverse effect. We investigated the ability of hydralazine to promote/stabilize the triplex DNA form of poly(dA).2poly(dT). Under conditions of low ionic strength, the polynucleotide melted as a double helix with a melting temperature (Tm) of 55.3 degrees C. Hydralazine destabilized this duplex form by reducing its Tm to 52.5 degrees C. Spermidine (2.5 microM), a natural polyamine, provoked the triplex form of poly(dA)-.2poly(dT) with two melting transitions, Tm1 of 42.8 degrees C corresponding to triplex-->duplex+single-stranded DNA and Tm2 of 65.4 degrees C, corresponding to duplex melting. Triplex DNA thus formed in the presence of spermidine was further stabilized by hydralazine (250 microM) with a Tm1 of 53.6 degrees C. A similar stabilization effect of hydralazine was found on triplex DNA formed in the presence of 5 mM Mg2+. CD spectra revealed conformational perturbations of DNA in the presence of spermidine and hydralazine. These results support the hypothesis that hydralazine is capable of stabilizing unusual forms of DNA. In contrast with the weak immunogenicity of DNA in its right-handed B-DNA conformation, these unusual forms are immunogenic and have the potential to elicit anti-DNA antibodies. To test this possibility, we analysed sera from a panel of 25 hydralazine-treated patients for anti-(triplex DNA) antibodies using an ELISA. Our results showed that 72% of sera from hydralazine-treated patients contained antibodies reacting toward the triplex DNA. In contrast, there was no significant binding of normal human sera to triplex DNA. Taken together our data indicate that hydralazine and related drugs might exert their action by interacting with DNA and stabilizing higher-order structures such as the triplex DNA.
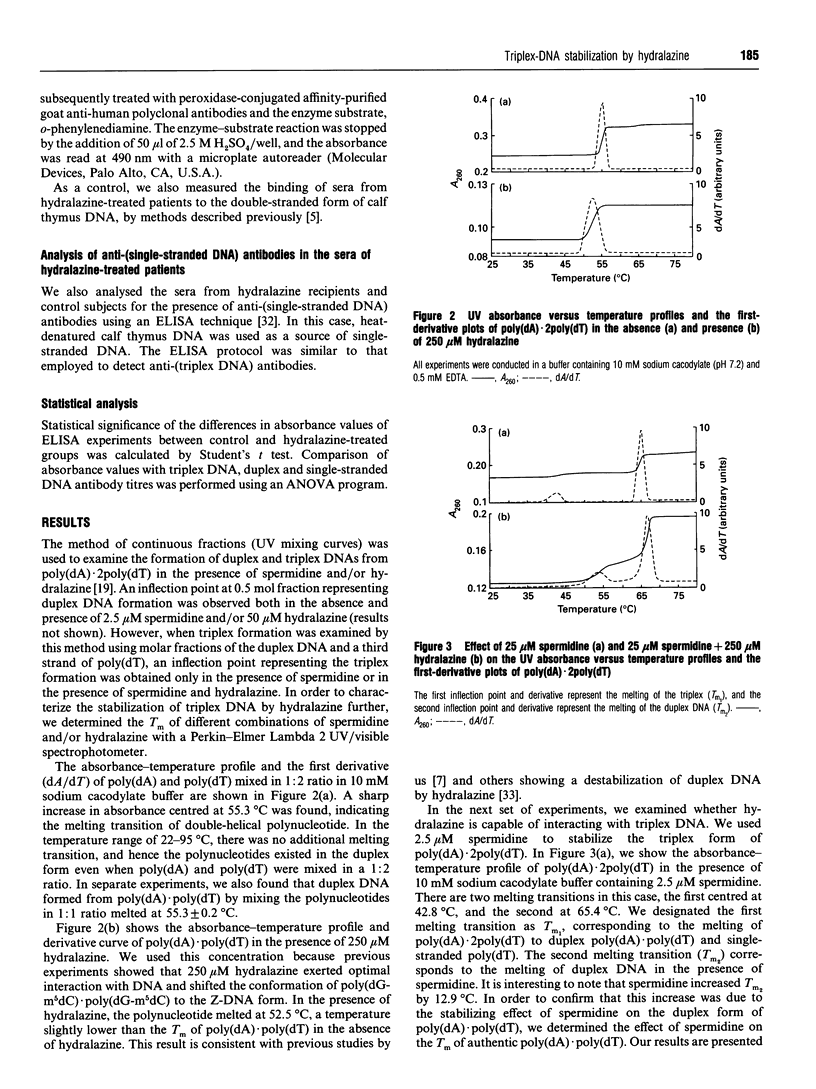
Full text
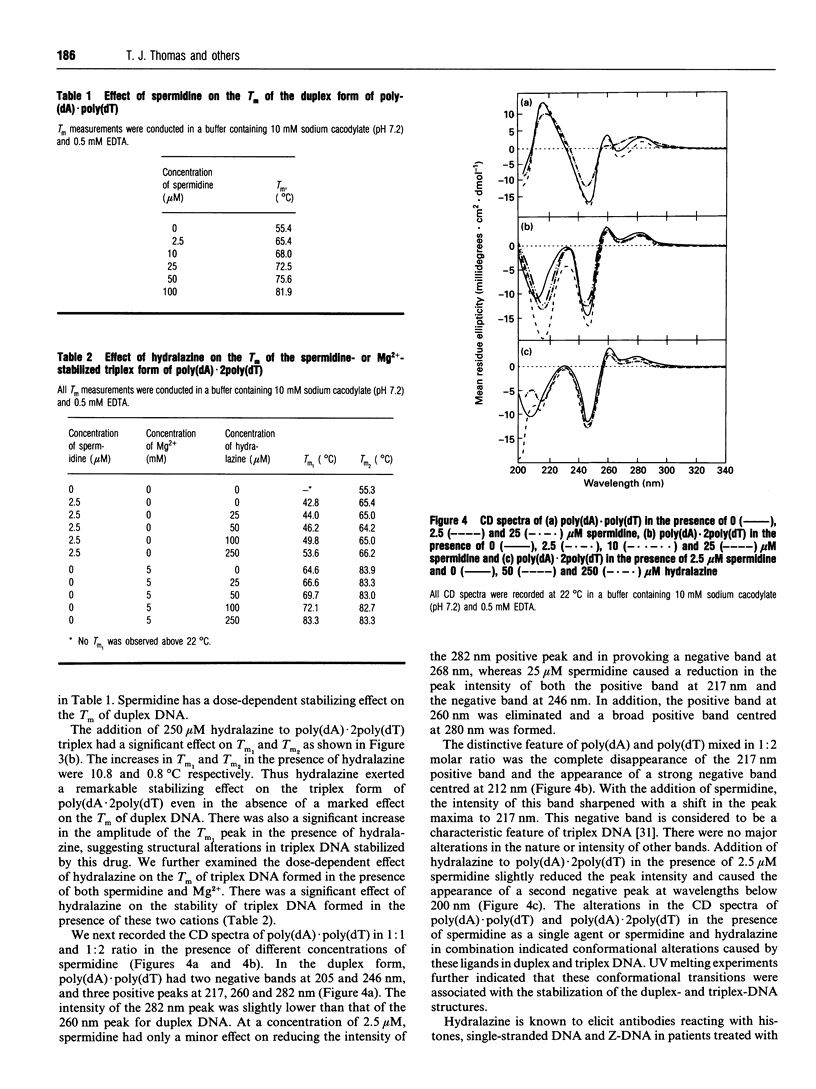
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. E., Hess E. V. Drug-related lupus. Incidence, mechanisms and clinical implications. Drug Saf. 1991 Nov-Dec;6(6):431–449. doi: 10.2165/00002018-199106060-00004. [DOI] [PubMed] [Google Scholar]
- Blume S. W., Gee J. E., Shrestha K., Miller D. M. Triple helix formation by purine-rich oligonucleotides targeted to the human dihydrofolate reductase promoter. Nucleic Acids Res. 1992 Apr 11;20(7):1777–1784. doi: 10.1093/nar/20.7.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder G. D., Latimer L. J., Lee J. S. Immunofluorescent staining of mammalian nuclei and chromosomes with a monoclonal antibody to triplex DNA. Chromosoma. 