Abstract
PURPOSE
Cabozantinib and nivolumab (CaboNivo) alone or with ipilimumab (CaboNivoIpi) have shown promising efficacy and safety in patients with metastatic urothelial carcinoma (mUC), metastatic renal cell carcinoma (mRCC), and rare genitourinary (GU) tumors in a dose-escalation phase I study. We report the final data analysis of the safety, overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) of the phase I patients and seven expansion cohorts.
METHODS
This is an investigator-initiated, multicenter, phase I trial. CaboNivo doublet expansion cohorts included (1) mUC, (2) mRCC, and (3) adenocarcinoma of the bladder/urachal; CaboNivoIpi triplet expansion cohorts included (1) mUC, (2) mRCC, (3) penile cancer, and (4) squamous cell carcinoma of the bladder and other rare GU tumors (ClinicalTrials.gov identifier: NCT02496208).
RESULTS
The study enrolled 120 patients treated with CaboNivo (n = 64) or CaboNivoIpi (n = 56), with a median follow-up of 49.2 months. In 108 evaluable patients (CaboNivo n = 59; CaboNivoIpi n = 49), the ORR was 38% (complete response rate 11%) and the median duration of response was 20 months. The ORR was 42.4% for mUC, 62.5% for mRCC (n = 16), 85.7% for squamous cell carcinoma of the bladder (n = 7), 44.4% for penile cancer (n = 9), and 50.0% for renal medullary carcinoma (n = 2). Grade ≥ 3 treatment-related adverse events occurred in 84% of CaboNivo patients and 80% of CaboNivoIpi patients.
CONCLUSION
CaboNivo and CaboNivoIpi demonstrated clinical activity and safety in patients with multiple GU malignancies, especially clear cell RCC, urothelial carcinoma, and rare GU tumors such as squamous cell carcinoma of the bladder, small cell carcinoma of the bladder, adenocarcinoma of the bladder, renal medullary carcinoma, and penile cancer.
INTRODUCTION
Approximately 465,000 new cases of genitourinary (GU) tumors were diagnosed in 2023 in the United States.1 Immunotherapy is now part of the standard of care for patients with metastatic urothelial carcinoma (mUC)2-5 and renal cell carcinoma (RCC),3 and is being clinically tested in several other GU tumors.4 However, most patients will eventually relapse or not respond to these treatments. In addition, some rare GU tumors lack standard treatment options.5 For these reasons, ongoing research is needed to develop effective treatments for these rare tumors.
CONTEXT
Key Objective
We aimed to evaluate the safety and clinical activity of cabozantinib in combination with nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in a larger number of patients with metastatic urothelial carcinoma and several expansion cohorts of rare genitourinary (GU) tumors.
Knowledge Generated
We report an overall response rate of 38%, with notable efficacy in rare tumor types, such as penile carcinoma, bladder adenocarcinoma, and squamous cell bladder cancer, that lack treatment options. Our immune cell subset correlates show differential immunomodulation between CaboNivo and CaboNivoIpi. This trial has prompted several larger trials in multiple GU tumors, including a larger phase II study (ICONIC) in rare GU tumors.
Relevance (M.A. Carducci)
This clinical summary updates and confirms the clinical activity of this novel combination approach for rare GU cancers. The prior JCO report laid the foundation for these regimens and with cohort expansion provides options for late stage clinical testing and potential use in rare GU cancers such as penile cancer, urachal, and squamous cell cancer of the urinary bladder.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FASCO, FACP.
Combining checkpoint inhibitors (CPIs) with other agents that could potentially alter the tumor microenvironment (TME) or act synergistically to enhance response to immunotherapy is now a common strategy against many types of cancer.6 Single-agent cabozantinib has clinical activity in platinum-refractory mUC,7 and we reported safety and early efficacy of cabozantinib and nivolumab (CaboNivo) and cabozantinib, nivolumab, and ipilimumab (CaboNivoIpi) in the dose-escalation portion of this phase I study.8 We also reported the efficacy of CaboNivo in a small cohort of patients with urothelial carcinoma (UC) previously treated and refractory to CPI.9 On the basis of the early efficacy signal of this study, several larger studies have been initiated in RCC,10 UC,11 and rare GU tumors12 using these or similar combinations. The CheckMate-9ER phase III study10,13,14 of first-line CaboNivo versus sunitinib in clear cell RCC (ccRCC) was developed on the basis of the safety and efficacy outcomes from this study, and the combination is now a standard of care for this disease. The Alliance PEDIGREE phase III study11 uses an innovatively adapted design to treat patients with ccRCC in the first-line setting. The COSMIC-313 study15 tests the triple combination of CaboNivoIpi versus NivoIpi in the first-line treatment of ccRCC. Another trial developed on the basis of the preliminary efficacy seen in this phase I study is the phase II Alliance ICONIC study of CaboNivoIpi, which includes 12 cohorts of rare GU tumors.14
Here, we present the results of seven expansion cohorts of patients with several GU tumors pooled with the dose-escalation outcomes for the phase I study of CaboNivo and CaboNivoIpi. We also report preplanned correlative analyses of peripheral immune subsets, providing novel insights into the immunologic response to single versus dual immune CPI paired with cabozantinib.
METHODS
Methods regarding patient selection/eligibility, study design, treatments, outcomes, statistical analysis, and correlative studies (peripheral immune subsets and cytokine/angiogenesis markers) are presented in Appendix 1 (online only) and Appendix Figure A1A.
RESULTS
Patient Characteristics
Patients (N = 120) were enrolled between July 2015 and July 2020. Fifty-four patients were enrolled in the phase I portion of the trial and 66 in the dose-expansion cohorts. At data cutoff (June 15, 2021), median follow-up was 49.2 months. The study design, baseline characteristics of all patients, and distribution of patients in the expansion cohorts are described in Appendix Figures A1A, A1B, and Table 1. Baseline characteristics for all enrolled mUC participants are described in Appendix Table A1.
TABLE 1.
Patient Characteristics
| Characteristic | All (N = 120) |
|---|---|
| Median age, years (range) | 59 (20-82) |
| Sex, No. (%) | |
| Male | 97 (80.8) |
| Female | 23 (19.2) |
| Race, No. (%) | |
| White | 103 (85.8) |
| Black or African American | 11 (9.2) |
| Asian | 5 (4.2) |
| Other | 1 (0.8) |
| Karnofsky performance status, %, No. (%) | |
| 100 | 15 (12.5) |
| 90 | 63 (52.5) |
| 80 | 32 (26.7) |
| 70 | 10 (8.3) |
| Smoking history, No. (%) | |
| Current | 10 (8.3) |
| Previous | 61 (50.9) |
| Never | 49 (40.8) |
| Cohorts, No. (%) | |
| Phase I | 54 (45) |
| Urothelial carcinoma | 24 (20) |
| RCC | 12 (10) |
| Adenocarcinoma of bladder | 10 (8.3) |
| Rare GU tumors treated | 12 (10) |
| Penile carcinoma treated | 8 (6.7) |
| Type of tumor, No. (%) | |
| Urothelial carcinoma | 39 (32.5) |
| Clear cell RCCa | 16 (13.3) |
| Adenocarcinoma bladder/urachal | 15 (12.5) |
| Penile carcinoma | 11 (9.2) |
| Prostate adenocarcinoma | 10 (8.3) |
| Squamous cell carcinoma bladder | 8 (6.7) |
| Germ cell tumor | 6 (5.0) |
| Small cell carcinoma of bladder/renal pelvis | 4 (3.3) |
| Renal medullary carcinoma | 3 (2.5) |
| Testicular primitive neuroectodermal tumor | 2 (1.7) |
| Trophoblastic tumor | 1 (0.83) |
| Sertoli cell tumor | 1 (0.83) |
| Collecting duct carcinoma | 1 (0.83) |
| Chromophobe RCC | 1 (0.83) |
| Papillary RCC | 1 (0.83) |
| Sarcomatoid bladder | 1 (0.83) |
| Metastasis location, No. (%) | |
| Lymph node only | 15 (12.5) |
| Bone | 19 (15.8) |
| Visceral disease | 80 (66.7) |
| Liver | 37 (30.8) |
| Lung | 54 (45.0) |
| Median number of previous treatments (range) | 1 (0-8) |
| No. of previous treatments, No. (%) | |
| 0 | 21 (17.5) |
| 1 | 54 (45.0) |
| 2 | 22 (18.3) |
| ≥3 | 23 (19.2) |
Abbreviations: CaboNivo, cabozantinib + nivolumab; CaboNivoIpi, cabozantinib + nivolumab + ipilimumab; GU, genitourinary; RCC, renal cell carcinoma.
Three patients with clear cell RCC had >50% sarcomatoid features.
Of the 120 patients, 108 were evaluable for response. Of the 12 patients who were not evaluable for response, seven had early disease progression, one withdrew from the study, two interrupted treatment because of toxicity before completing the first cycle, one refused treatment, and one had not reached the first restaging scan at the time of data cutoff.
Efficacy
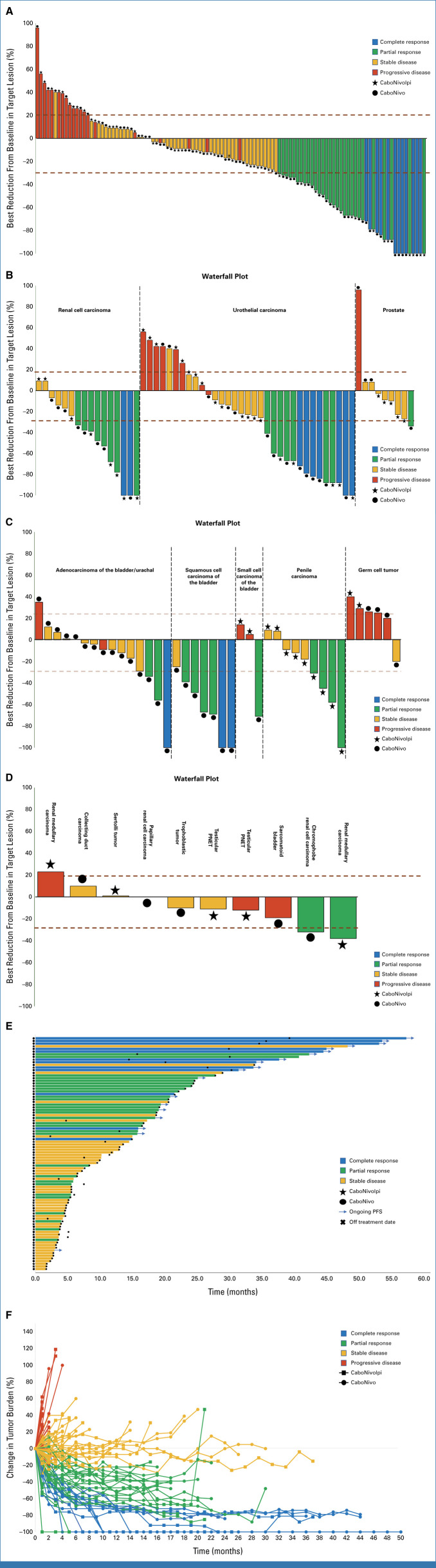
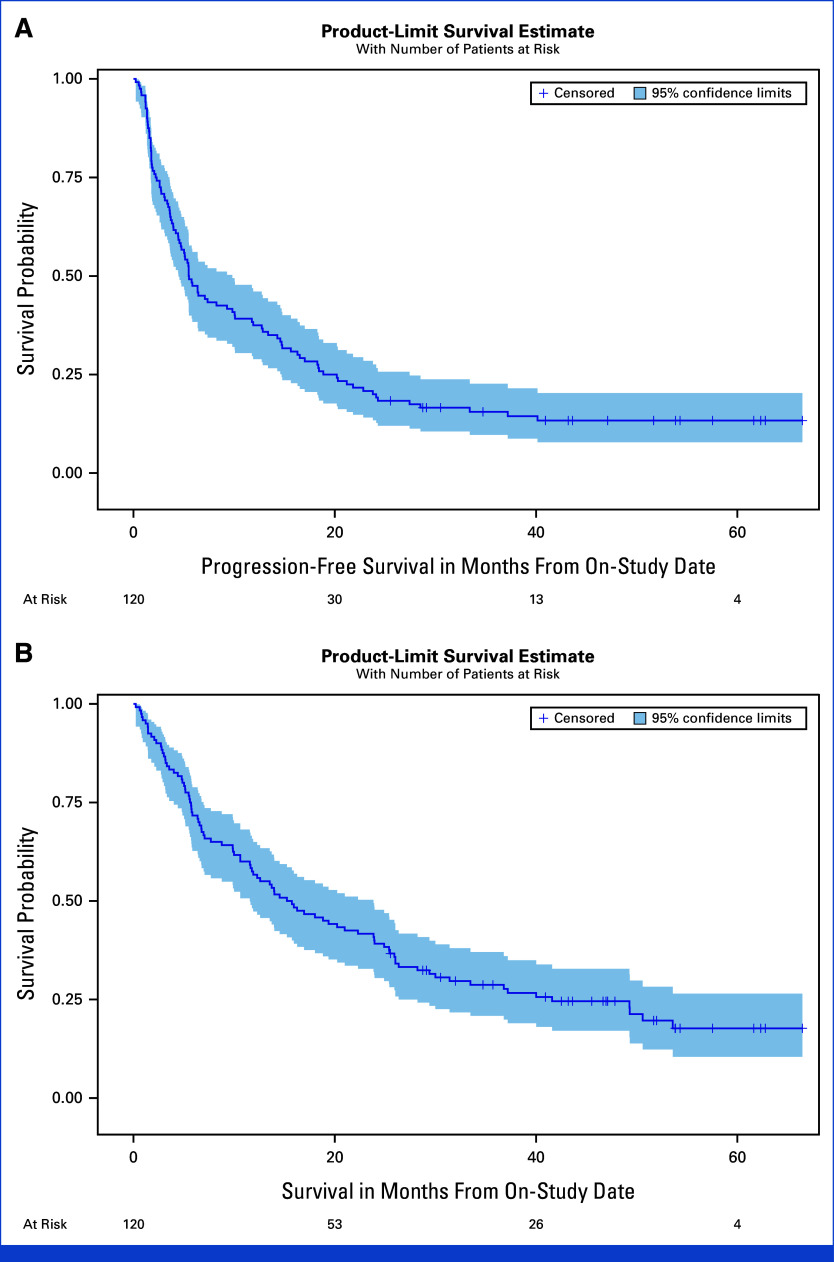
The overall response rate (ORR) for the 108 evaluable patients was 38.0% (95% CI, 28.8 to 47.8); 12 patients (11.1%) achieved a complete response (CR). The disease control rate (DCR; CR + partial response [PR] + stable disease [SD]) was 81.5% (88/108 patients; 95% CI, 72.9 to 88.3; Table 2, Figs 1A, 1E, 1F). At data cutoff, 110 patients had completed the treatment and 10 were still being treated. The median duration of treatment for the 110 patients who completed the treatment was 4.73 months (range: 0.97-38.63 months). Among the 41 patients who achieved a CR or PR, the median time to response was 2.14 months (range: 1.18-31.11 months). Median overall survival (OS) for the entire study population was 15.5 months (95% CI, 11.6 to 23.9; Table 2, Fig 2). Among the 41 responder patients (patients who achieved a CR or PR as best response), the median OS from date of response was 51.8 months (95% CI, 27.1 months to not estimable; Appendix Fig A2A). The median duration of response (DoR) for responder patients was 20.2 months (95% CI, 14.4 to not estimable; Appendix Fig A2B). The median progression-free survival (PFS) for the intention-to-treat (ITT) population (N = 120) was 5.5 months (95% CI, 4.5 to 9.8 months), and the 12-month PFS probability was 37.5% (95% CI, 28.9 to 46.1; Fig 2A, Table 2).
TABLE 2.
Clinical Activity by Treatment and Tumor Type
| Treatment/Tumor Type | No.a | CR, No. (%) (95% CI) |
PR, No. (%) (95% CI) |
SD, No. (%) (95% CI) |
PD, No. (%) (95% CI) |
ORR CR+ PR, No. (%) (95% CI) |
CR + PR + SD Disease Control, No. (%) (95% CI) |
No. | Median OS (months) (95% CI) |
Median PFS (months) (95% CI) |
12-Month OS (%) (95% CI) |
Median DoR (months) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 108 | 12 (11.1) (5.9 to 18.6) |
29 (26.9) (18.8 to 36.2) |
47 (43.5) (34.0 to 53.4) |
20 (18.5) (11.7 to 27.1) |
41 (38.0) (28.8 to 47.8) |
88 (81.5) (72.9 to 88.3) |
120 | 15.5 (11.6 to 23.9) | 5.5 (4.5 to 9.8) | 56.7 (47.3 to 65.0) | 20.2 (14.4 to NE) |
| CaboNivo | 59 | 9 (15.3) (7.2 to 27.0) |
17 (28.8) (17.8 to 42.1) |
24 (40.7) (28.1 to 54.3) |
9 (15.3) (7.2 to 27.0) |
26 (44.1) (31.2 to 57.0) |
50 (84.7) (73.0 to 92.8) |
64 | 24.4 (14.0 to 36.8) | 11.0 (5.5 to 18.4) | 68.8 (55.9 to 78.6) | 25.8 (14.7 to NE) |
| CaboNivoIpi | 49 | 3 (6.1) (1.3 to 16.9) |
12 (24.5) (13.3 to 38.9) |
23 (46.9) (32.5 to 61.7) |
11 (22.5) (11.8 to 36.6) |
15 (30.6) (18.3 to 45.4) |
38 (77.6) (63.4 to 88.2) |
56 | 9.3 (5.8 to 15.9) | 4.5 (3.1 to 5.8) | 42.9 (29.8 to 55.3) | 14.5 (3.6 to 21.6) |
| Cabozantinib 40 mg daily/Nivo ± Ipib | 91 | 10 (11.0) (5.4 to 19.3) |
25 (27.5) (18.6 to 37.8) |
38 (41.8) (31.5 to 52.6) |
18 (19.8) (12.2 to 29.5) |
35 (38.5) (28.5 to 49.3) |
73 (80.2) (70.6 to 87.8) |
102 | 15.8 (9.9 to 23.9) | 5.5 (3.9 to 10.1) | 57.8 (47.7 to 66.7) | 20.2 (14.5 to NE) |
| Cabozantinib 60 mg daily/Nivo ± Ipic | 17 | 2 (11.8) (1.5 to 36.4) |
4 (23.5) (6.8 to 49.9) |
9 (52.9) (27.8 to 77.0) |
2 (11.8) (1.5 to 36.4) |
6 (35.3) (14.2 to 61.7) |
15 (88.2) (63.6 to 98.5) |
18 | 12.9 (10.0 to 26.0) | 6.7 (4.7 to 12.8) | 50.0 (25.9 to 70.1) | 14.8 (2.4 to NE) |
| Tumor type | ||||||||||||
| Urothelial carcinoma | 33 | 7 (21.2) (9.0 to 38.9) |
7 (21.2) (9.0 to 38.9) |
12 (36.4) (20.4 to 54.9) |
7 (21.2) (9.0 to 38.9) |
14 (42.4) (25.5 to 60.8) |
26 (78.8) (61.1 to 91.0) |
39 | 24.9 (11.8 to 41.6) | 10.1 (2.2 to 21.2) | 66.7 (49.6 to 79.1) | 28.0 (11.5 to NE) |
| Clear cell RCC | 16 | 2 (12.5) (1.6 to 38.4) |
8 (50.0) (24.7 to 75.4) |
6 (37.5) (15.2 to 64.6) |
0 | 10 (62.5) (35.4 to 84.8) |
16 (100) (79.4 to 100) |
16 | 43.6 (19.4 to NE) | 16.3 (6.4 to 21.8) | 81.3 (52.5 to 93.5) | 16.7 (3.6 to NE) |
| Penile carcinoma | 9 | 0 | 4 (44.4) (13.7 to 78.8) |
5 (55.6) (21.2 to 86.3) |
0 | 4 (44.4) (13.7 to 78.8) |
9 (100) (66.4 to 100) |
11 | 6.7 (5.2 to 23.9) | 4.8 (3.1 to 6.4) | 36.4 (11.2 to 62.7) | 9.2 (2.4 to NE) |
| Prostate adenocarcinoma | 9 | 0 | 1 (11.1) (0.3 to 48.3) |
7 (77.8) (40.0 to 97.2) |
1 (11.1) (0.3 to 48.3) |
1 (11.1) (0.3 to 48.3) |
8 (88.9) (51.8 to 99.7) |
10 | 11.1 (6.6 to 17.0) | 4.5 (1.6 to 5.1) | 40.0 (12.3 to 67.0) | 3.7 |
| Adenocarcinoma bladder | 15 | 1 (6.7) (0.2 to 32.0) |
2 (13.3) (1.7 to 40.5) |
9 (60.0) (32.3 to 83.7) |
3 (20.0) (4.3 to 48.1) |
3 (20.0) (4.3 to 48.1) |
12 (80) (51.9 to 95.7) |
15 | 18.0 (5.8 to 28.2) | 10.1 (1.8 to 16.5) | 80.0 (50.0 to 93.1) | 14.7 (11.0 to NE) |
| Squamous cell carcinoma bladder | 7 | 2 (28.6) (3.7 to 71.0) |
4 (57.1) (18.4 to 90.1) |
1 (14.3) (0.4 to 57.9) |
0 | 6 (85.7) (42.1 to 99.6) |
7 (100) (59.0 to 100) |
8 | 22.6 (1.4 to NE) | 16.5 (1.4 to NE) | 62.5 (22.9 to 86.1) | 25.8 (1.6 to NE) |
| Germ cell tumor | 6 | 0 | 0 | 1 (16.7) (0.4 to 64.1) |
5 (83.3) (35.9 to 99.6) |
0 | 1 (16.7) (0.4 to 64.1) |
|||||
| Small cell carcinoma of bladder/renal pelvis | 3 | 0 | 1 (33.3) (0.8 to 90.6) |
0 | 2 (66.7) (9.4 to 99.2) |
1 (33.3) (0.8 to 90.6) |
1 (33.3) (0.8 to 90.6) |
|||||
| Renal medullary carcinoma | 2 | 0 | 1 (50.0) | 0 | 1 (50.0) | 1 (50.0) | 1 (50.0) | |||||
| Testicular primitive neuroectodermal tumor | 2 | 0 | 0 | 1 (50.0) | 1 (50.0) | 0 | 1 (50.0) | |||||
| Trophoblastic tumor | 1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | |||||
| Sertoli cell tumor | 1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | |||||
| Collecting duct carcinoma | 1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | |||||
| Chromophobe RCC | 1 | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | |||||
| Papillary RCC | 1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | |||||
| Sarcomatoid bladder | 1 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) |
Abbreviations: CR, complete response; DoR, duration of response; NE, not estimable; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RCC, renal cell carcinoma; SD, stable disease.
Number evaluable for response.
Includes patients receiving varying doses of nivo +/− ipi in the dose escalation and expansion cohorts, see Appendix Fig A1B.
Includes patients receiving varying doses of nivo +/− ipi in the dose escalation cohorts, see Appendix Fig A1B.
FIG 1.