1988 Nov;97(3):185–192. doi: 10.1007/BF00292959. [DOI] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L. Drug-induced anti-histone autoantibodies display two patterns of reactivity with substructures of chromatin. J Clin Invest. 1991 Aug;88(2):680–690. doi: 10.1172/JCI115353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Breslauer K. J., Hogan M. E., Kessler D. J., Austin R. H., Ojemann J., Passner J. M., Wiles N. C. Physical studies of DNA premelting equilibria in duplexes with and without homo dA.dT tracts: correlations with DNA bending. Biochemistry. 1990 Jul 3;29(26):6161–6171. doi: 10.1021/bi00478a008. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Morris D. R., Coffino P. Sequestered end products and enzyme regulation: the case of ornithine decarboxylase. Microbiol Rev. 1992 Jun;56(2):280–290. doi: 10.1128/mr.56.2.280-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubroff L. M., Reid R. J., Jr Hydralazine-pyrimidine interactions may explain hydralazine-induced lupus erythematosus. Science. 1980 Apr 25;208(4442):404–406. doi: 10.1126/science.7367866. [DOI] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- EVANS D. A., WHITE T. A. HUMAN ACETYLATION POLYMORPHISM. J Lab Clin Med. 1964 Mar;63:394–403. [PubMed] [Google Scholar]
- Egli M., Williams L. D., Gao Q., Rich A. Structure of the pure-spermine form of Z-DNA (magnesium free) at 1-A resolution. Biochemistry. 1991 Dec 3;30(48):11388–11402. doi: 10.1021/bi00112a005. [DOI] [PubMed] [Google Scholar]
- Eldredge N. T., van Robertson W. B., Jiller J. J., 3rd The interaction of lupus-inducing drugs with deoxyribonucleic acid. Clin Immunol Immunopathol. 1974 Nov;3(2):262–271. doi: 10.1016/0090-1229(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Flescher E., Bowlin T. L., Ballester A., Houk R., Talal N. Increased polyamines may downregulate interleukin 2 production in rheumatoid arthritis. J Clin Invest. 1989 Apr;83(4):1356–1362. doi: 10.1172/JCI114023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. E., Blume S., Snyder R. C., Ray R., Miller D. M. Triplex formation prevents Sp1 binding to the dihydrofolate reductase promoter. J Biol Chem. 1992 Jun 5;267(16):11163–11167. [PubMed] [Google Scholar]
- Gunnia U. B., Thomas T., Thomas T. J. The effects of polyamines on the immunogenicity of polynucleotides. Immunol Invest. 1991 Jul;20(4):337–350. doi: 10.3109/08820139109057760. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel K. J., Ashley C., Lee J. S. Kilobase-range communication between polypurine.polypyrimidine tracts in linear plasmids mediated by triplex formation: a braided knot between two linear duplexes. Biochemistry. 1994 May 17;33(19):5674–5681. doi: 10.1021/bi00185a002. [DOI] [PubMed] [Google Scholar]