Antitumor activity and response by measurable disease. (A) Waterfall plot of change in target lesions from baseline. Horizontal upper and lower dashed lines represent RECIST boundaries of 20% increase and 30% decrease in size of target lesions, respectively. (B) Waterfall plots for renal cell carcinoma, urothelial carcinoma, and prostate cohorts. Tumor type for each cohort is listed for each group. (C) Waterfall plots for adenocarcinoma, squamous cell carcinoma of the bladder/urinary tract, small cell carcinoma of the bladder/urinary tract, penile carcinoma, and germ cell tumor cohorts. Tumor type for each cohort is listed for each group. (D) Waterfall plot for rare genitourinary tumor cohort. Tumor type for each individual is listed above each bar. (E) Swimmer's plot showing duration of response, treatment arm, and response. (F) Spider plot depicting response and change in target lesion size (tumor burden) from baseline for individual participants. Red bars/lines represent PD, yellow bars represent SD, green bars represent PR, and blue bars represent CR as best response. CaboNivo, cabozantinib + nivolumab; CaboNivoIpi, cabozantinib + nivolumab + ipilimumab; CR, complete response; PR, partial response; SD, stable disease.
FIG 2.
Kaplan-Meier estimates for PFS and OS for phase I and expansion cohorts ITT population (n = 120). (A) Median PFS of 5.5 months (95% CI, 4.5 to 9.8 months). (B) Median OS of 15.5 months (95% CI, 11.6 to 23.9). ITT, intention-to-treat; OS, overall survival; PFS, progression-free survival.
There was no statistically significant difference in ORR, median OS, or median PFS between patients treated with daily cabozantinib 40 mg plus Nivo (1 or 3 mg/kg every 2 weeks) alone or NivoIpi (Nivo 1 or 3 mg/kg every 3 weeks plus Ipi 1 or 3 mg/kg every 3 weeks x 4 doses; includes patients receiving varying doses of nivo and ipi in the dose escalation and expansion cohorts) versus daily cabozantinib 60 mg plus Nivo (1 or 3 mg/kg every 2 weeks) alone or NivoIpi (Nivo 1 or 3 mg/kg every 3 weeks plus Ipi 1 or 3 mg/kg every 3 weeks x 4 doses; includes patients in the dose escalation cohorts only; see Appendix Fig A1B) (n = 91 and n = 17, respectively; Table 2 and Appendix Fig A2C).
Among patients who received CaboNivo (n = 59), the ORR was 44.1% (95% CI, 31.2 to 57.0). Among patients who received CaboNivoIpi (n = 49), the ORR was 30.6% (95% CI, 18.3 to 45.4). Additional survival (mPFS and mOS) and DoR data are summarized in Table 2 and Appendix Figures A2E, A2F.
Efficacy and survival data for each tumor histology in each treatment arm (CaboNivo or CaboNivoIpi) are summarized in Table 2, Figures 2B‐2D, and Appendix Fig A2D. For patients with UC (n = 33), the ORR was 42.4% (95% CI, 25.5 to 60.8), with seven CRs (21.2%). The median DoR was 28.0 months (95% CI, 11.5 to not estimable), median OS was 24.9 months (95% CI, 11.8 to 41.6 months; Appendix Fig A2D), and median PFS was 10.1 months (95% CI, 2.2 to 21.2). For patients with ccRCC (n = 16), the ORR was 62.5% (95% CI, 35.4 to 84.8), with two CRs (12.5%). The median DoR was 16.7 months (95% CI, 3.6 to not estimable), median OS was 43.6 months (95% CI, 19.4 to not estimable), and median PFS was 16.3 months (95% CI, 6.4 to 21.8 months). Patients with squamous cell carcinoma of the bladder (n = 6) had an ORR of 85.7% (95% CI, 42.1 to 99.6), a CR rate of 28.6%, median DoR of 25.8 months (95% CI, 1.6 to not estimable), median OS of 22.6 months (95% CI, 1.4 months to not estimable), and median PFS of 16.5 months (95% CI, 1.4 months to not estimable). Patients with penile carcinoma (n = 9) had an ORR of 44.4% (95% CI, 13.7 to 78.8) and a DCR of 100% (95% CI, 66.4 to 100); for this histology, median PFS was 4.8 months (95% CI, 3.1 to 6.4 months) and median OS was 6.7 months (95% CI, 5.2 to 23.9 months). Patients with adenocarcinoma of the bladder (n = 15) had an ORR of 20% (95% CI, 4.3 to 48.1) and a DCR of 80% (95% CI, 51.9 to 95.7); for this histology, median PFS was 10.1 months (95% CI, 1.8 to 16.5 months) and median OS was 18.0 months (95% CI, 5.8 to 28.2 months).
Safety and Tolerability
Treatment-related adverse events (TRAEs) of any grade occurred in patients treated with at least one dose of CaboNivo (n = 64; 91%) and CaboNivoIpi (n = 56; 93%; Table 3). Nonimmune-related TRAEs of any grade occurred in 91% of patients receiving CaboNivo and 93% receiving CaboNivoIpi. Immune-related adverse events (irAEs) occurred in 13 patients (20%) receiving CaboNivo and 13 patients (23%) receiving CaboNivoIpi; 16% of patients receiving CaboNivo and 20% receiving CaboNivoIpi required systemic corticosteroids for irAEs (Appendix Table A2). There were no grade 5 TRAEs.
TABLE 3.
Adverse Events
| Preferred Term AE | CaboNivo (n = 64) | CaboNivoIpi (n = 56) | ||
|---|---|---|---|---|
| G1/G2, No. (%) | G3/G4, No. (%) | G1/G2, No. (%) | G3/G4, No. (%) | |
| Fatigue | 41 (64) | 8 (13) | 30 (54) | 9 (16) |
| Diarrhea | 36 (56) | 2 (3) | 27 (48) | 4 (7) |
| Anorexia | 26 (41) | 0 | 24 (43) | 1 (2) |
| Skin toxicity | 35 (55) | 1 (2) | 27 (48) | 0 |
| Dysphonia | 19 (30) | 0 | 11 (20) | 0 |
| Nausea | 34 (53) | 2 (3) | 22 (40) | 2 (3) |
| Myalgia | 17 (27) | 0 | 8 (14) | 0 |
| Mucositis | 38 (59) | 0 | 19 (34) | 1 (2) |
| Dysgeusia | 25 (39) | 0 | 13 (23) | 0 |
| Weight loss | 19 (3) | 1 (2) | 21 (38) | 0 |
| Vomiting | 16 (25) | 2 (3) | 10 (18) | 0 |
| PPE | 32 (50) | 0 | 12 (21) | 0 |
| Abdominal pain | 18 (28) | 1 (2) | 9 (16) | 1 (2) |
| Hypertension | 15 (23) | 8 (13) | 6 (11) | 6 (11) |
| Headache | 13 (20) | 0 | 3 (5) | 1 (2) |
| Cough | 9 (14) | 0 | 12 (21) | 0 |
| Muscle weakness | 1 (2) | 1 (2) | 6 (11) | 0 |
| Dehydration | 6 (9) | 4 (6) | 9 (16) | 3 (5) |
| Infection | 7 (11) | 1 (2) | 5 (9) | 0 |
| Thromboembolic event | 5 (8) | 4 (6) | 1 (2) | 3 (5) |
| Fever | 3 (5) | 0 | 5 (9) | 1 (2) |
| Dyspnea | 9 (14) | 1 (2) | 7 (13) | 0 |
| Vaginal or rectal fistula | 1 (2) | 2 (3) | 0 | 0 |
| Chronic kidney disease | 0 | 1 (2) | 3 (5) | 1 (2) |
| Dry mouth | 12 (19) | 0 | 11 (20) | 0 |
| Dizziness | 7 (11) | 0 | 6 (11) | 0 |
| Rhinitis | 5 (8) | 0 | 6 (11) | 0 |
| Arthralgia | 6 (9) | 0 | 7 (13) | 0 |
| Hematology | ||||
| White blood cell count decrease | 20 (31) | 3 (5) | 11 (20) | 0 |
| Neutrophil count decrease | 13 (20) | 7 (11) | 3 (5) | 1 (2) |
| Lymphocyte count decrease | 22 (34) | 2 (3) | 7 (13) | 5 (9) |
| Anemia | 16 (25) | 4 (6) | 20 (36) | 3 (5) |
| Platelet count decrease | 20 (31) | 2 (3) | 12 (21) | 1 (2) |
| Electrolytes | ||||
| Hypocalcemia | 17 (27) | 0 | 10 (18) | 2 (4) |
| Hyponatremia | 17 (27) | 3 (5) | 10 (18) | 4 (7) |
| Hypophosphatemia | 26 (41) | 7 (11) | 17 (30) | 8 (14) |
| Hypomagnesemia | 16 (25) | 1 (1.5) | 10 (18) | 0 |
| Hypokalemia | 12 (19) | 0 | 9 (16) | 0 |
| Renal | ||||
| Proteinuria | 12 (19) | 2 (3) | 10 (18) | 0 |
| Hepatic | ||||
| ALT elevation | 31 (48) | 3 (5) | 17 (30) | 3 (5) |
| AST elevation | 28 (44) | 4 (6) | 17 (30) | 2 (4) |
| Alkaline phosphatase elevation | 8 (13) | 2 (3) | 9 (16) | 1 (2) |
| Hypoalbuminemia | 16 (25) | 0 | 10 (18) | 0 |
| PTT prolongation | 2 (3) | 1 (2) | 3 (5) | 0 |
| Pancreatic | ||||
| Amylase elevation | 12 (19) | 4 (6) | 12 (21) | 2 (4) |
| Lipase elevation | 15 (23) | 9 (14) | 11 (20) | 11 (20) |
| Endocrine | ||||
| Hyperthyroidism | 4 (6) | 1 (2) | 9 (16) | 0 |
| Hypothyroidism | 21 (33) | 2 (3) | 13 (23) | 0 |
Abbreviations: AE, adverse event; PPE, palmar-plantar erythrodyselthesia; PTT, partial thromboplastin time.
Peripheral Immune Subset Analysis
Cabozantinib's immunomodulatory properties7 provided the rationale for combination with CPIs and for preplanned exploratory analyses on peripheral blood immune subsets.
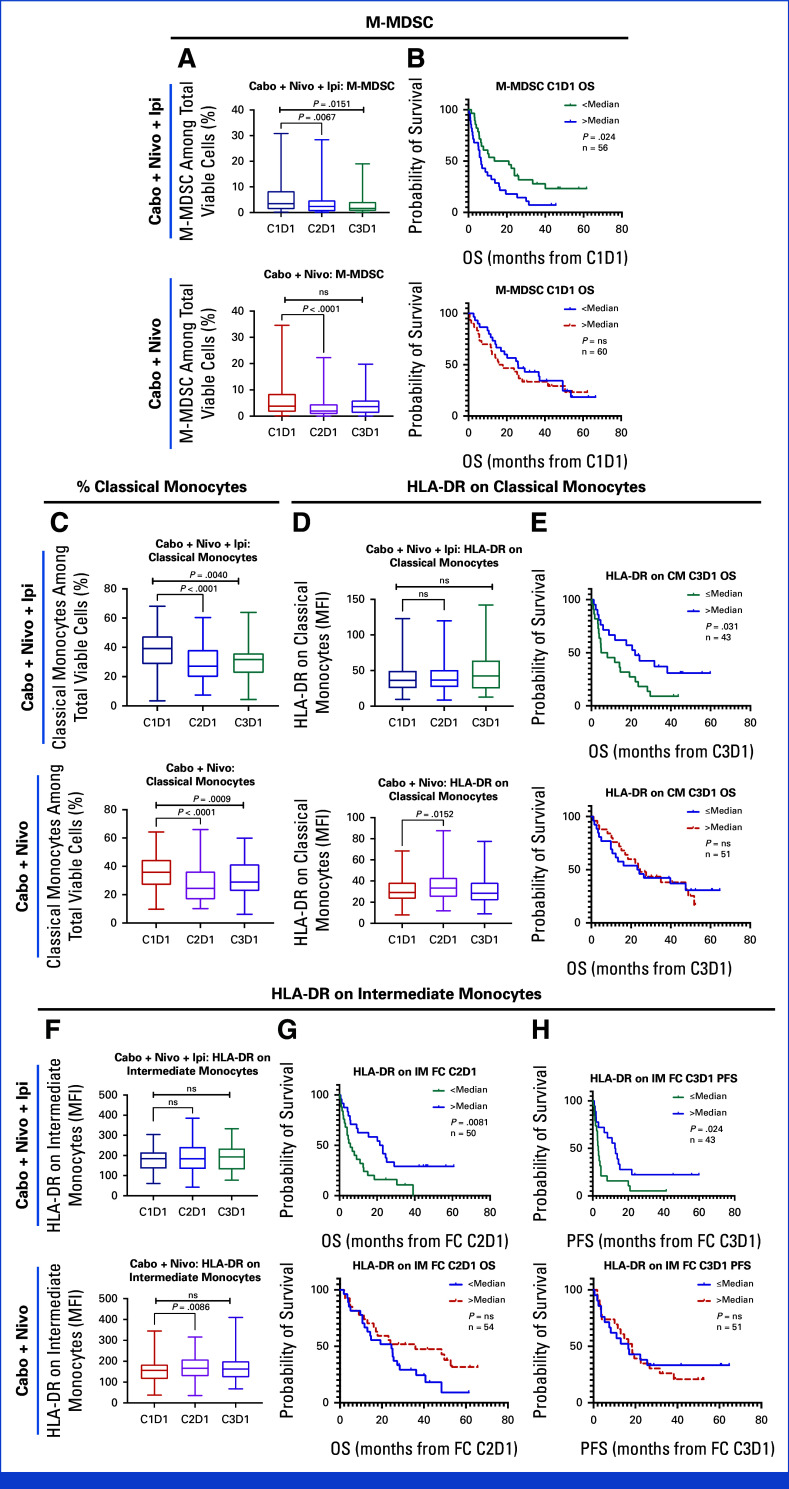
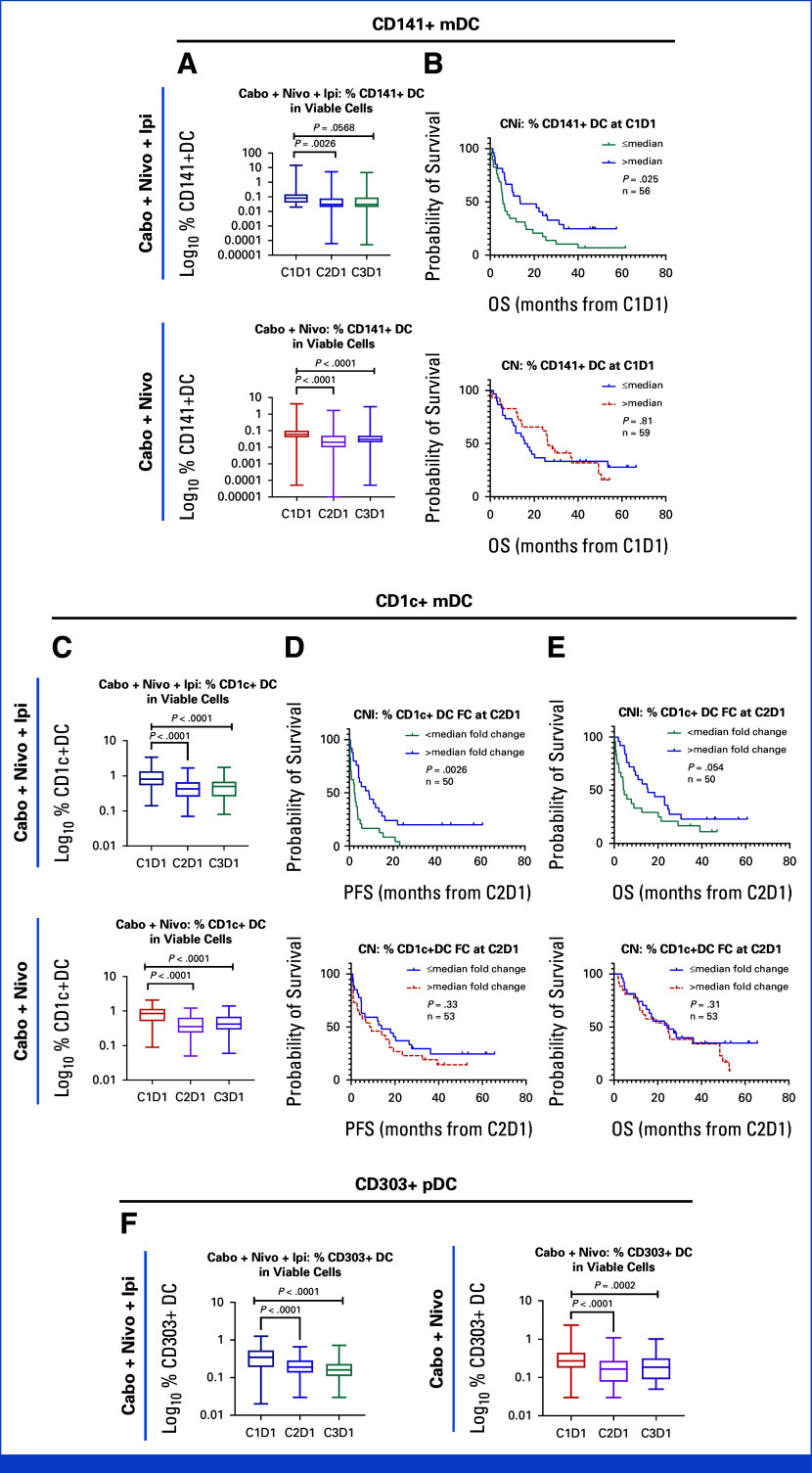
Dynamics of monocytic-myeloid-derived suppressor cells (M-MDSCs) and classical monocytes (including HLA-DR expression) are summarized in Appendix Fig A3. Lower M-MDSCs at baseline was associated with better OS in the CaboNivoIpi-treated group; however, there was no statistically significant association between baseline M-MDSCs and OS in the CaboNivo-treated group (Appendix Fig A3B). Higher HLA-DR expression on classical monocytes at C3D1 and a higher fold change of HLA-DR expression on intermediate monocytes at C2D1 or C3D1 were associated with improved PFS and OS in the CaboNivoIpi arm but not in the CaboNivo arm (Appendix Figs A3E, A3G, A3H).
Differential post-treatment changes and correlation with PFS and OS with CaboNivo and CaboNivoIpi for activated proliferative T-cell subsets (CD4+ and CD8+ T cells), dendritic cell (DC) subsets, and effector Tregs (eTregs) are shown in Appendix Figs A4-A6.
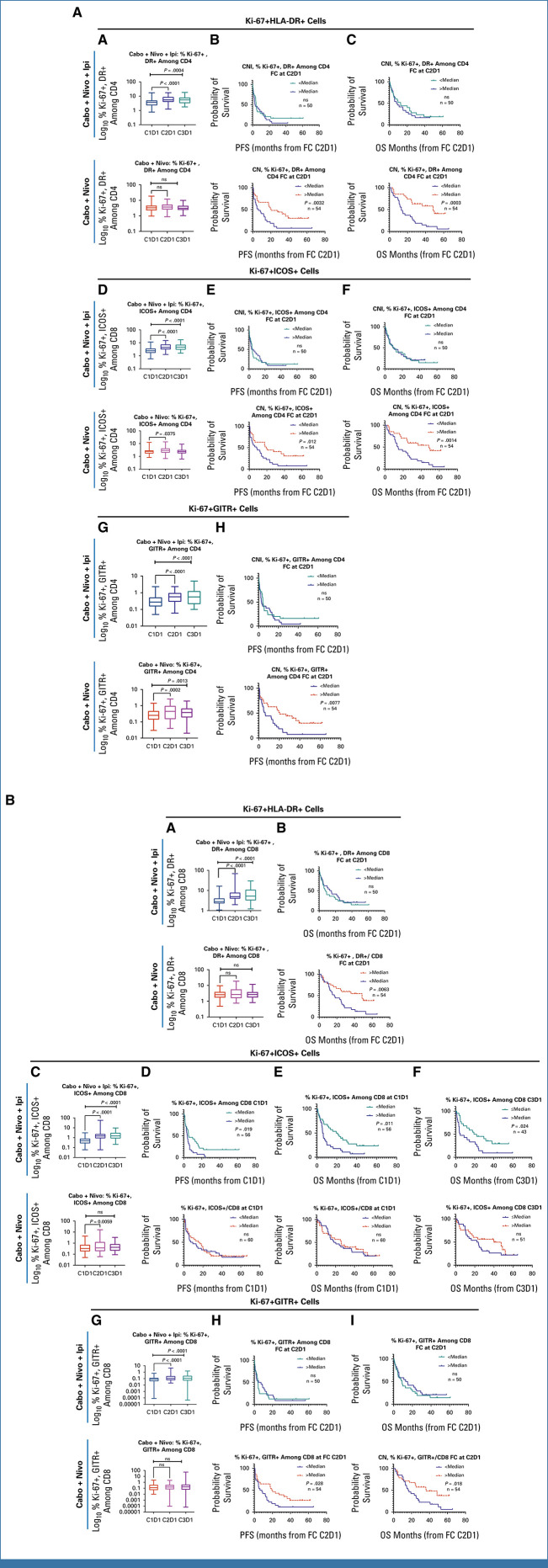
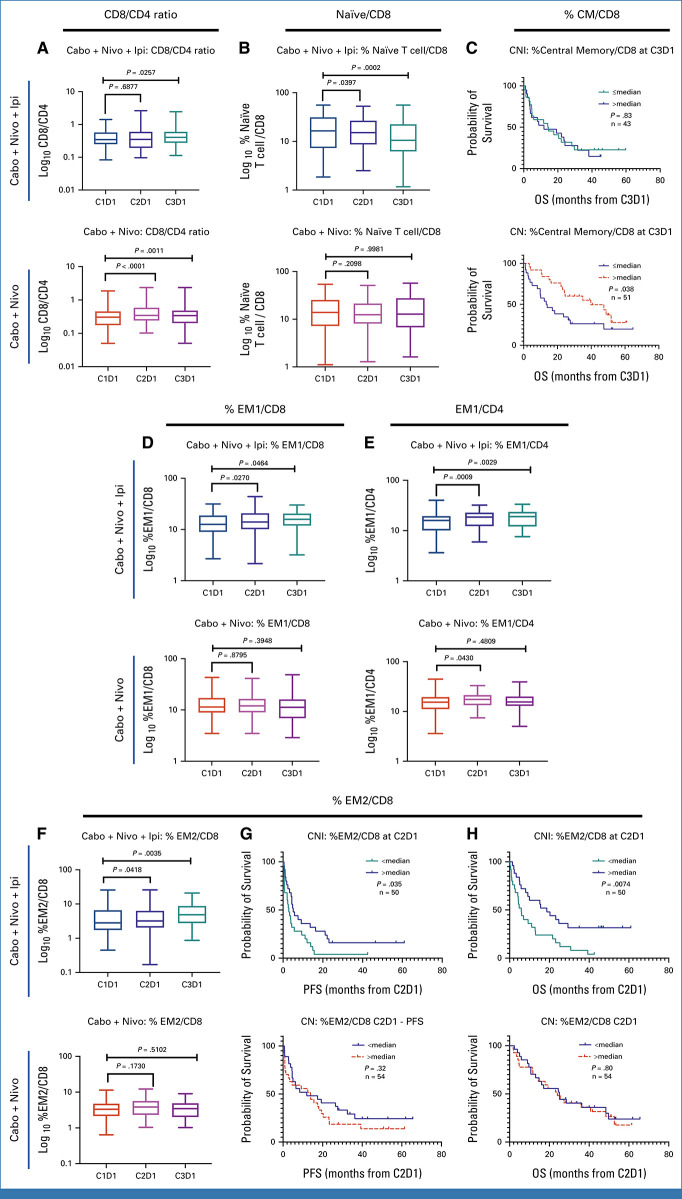
The ratio of CD8+ to CD4+ T cells increased with both CaboNivoIpi treatment and CaboNivo treatment (Appendix Fig A7A). Among CD8+ T cells, naïve cells decreased (Appendix Fig A7B). Effector memory (EM) 1 (Appendix Fig A7D) and EM2 cells increased (Appendix Fig A7F) with CaboNivoIpi treatment but not with CaboNivo treatment. A higher percentage of central memory (CM) cells among CD8+ T cells at C3D1 in the CaboNivo arm was associated with better OS (Appendix Fig A7C). A higher percentage of EM2 cells at C2D1 in the CaboNivoIpi arm was associated with improved PFS and OS (Appendix Figs A7G, A7H).