- Hampel K. J., Crosson P., Lee J. S. Polyamines favor DNA triplex formation at neutral pH. Biochemistry. 1991 May 7;30(18):4455–4459. doi: 10.1021/bi00232a012. [DOI] [PubMed] [Google Scholar]
- Hsu H. C., Seibold J. R., Thomas T. J. Regulation of ornithine decarboxylase in the kidney of autoimmune mice with the lpr gene. Autoimmunity. 1994;19(4):253–264. doi: 10.3109/08916939409071351. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Kessler D. J., Murphy M., Jayaraman K., Zendegui J. G., Hogan M. E., O'Malley B. W., Tsai M. J. In vivo transcription of a progesterone-responsive gene is specifically inhibited by a triplex-forming oligonucleotide. Nucleic Acids Res. 1993 Jun 25;21(12):2789–2796. doi: 10.1093/nar/21.12.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Burkholder G. D., Latimer L. J., Haug B. L., Braun R. P. A monoclonal antibody to triplex DNA binds to eucaryotic chromosomes. Nucleic Acids Res. 1987 Feb 11;15(3):1047–1061. doi: 10.1093/nar/15.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin A., Adams L. E., Zimmer H., Foad B., Loggie J. H., Hess E. V. Prospective study of immunologic effects of hydralazine in hypertensive patients. Clin Pharmacol Ther. 1981 Apr;29(4):447–456. doi: 10.1038/clpt.1981.62. [DOI] [PubMed] [Google Scholar]
- Mergny J. L., Duval-Valentin G., Nguyen C. H., Perrouault L., Faucon B., Rougée M., Montenay-Garestier T., Bisagni E., Hélène C. Triple helix-specific ligands. Science. 1992 Jun 19;256(5064):1681–1684. doi: 10.1126/science.256.5064.1681. [DOI] [PubMed] [Google Scholar]
- Mongey A. B., Donovan-Brand R., Thomas T. J., Adams L. E., Hess E. V. Serologic evaluation of patients receiving procainamide. Arthritis Rheum. 1992 Feb;35(2):219–223. doi: 10.1002/art.1780350216. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S., Brushaber V. M., Anderson C. F., Record M. T., Jr Relative affinities of divalent polyamines and of their N-methylated analogues for helical DNA determined by 23Na NMR. Biochemistry. 1991 Jul 30;30(30):7550–7559. doi: 10.1021/bi00244a026. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Reidenberg M. M. Aromatic amines and the pathogenesis of lupus erythematosus. Am J Med. 1983 Dec;75(6):1037–1042. doi: 10.1016/0002-9343(83)90885-9. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Bell S. A., Burlingame R. W. Autoantibodies associated with lupus induced by diverse drugs target a similar epitope in the (H2A-H2B)-DNA complex. J Clin Invest. 1992 Jul;90(1):165–173. doi: 10.1172/JCI115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaria P. V., Shafer R. H. Binding of ethidium bromide to a DNA triple helix. Evidence for intercalation. J Biol Chem. 1991 Mar 25;266(9):5417–5423. [PubMed] [Google Scholar]
- Sinha B. K., Patterson M. A. Free radical metabolism of hydralazine. Binding and degradation of nucleic acids. Biochem Pharmacol. 1983 Nov 15;32(22):3279–3284. doi: 10.1016/0006-2952(83)90351-9. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Ionic and structural effects on the thermal helix-coil transition of DNA complexed with natural and synthetic polyamines. Biopolymers. 1984 Jul;23(7):1295–1306. doi: 10.1002/bip.360230713. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Meryhew N. L., Messner R. P. DNA sequence and conformation specificity of lupus autoantibodies. Preferential binding to the left-handed Z-DNA form of synthetic polynucleotides. Arthritis Rheum. 1988 Mar;31(3):367–377. doi: 10.1002/art.1780310308. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Effects of lupus-inducing drugs on the B to Z transition of synthetic DNA. Arthritis Rheum. 1986 May;29(5):638–645. doi: 10.1002/art.1780290508. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Structural specificity of polyamines in left-handed Z-DNA formation. Immunological and spectroscopic studies. J Mol Biol. 1988 May 20;201(2):463–467. doi: 10.1016/0022-2836(88)90155-6. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Seibold J. R., Adams L. E., Hess E. V. Hydralazine induces Z-DNA conformation in a polynucleotide and elicits anti(Z-DNA) antibodies in treated patients. Biochem J. 1993 Sep 1;294(Pt 2):419–425. doi: 10.1042/bj2940419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Thomas T. J. Selectivity of polyamines in triplex DNA stabilization. Biochemistry. 1993 Dec 21;32(50):14068–14074. doi: 10.1021/bi00213a041. [DOI] [PubMed] [Google Scholar]
- Tripathi J., Brahmachari S. K. Distribution of simple repetitive (TG/CA)n and (CT/AG)n sequences in human and rodent genomes. J Biomol Struct Dyn. 1991 Oct;9(2):387–397. doi: 10.1080/07391102.1991.10507919. [DOI] [PubMed] [Google Scholar]
- Van Helden P. D. Potential Z-DNA-forming elements in serum DNA from human systemic lupus erythematosus. J Immunol. 1985 Jan;134(1):177–179. [PubMed] [Google Scholar]