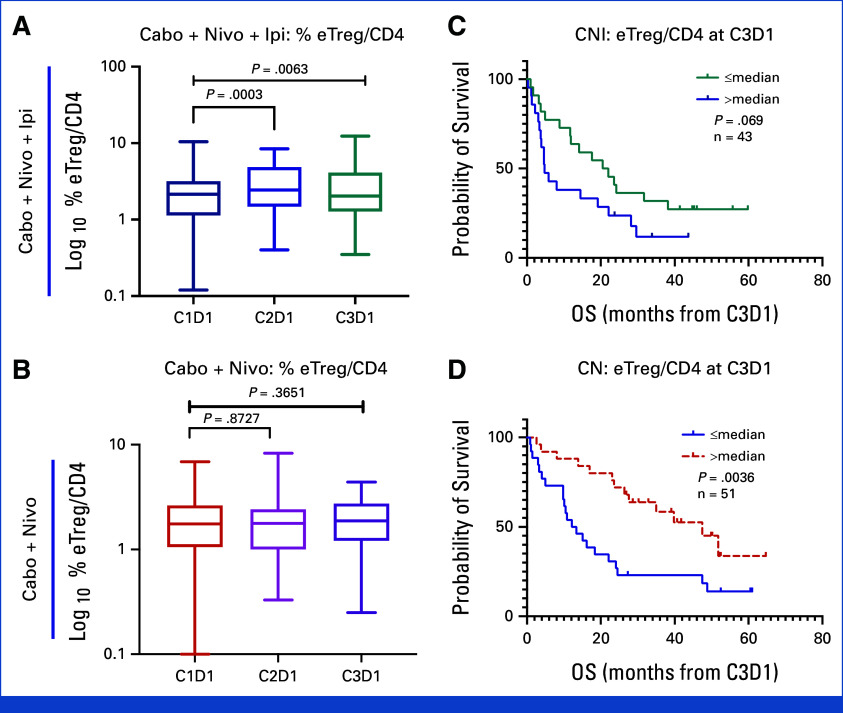
Among CD4+ T cells, the percentage of EM1 cells increased with both CaboNivoIpi treatment and CaboNivo treatment (Appendix Fig A7E), as did expression of CTLA-4 and TIM-3 on CD4+ and CD8+ T cells (Appendix Figs A8A-A8D). Higher expression of CTLA-4 on CD4+ T cells at C2D1 was associated with better OS with CaboNivo, and higher expression of CTLA-4 on CD4+ T cells at C3D1 was associated with better PFS and OS with CaboNivoIpi (Appendix Figs A8E-A8H). We have summarized the results of the immune subset analyses in Figure 3.
FIG 3.
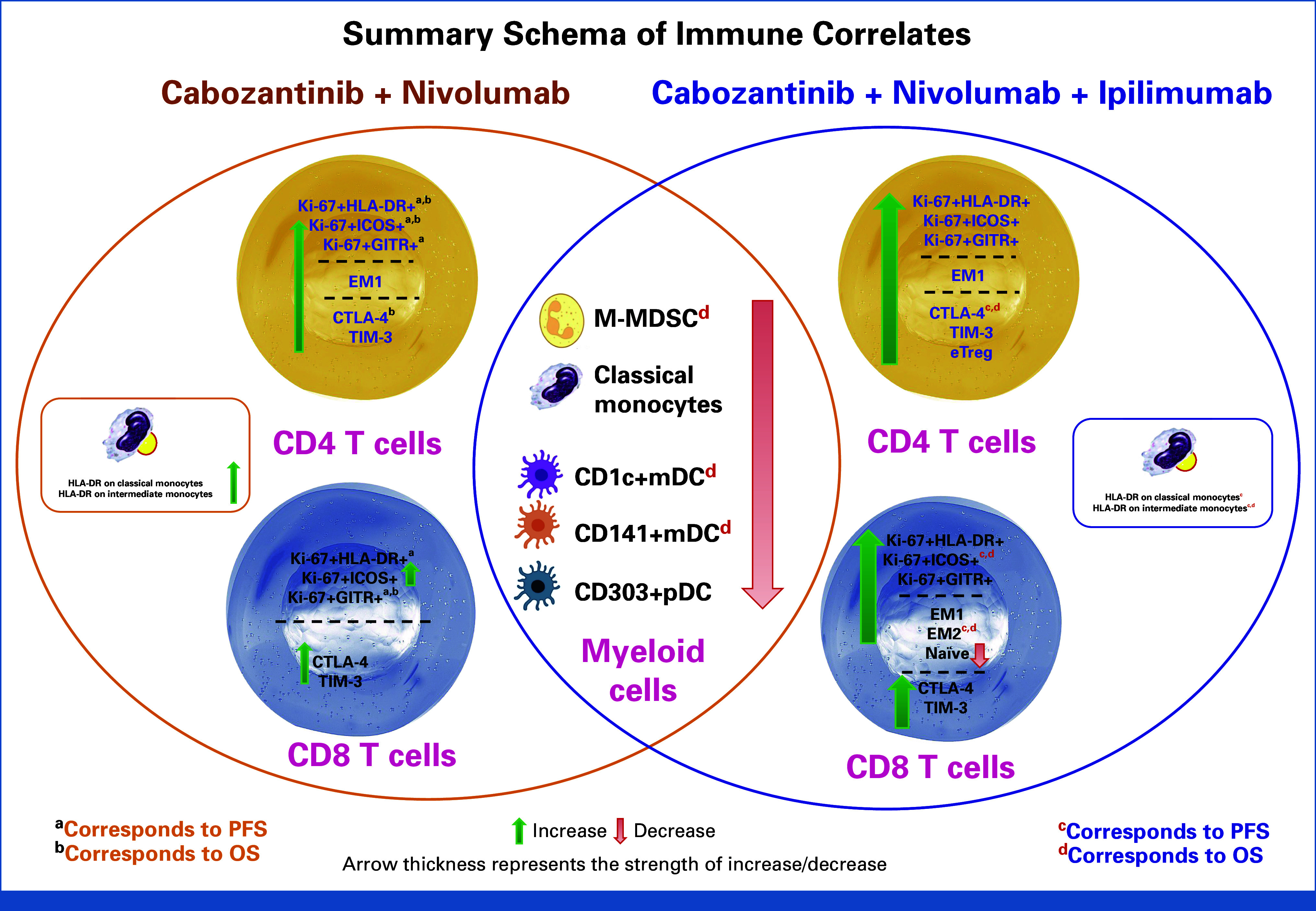
Summary schema of immune correlative results. This figure summarizes peripheral blood immune subset and functional marker changes by CaboNivo and CaboNivoIpi treatments and association with PFS and OS. CaboNivo and CaboNivoIpi treatments had common and differential impacts on immune subsets. In brief, myeloid cells, including M-MDSC, classical monocytes, and dendritic cells, decreased by both treatments, but association with OS was observed only in CaboNivoIpi treatment group. The activated (Ki67+HLA-DR+, Ki67+ICOS+, or Ki67+GITR+) T cells were robustly increased by CaboNivoIpi treatment; however, association with PFS and OS was observed mostly in the CaboNivo treatment group. The associations with PFS and OS are denoted with (a, b) in the CaboNivo group and with (c, d) in the CaboNivoIpi group. EM, effector memory cells; eTreg, effector regulatory T cells; M-MDSC, monocytic myeloid-derived suppressor cells; mDC, myeloid dendritic cells; OS, overall survival; pDC, plasmacytoid dendritic cells; PFS, progression-free survival.
Cytokine Analysis
There were 100 patients with all the time points available (37 responders [CR and PR] and 63 nonresponders [progressive disease and SD]). Plasma markers of immune activation increased at C2D1 and C3D1 (Appendix Table A3, Fig A9). Higher baseline levels of placenta growth factor (PlGF), IL-10, and TNFα were seen in nonresponders (combined CaboNivo and CaboNivoIpi treatment arms), and high baseline PIGF and TNFα were associated with lower PFS and OS. High IL-8 levels at baseline were associated with lower PFS and OS (Appendix Fig A10). IFNγ, IL-6, and vascular endothelial growth factor levels at baseline did not correlate with response or survival (Appendix Figs A9-A11).
DISCUSSION
The combinations of CaboNivo and CaboNivoIpi have significant clinical activity in patients with multiple GU malignancies, especially ccRCC (ORR, 62.5%), UC (ORR, 42.4%), and rare GU tumors such as squamous cell carcinoma of the bladder (ORR, 85.7%), small cell carcinoma of the bladder (ORR, 33.3%), adenocarcinoma of the bladder (ORR, 20.0%), renal medullary carcinoma (ORR, 50.0%), and penile cancer (ORR, 44.4%). The expansion cohorts reported here were triggered on the basis of the early, exciting ORRs found in our phase I study8 where we saw high activity in many GU tumors. Although these expansion cohorts are small and hypothesis-generating, they confirmed the efficacy seen in the phase I study in multiple GU tumors. Given that many of the tumors in the expansion cohorts are rare and lack prospective trials assessing effective therapies, this study provides clinical trial evidence of the efficacy of a tyrosine kinase inhibitor plus CPI or double CPI in rare GU tumors and warrants further study.
In this study, the activity of CaboNivo and CaboNivoIpi in platinum-refractory UC was remarkable, showing an ORR of 42% and a CR rate of 21% in the second-line setting. We have also previously reported an ORR of 16% with CaboNivo in UC refractory to CPI.9 The treatment landscape for mUC is rapidly changing with the recent US Food and Drug Administration approval of the combination of enfortumab vedotin (EV) plus pembrolizumab on the basis of the EV-302/Keynote A39 trial.16 This trial follows the earlier approval of EV in the second-line setting post-CPI and the third-line setting post-CPI and postplatinum, along with the accelerated approval of the combination of EV plus pembrolizumab for first-line treatment of cisplatin-ineligible patients with locally advanced or mUC.17 On the basis of the activity of CaboNivo in patients with UC in this study, the best setting for including the combination is in patients with platinum-refractory UC who plan to receive CPI as a monotherapy. The combination of cabozantinib plus avelumab as maintenance therapy in patients with mUC postplatinum is under investigation in the Alliance MAIN-CAV study. The rapidly evolving treatment landscape of UC has made it challenging to find the best setting to apply this concept and expand on the high activity seen with CaboNivo in UC. Trials in mUC now need to be developed post-EV plus pembrolizumab.
Although no new safety signals were observed in this study, these combinations have significant toxicity that require careful follow-up and management.18 Optimizing toxicity management can maintain tolerability, leading to longer treatment and possibly greater efficacy in patients receiving CaboNivo and CaboNivoIpi.
Limitations of this study include lack of randomization between the CaboNivo and CaboNivoIpi treatment arms; also, the small sample size limits comparison between treatments. When we evaluated the patients with UC and the patients with RCC treated with CaboNivo versus CaboNivoIpi (Appendix Figs A2E, A2F), we did not see a benefit from the addition of Ipi in terms of ORR, PFS, or OS. However, results from the COSMIC-31315 study showed significantly improved PFS with CaboNivoIpi versus NivoIpi/placebo (not reached v 11.3 months, respectively; HR, 0.73, P = .0131). OS results are pending. Given the increased adverse events (AEs) with CaboNivoIpi versus NivoIpi/placebo, wide adoption of the triplet regimen will depend on demonstrated OS improvement. However, the efficacy of CaboNivo versus CaboNivoIpi or even CaboNivo versus NivoIpi is still a question. Prospective randomized trials are needed to understand the contributions of each combination, to optimize the dosing and sequence, and to assess the CPI-refractory activity, including post-EV plus pembrolizumab.19-21 The CheckMate 9ER study initially included a triple arm (ie, CaboNivoIpi)22 that closed early after it accrued 55 participants of a planned 338. The ORR was 44% (95% CI, 30 to 58.7), with 4/55 (8%) attaining a CR. The experience of the CheckMate 9ER triplet arm exemplifies the challenges of conducting clinical trials in a rapidly changing treatment landscape.23 Remarkably, the triplet CaboNivoIpi in the CheckMate 9ER study did not perform better than the doublet CaboNivo. Although the arms were randomized, it is difficult to directly compare these arms as the arm triplet closed early in the trial.
The clinical results of this study are paired with exploratory peripheral blood immune subset data to elucidate the contribution of ipilimumab (Fig 3). These immune-subset analyses are important, given our previous published work showing cabozantinib's immunomodulatory properties, which provided the rationale for these combinations. We hoped to harness these immunomodulatory properties to enhance the antitumor response when given in combination with nivolumab alone or with nivolumab and ipilimumab. The markers were chosen to reflect highly specific subsets that could explain immunologic response or tolerance, and might help to elucidate differences in antitumor responses to CaboNivo versus CaboNivoIpi.
The M-MDSCs and classical monocytes decreased in our previous cabozantinib monotherapy7 and CPI-refractory UC patient studies.7,9 A reduction in the frequency of M-MDSCs in the periphery by CPI has been reported in other studies.24 In the CaboNivoIpi-treated patients, a higher percentage of CD141+ myeloid dendritic cells (mDCs) or a higher fold change of CD1c+ mDCs was associated with improved survival. There is a lack of evidence on the role of CPI on blood DCs, but there are several studies that show a better prognosis when DCs are abundantly infiltrated into tumor.25,26
Several studies have shown that CPI induces activated proliferative T cells in peripheral blood.27-29 Interestingly, while CaboNivo treatment moderately increased proliferative activated T-cell subsets at C2D1 but not C3D1, this increase was highly associated with better PFS and OS, while CaboNivoIpi treatment strongly increased these subsets at both C2D1 and C3D1 without association with better survival or response.
Although myeloid populations appear to be closely associated with survival in response to CaboNivoIpi, a robust increase in activated proliferating T cells did not appear to benefit patients. Overstimulation of T cells by adding ipilimumab may have masked survival outcome, compared with the moderate increase of T cells in response to CaboNivo. Previous studies investigating CPI have identified the potential role of T-cell exhaustion, particularly in the setting of dual anti–PD-(L)1/anti–CTLA-4 CPI, with suboptimal antitumor response. Overstimulation of T cells, driven in part by IFNγ signaling, can lead to loss of tumor-specific T cells and reduced memory T cells in the setting of lower tumor burden.30 A similar association was demonstrated for positive responses with the combination of optimal reinvigoration of T-cell responses and low tumor burden with single-agent CPI.27
Previous studies have demonstrated dose-related increases in toxicity with ipilimumab,31-34 leading investigators to examine whether regimens with a lower dosage and/or frequency of ipilimumab could reduce toxicity while maintaining clinical efficacy.23
A limitation of our correlative studies is the lack of tumor-tissue profiling of T cells and MDSCs in the TME, which would help determine if the observed peripheral blood immune effects relate to immune-cell trafficking to the TME or direct effects on peripheral blood immune cell phenotypes. Another limitation in the immune analysis is attributed to the different GU malignancies in the patients, indicating that a more streamlined approach is warranted. The cytokine correlative data demonstrated a general induction of cytokines and angiogenic factors after the treatment, with the changes in PlGF and IFNγ being the most prominent. Elevated baseline PlGF and TNFα are strongly associated with poor PFS and OS.
In conclusion, CaboNivo and CaboNivoIpi are active combinations in UC, RCC, and a range of rare GU tumors. Larger trials stemming from this study have confirmed both these combinations to be highly effective in ccRCC. Although treatment landscapes will continue to evolve, and there have been significant changes and more treatment options in both RCC and UC, there is still no standard of care for penile cancer, adenocarcinoma of the bladder/urachal, small cell bladder cancer, and squamous cell carcinoma of the bladder, all rare GU tumors included in the expansion cohorts and showing clinical activity with CaboNivo and CaboNivoIpi. The ongoing Alliance ICONIC study of CaboNivoIpi in rare GU tumors will test the triplet combination in a larger cohort of 12 rare GU tumors, which aims to further extend the clinical data generated in this study. Our immune correlative data suggest that the combination of two CPIs with cabozantinib likely weakens the beneficial outcome in patients with CPI-naïve GU tumor. A more nuanced approach to immunomodulation is needed to maximize therapy benefit. Ongoing research is warranted to further refine the strategy for optimal immune activation to achieve safe, effective, and durable antitumor responses.
ACKNOWLEDGMENT
The authors acknowledge the contributions from Jane Trepel, formerly of the Developmental Therapeutics Branch at the National Cancer Institute, who provided valuable insights on the immune cell subset correlatives and critical appraisal of this manuscript.
APPENDIX 1. METHODS
Patient Selection
Eligibility criteria for the phase I portion of this study have been previously published.8 Four expansion cohorts for CaboNivo, including urothelial carcinoma (UC), clear cell renal cell carcinoma (ccRCC), adenocarcinoma or urachal carcinoma of the bladder, and rare genitourinary (GU) histologies, and three expansion cohorts for CaboNivoIpi, including UC, ccRCC, and squamous cell carcinoma of the penis. Patients must have failed at least one line of standard therapy for advanced disease, except for histologies lacking an approved standard of care with survival benefit. Patients with UC who were treatment-naïve for metastatic disease were accepted only if they were ineligible for cisplatin. There was no limit to the number of previous lines of therapy for metastatic disease, and previous treatment with CPIs was not permitted. Patients were required to have at least one measurable site of disease according to RECIST v1.1.35
This multicenter study was conducted at six institutions in the United States, and the protocol was approved by institutional review boards at all participating institutions (ClinicalTrials.gov identifier: NCT02496208). Patients were enrolled according to international standards of good clinical practice and institutional safety monitoring, and all patients provided written informed consent before study entry.
Study Design
The phase I portion of this study, with dose escalation, has been published, and the recommended phase II dose (RP2D) has been defined.8 After determining the RP2D for the combinations of CaboNivo and CaboNivoIpi, seven expansion cohorts were designed to further evaluate the efficacy of these combinations on the basis of the responses seen in the phase I dose-escalation study (Appendix Fig A1B). Patients were not randomly assigned between the CaboNivo versus CaboNivoIpi treatment arms. For the dose escalation and the UC and RCC expansions, the CaboNivo cohorts were filled first, followed by enrollment in the CaboNivoIpi cohorts.
Treatment
For patients receiving CaboNivo, treatment consisted of continuous daily oral cabozantinib at a dose of 40 or 60 mg and intravenous (IV) nivolumab 3 mg/kg or 1 mg/kg every 2 weeks for a 28-day cycle. After cycle 21, nivolumab was given at a maintenance dose of 480 mg every 4 weeks with daily cabozantinib 40 or 60 mg, see Appendix Fig A1B. Restaging scans were performed every two cycles (every 8 weeks).
For patients receiving CaboNivoIpi, treatment consisted of continuous daily oral cabozantinib at a dose of 40 or 60 mg, IV nivolumab 3 mg/kg or 1 mg/kg, and IV ipilimumab 1 mg/kg or 3 mg/kg every 3 weeks for the first four cycles. After the first four cycles, ipilimumab was stopped and patients continued receiving oral cabozantinib 40 or 60 mg daily and IV nivolumab 3 mg/kg or 1 mg/kg every 14 days. After 21 cycles, nivolumab was given at a maintenance dose of 480 mg every 4 weeks along with daily cabozantinib 40 or 60 mg, see Appendix Fig A1B. Restaging was performed every two cycles (every 6 weeks during the first four cycles while on ipilimumab, then every 8 weeks thereafter).
Patients could discontinue treatment because of disease progression, unacceptable toxicity, withdrawal of consent, or investigator's clinical judgment. Patients had the option to discontinue the study therapy after 2 years of confirmed complete response (CR) or partial response (PR).
Dose reductions for daily cabozantinib (40 mg, 20 mg, then 20 mg every other day) and interruptions of study treatment were specified for management of adverse events. No dose modification was allowed for nivolumab and ipilimumab. Treatment beyond disease progression was permitted if patients tolerated treatment and the investigator considered the patient would benefit clinically.
Outcomes
For the phase I portion, the primary end point was to determine dose-limiting toxicity and the RP2D of CaboNivo and CaboNivoIpi in patients with metastatic GU tumors, primarily metastatic urothelial carcinoma. For the expansion cohorts, the primary objective was to evaluate the efficacy of these combinations in patients with metastatic GU histology measured as overall response rate (ORR), which was defined as the proportion of patients with a confirmed CR or PR as best response on the basis of investigator-assessed response per RECIST v.1.1. Secondary end points included progression-free survival (PFS), overall survival (OS), and duration of response (DoR). Safety and toxicity profiles were also secondary end points and were assessed with National Cancer Institute Common Terminology for Adverse Events v.5.0. Additional preplanned exploratory end points included peripheral blood immune subset and cytokine analysis.
Statistical Analysis
Follow-up was calculated as the median of the follow-up intervals for each patient from the on-study date until the date that data were locked (June 15, 2021). Patients were considered evaluable for response assessment if they had received at least one cycle of the study regimens. The ORR was calculated as the number of patients who achieved a CR or PR per RECIST divided by the total number of evaluable patients. The ORR was estimated along with a 95% CI, which was determined using the exact Clopper-Pearson method. The DoR was defined as the date the response was first noted until the date of radiologic progression, clinical progression, or death. Survival analyses (PFS and OS) were estimated using Kaplan-Meier methodology, with the significance of the difference between curves determined by a two-tailed log-tank test. The PFS was calculated starting at the on-study date until progression, death without previous progression, or last follow-up. The OS was calculated starting at the on-study date until death or last follow-up, as appropriate. Survival analyses were performed using SAS v.9.4 software. Safety and clinical activity of CaboNivo and CaboNivoIpi were analyzed in all patients.
Immune Subset Analysis
Peripheral immune subsets were analyzed at baseline and before C2D1 and C3D1. Peripheral blood samples were collected in Cell Preparation Tubes with sodium citrate (BD Vacutainer CPT Tubes, BD Biosciences, San Jose, CA). Peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation and viably frozen until analysis. Multiparameter flow cytometric analysis was performed on PBMCs. Cells were incubated with Fc receptor blocking agent (Miltenyi Biotec, Germany) and stained for 20-30 min at 4°C with monoclonal antibodies. For analysis of Foxp3 and Ki-67 expression, cells were fixed and permeabilized using a Fix/Perm buffer (eBioscience, San Diego, CA) according to the manufacturer's instructions, then stained with anti-Foxp3 antibody. Live cells were discriminated by means of LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies, San Diego, CA) and dead cells were excluded from all analyses. All flow cytometric analyses were performed using a MACSQuant Analyzer (Miltenyi Biotec, San Jose, CA). Flow cytometric data were quantified either as the median fluorescence intensity or as a percentage of cells, as indicated. Data were analyzed using FlowJo software version 10.6.1. (FlowJo, Ashland, OR).
The following immunophenotypic markers were used to define immune subsets:
M-MDSC: CD14+ CD11b+ HLA–DRlow/– CD15–
Classical monocytes: CD14+ CD16–
Intermediate monocytes: CD14+CD16–
non-classical monocytes: CD14dim CD16+
CD1c+ myeloid DC (mDC): lineage (CD3, CD19, CD56)–HLADR+CD11c+CD1c+
CD141+ mDC: lineage–HLA–DR+ CD11c+ CD141+
CD303+ plasmacytoid DC (pDC): lineage-HLA–DR+ CD11c+ CD303+
eTreg: CD8– CD4+CD45RA-Foxp3high
Naïve T cells: CD45RA+CCR7+ CD28+ CD27+ CD3+ (CD4+ or CD8+)
Effector T cells: CD45RA+CCR7– CD28– CD27– CD3+ (CD4+ or CD8+)
EM1 T cells: CD45RA–CCR7– CD28+ CD27+CD3+ (CD4+ or CD8+)
EM2 T cells: CD45RA–CCR7– CD28– CD27+ CD3+ (CD4+ or CD8+)
CM T cells: CD45RA–CCR7+ CD28+ CD27+ CD3+ (CD4+ or CD8+)
The following monoclonal antibodies were used:
CD14 clone HCD14, CD16 clone 3G8, HLA-DR clone LN3, CD3 clone OKT3, CD56
clone MEM-188, CD19 clone HIB19, CD11b clone ICRF44, CD15 clone W6D3,
CD33 clone WM53, CD11c clone Bu15, CD1c clone L161, CD141 clone M80,
CD303 clone 201A, CD83 clone HB15e. CD8 clone SK1, CD4 clone RPAT4,
CD25 clone BC96, Foxp3 clone 206D, PD-1 clone EH12.2H7, CTLA-4 clone
L3D10, TIM-3 clone F38-2E2 ICOS clone C398.4A, CD45RA clone
HI100, CCR7 clone G043H7, CD27 clone LG.3A10 and CD28 clone CD28.2
(BioLegend, San Diego, CA) and Ki-67 clone B56 (BD Biosciences).
Statistical Analysis
The data are represented as median with 95% confidence interval for each time point. A two-tailed Wilcoxon matched-pairs signed rank test was used for change analysis. A two-tailed unpaired Mann-Whitney test was used to compare the distributions between groups in the disease response analysis. Probabilities of PFS and OS were determined by the Kaplan-Meier method and a log-rank was used to compare curves. All statistical analysis was done using GraphPad Prism version 6.01 and the differences were considered significant when P < .05.
Cytokines
Plasma levels of inflammatory cytokines and angiogenesis markers were analyzed at baseline, C2D1, and C3D1. After collection in EDTA tubes, samples were stored at –80°C until analysis. Analysis was conducted with MSD V-PLEX technology.
FIG A1.
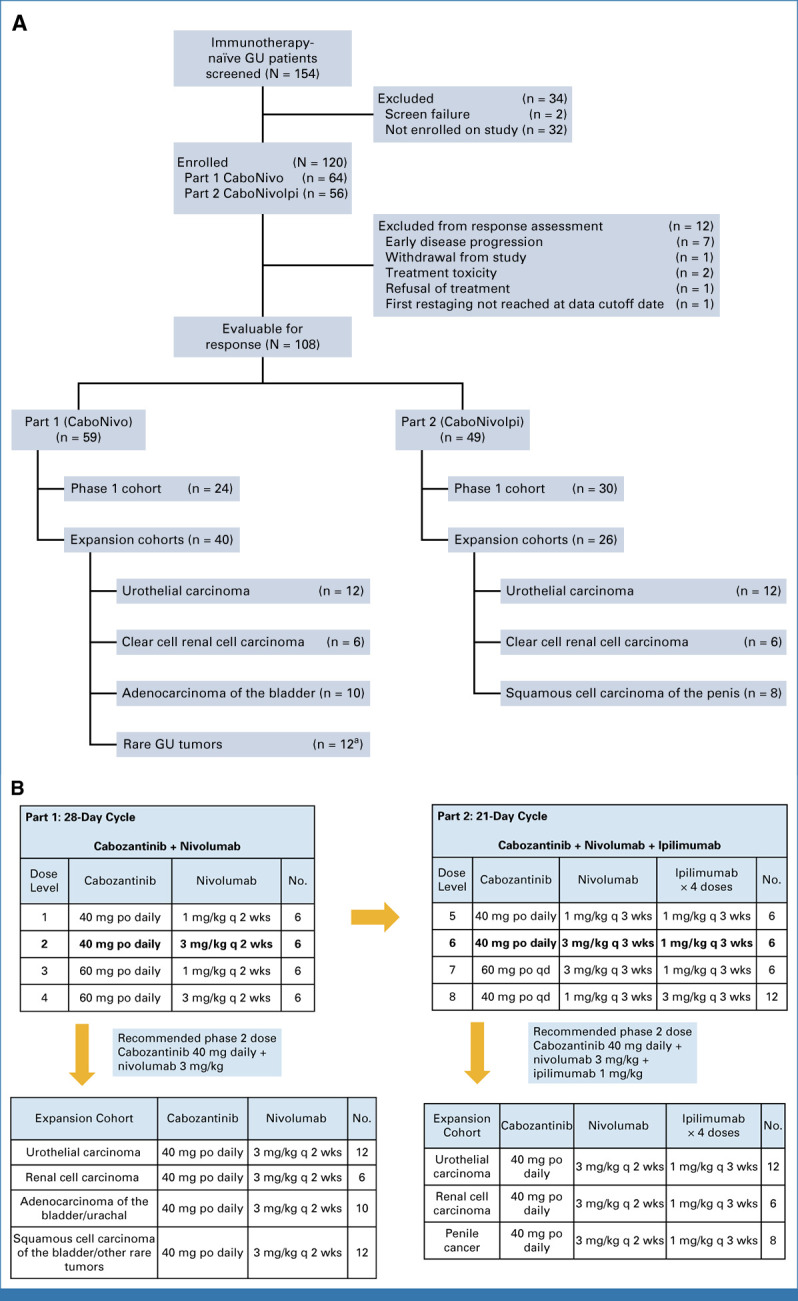
Study design and dose-escalation schematic for CaboNivo and CaboNivoIpi and respective expansion cohorts. (A) CONSORT diagram of patient flow. (B) Dose levels and corresponding doses for cabozantinib plus nivolumab (Part 1—CaboNivo) and cabozantinib, nivolumab, and ipilimumab (Part 2—CaboNivoIpi). Lower panels list the tumor histologies accrued within the expansion cohorts for CaboNivo and CaboNivoIpi. aHistologies enrolled into rare genitourinary tumors cohort included squamous cell carcinoma of the bladder (n = 5), collecting duct carcinoma of the kidney (n = 1), sarcomatoid RCC (n = 1), chromophobe RCC (n = 1), small cell carcinoma of the bladder (n = 1), papillary RCC (n = 1), bladder adenocarcinoma (n = 1), and sarcomatoid bladder (n = 1). GU, genitourinary; po, orally; q 2 wks, once every 2 weeks; q 3 wks, once every 3 weeks; qd, once daily.
FIG A2.
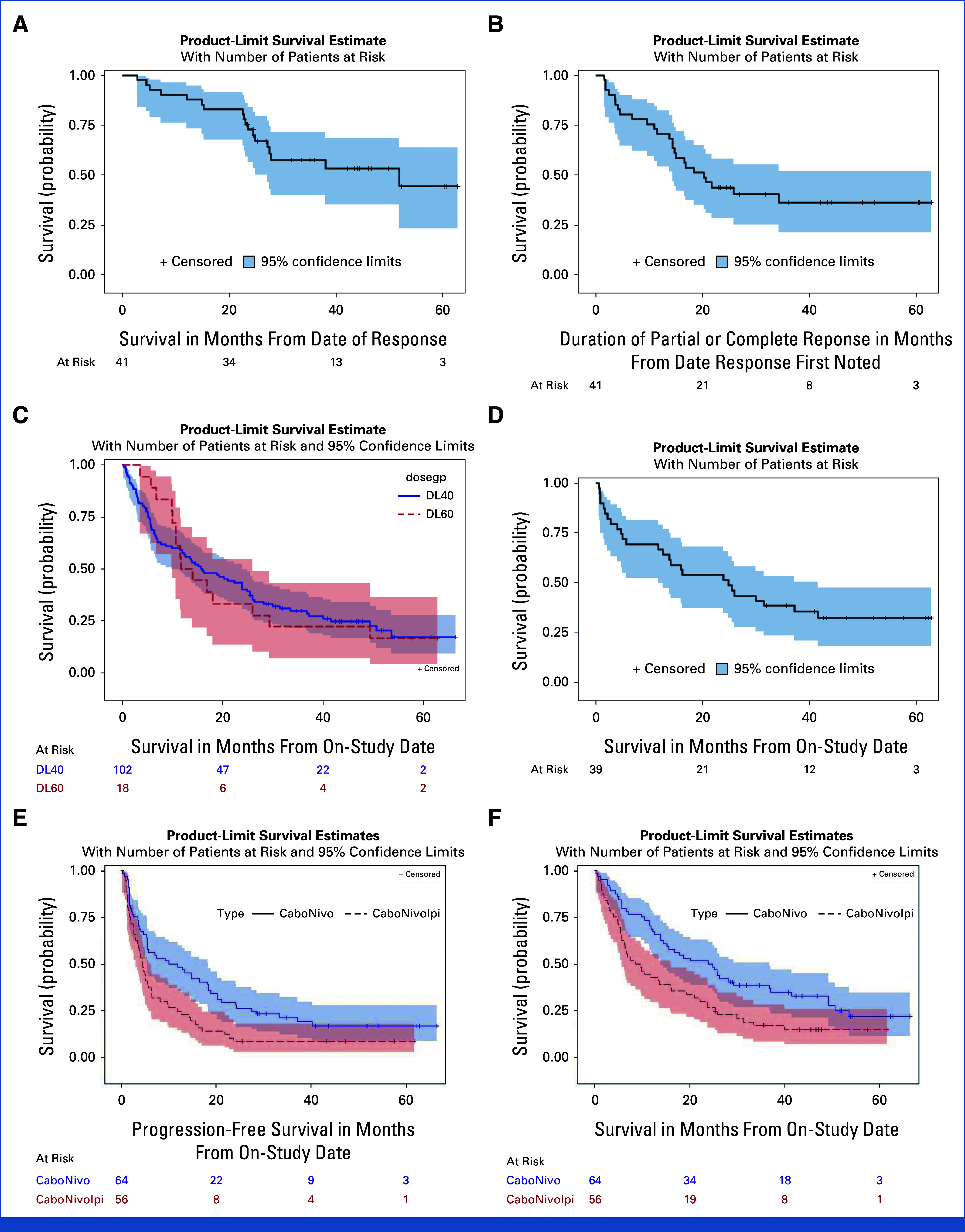
Exploratory Kaplan-Meier estimate of PFS, OS, and DoR for subgroups. (A) OS for responders (CR + PR) (n = 41) (median OS: 51.8 months [95% CI, 27.1 months to not estimable]; 6 month OS: 92.7% [95% CI, 79.0% to 97.6%]; 12 month OS: 90.2% [95% CI, 76.1% to 96.2%]; 24 month OS: 72.8% [95% CI, 56.3% to 86.6%]). (B) Median DoR for responders (median duration of PR, CR: 20.2 months [95% CI, 14.4 months to not estimable]; 6 month DOR: 80.5% [95% CI, 64.8% to 89.7%]; 12 month DOR: 70.7% [95% CI, 54.3% to 82.2%]; 24 month DOR: 43.9% [95% CI, 28.6% to 58.2%]). (C) OS (N = 120) by cabozantinib dose 40 mg (median OS: 15.8 months [95% CI, 9.9 months to 23.9 months]; 6 month OS: 68.6% [95% CI, 58.7% to 76.7%]; 12 month OS: 57.8% [95% CI, 47.7% to 66.7%]; 24 month OS: 40.2% [95% CI, 30.7% to 49.5%]) and 60 mg (N = 120) (median OS 12.9 months [95% CI, 10.0 months to 26.0 months]; 88.9% [95% CI, 62.4% to 97.1%]; 50.0% [95% CI, 25.9% to 70.1%]; 33.3% [95% CI, 13.7% to 54.5%]). (D) OS for patients with UC (n = 39) (median OS: 24.9 months [95% CI, 11.8 months to 41.6 months]; 6 month OS: 69.2% [95% CI, 52.2% to 81.2%]; 12 month OS: 66.7% [95% CI, 49.6% to 79.1%]; 24 month OS: 51.3% [95% CI, 34.8% to 65.5%]). (E) PFS in patients treated with CaboNivo and CaboNivoIpi (median PFS: CaboNivo: 11.0 months [95% CI, 5.5 months to 18.4 months] CaboNivoIpi: 4.5 months [95% CI, 3.1 months to 5.8 months] P = 0.0069). (F) OS in patients treated with CaboNivo and CaboNivoIpi (median OS CaboNivo: 24.4 months [95% CI, 14.0 months to 36.8 months]; median OS CaboNivoIpi: 9.3 months [95% CI, 5.8 months to 15.9 months] P = 0.0072). CR, complete response; DoR, duration of response; OS, overall survival; PFS, progression-free survival; PR, partial response; UC, urothelial carcinoma.
FIG A3.
Effect on M-MDSCs and classical monocytes. (A) M-MDSCs decreased by CaboNivoIpi and CaboNivo treatment. (B) Lower M-MDSCs at baseline was associated with better OS with CaboNivoIpi but not with CaboNivo. (C) Classical monocytes significantly decreased by both CaboNivoIpi and CaboNivo. (D) HLA-DR expression on classical monocytes increased after 1 cycle of CaboNivo. (E) Higher HLA-DR expression on classical monocytes at C3D1 was associated with better OS with CaboNivoIpi. (F) HLA-DR expression on intermediate monocytes increased after one cycle of CaboNivo. (G, H) Higher fold change of HLA-DR expression on intermediate monocytes at (G) C2D1 or (H) C3D1 of CaboNivoIpi was associated with better OS and PFS. FC, fold change; IM, intermediate monocytes; MFI, median fluoroscense intensity; OS, overall survival; PFS, progression-free survival.
FIG A4.
(A) Effect on Ki-67+, activated CD4+ T cells. (A) Percentage of Ki-67+HLA-DR+ cells among total CD4+ T cells markedly increased at C2D1 and C3D1 with CaboNivoIpi. (B, C) Higher fold change in percentage of Ki-67+HLA-DR+ cells at C2D1 was associated with better (B) PFS and (C) OS with CaboNivo. (D) Percentage of Ki-67+ICOS+ cells markedly increased at C2D1 and C3D1 with CaboNivoIpi and increased at C2D1 with CaboNivo. (E, F) Lower-than-median percentage of Ki-67+ICOS+ cells at baseline was associated with poor (E) PFS and (F) OS with CaboNivo. (G) Percentage of Ki-67+GITR+ cells markedly increased at C2D1 and C3D1 with both treatments. (H) Higher fold change in percentage of Ki-67+GITR+ cells at C2D1 with CaboNivo was associated with better PFS. (B) Effect on Ki-67+, activated CD8+ T cells. (A) Percentage of Ki-67+HLA-DR+ cells among total CD8+ T cells greatly increased at C2D1 and C3D1 with CaboNivoIpi. (B) Higher fold change in percentage of Ki-67+HLA-DR+ T cells at C2D1 with CaboNivo was associated with better OS. (C) Percentage of Ki-67+ICOS+ cells among total CD8+ T cells greatly increased at C2D1 and C3D1 with CaboNivoIpi and increased at C2D1 with CaboNivo. (D, E) Higher-than-median percentage of Ki-67+ICOS+ cells at baseline was associated with poor (D) PFS and (E) OS with CaboNivoIpi. (F) Higher-than-median percentage of Ki-67+ICOS+ cells at C3D1 was associated with poor OS with CaboNivoIpi. (G) Percentage of Ki-67+GITR+ cells among total CD8+ T cells greatly increased at C2D1 and C3D1 with CaboNivoIpi. (H, I) Higher fold change in percentage of Ki-67+GITR+ cells at C2D1 with CaboNivo was associated with better (H) PFS and (I) OS. FC, fold change; OS, overall survival; PFS, progression-free survival.
FIG A5.
DCs decreased by treatment and association with survival in CaboNivoIpi. (A) Percentage of CD141+ mDCs decreased. (B) Higher percentage of CD141+ mDCs at baseline was associated with better OS with CaboNivoIpi. (C) Percentage of CD1c+ mDCs decreased. (D, E) Higher fold change in percentage of CD1c+ mDCs at C2D1 was associated with better (D) PFS and (E) OS with CaboNivoIpi. (F) Percentage of CD303+ pDCs decreased. FC, fold change; mDC, mDC, myeloid dendritic cells; OS, overall survival; PFS, progression-free survival.
FIG A6.
Effect on eTregs. (A) Percentage of eTregs among CD4+ T cells increased with CaboNivoIpi. (B) Higher percentage of eTregs at C3D1 was associated with better response with CaboNivo. (C) Percent eTreg/CD4 at C3D1 did not associate with OS in CaboNivoIpi treatment. (D) Higher percentage of eTregs at C3D1 was associated with better OS with CaboNivo treatment. OS, overall survival.
FIG A7.
Effect on naïve T cells and effector memory (EM) T cells. (A) CD8:CD4 ratio increased with treatment. (B) Percentage of naïve cells among CD8+ T cells decreased with CaboNivoIpi. (C) Higher percentage CM cells at C3D1 was associated with better OS with CaboNivo. (D) Percentage of EM1 CD8+ T cells increased with CaboNivoIpi. (E) Percentage of EM1 cells among CD4+ T cells increased with CaboNivoIpi. (F) Percentage of EM2 CD8+ T cells increased with CaboNivoIpi. (G, H) Higher percentage of EM2 CD8+ T cells at C2D1 was associated with better (G) PFS and (H) OS with CaboNivoIpi. OS, overall survival; PFS, progression-free survival.
FIG A8.
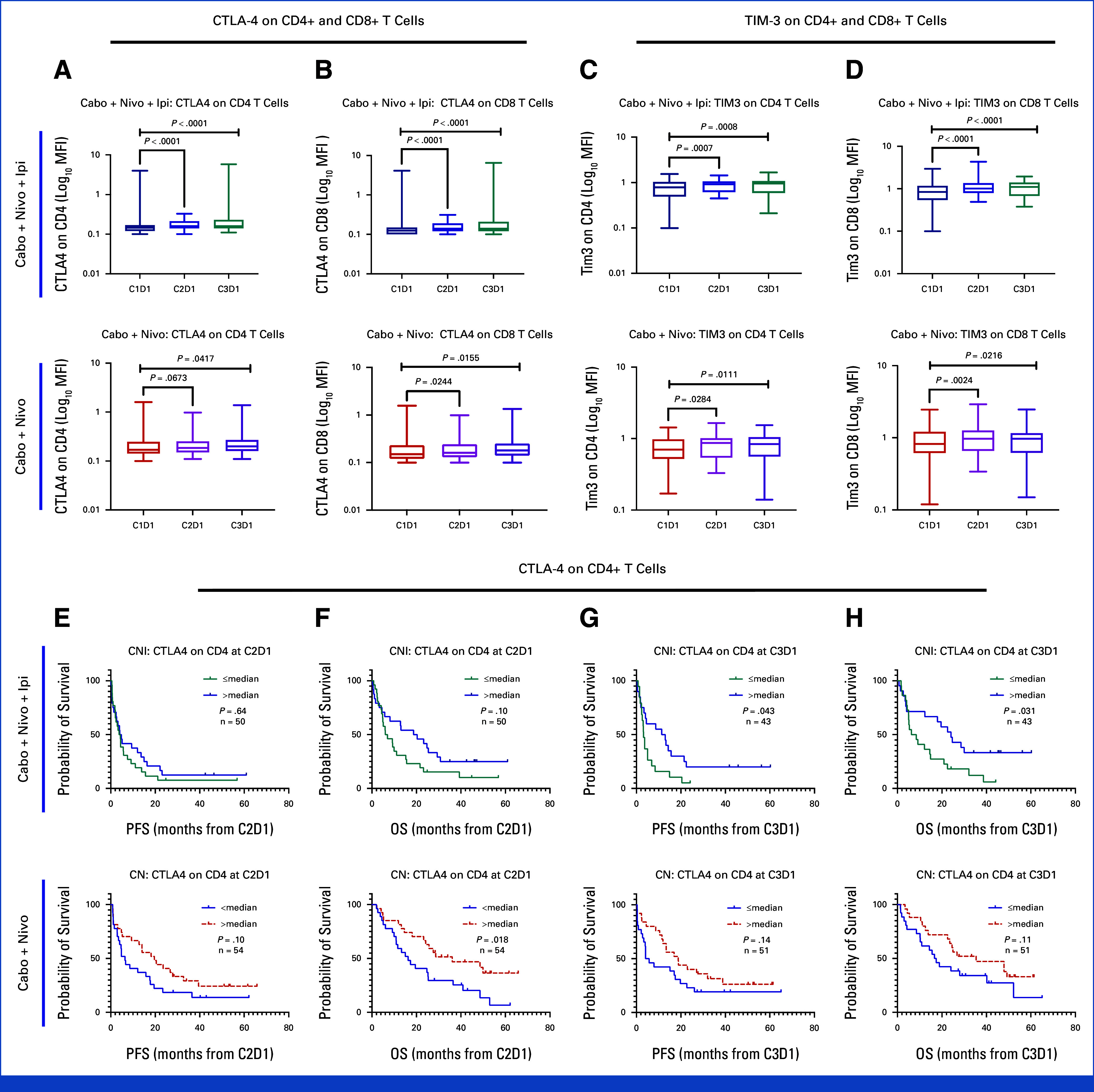
Effect on CTLA-4 and TIM-3 expression. Expression of CTLA-4 on (A) CD4+ T cells and on (B) CD8+ T cells increased with both CaboNivo and CaboNivoIpi. Expression of TIM-3 on (C) CD4+ T cells and on (D) CD8+ T cells increased with both treatments. Higher expression of CTLA-4 on CD4+ T cells at (E, F) C2D1 and at (G, H) C3D1 was associated with improved PFS and OS. OS, overall survival; PFS, progression-free survival.
FIG A9.
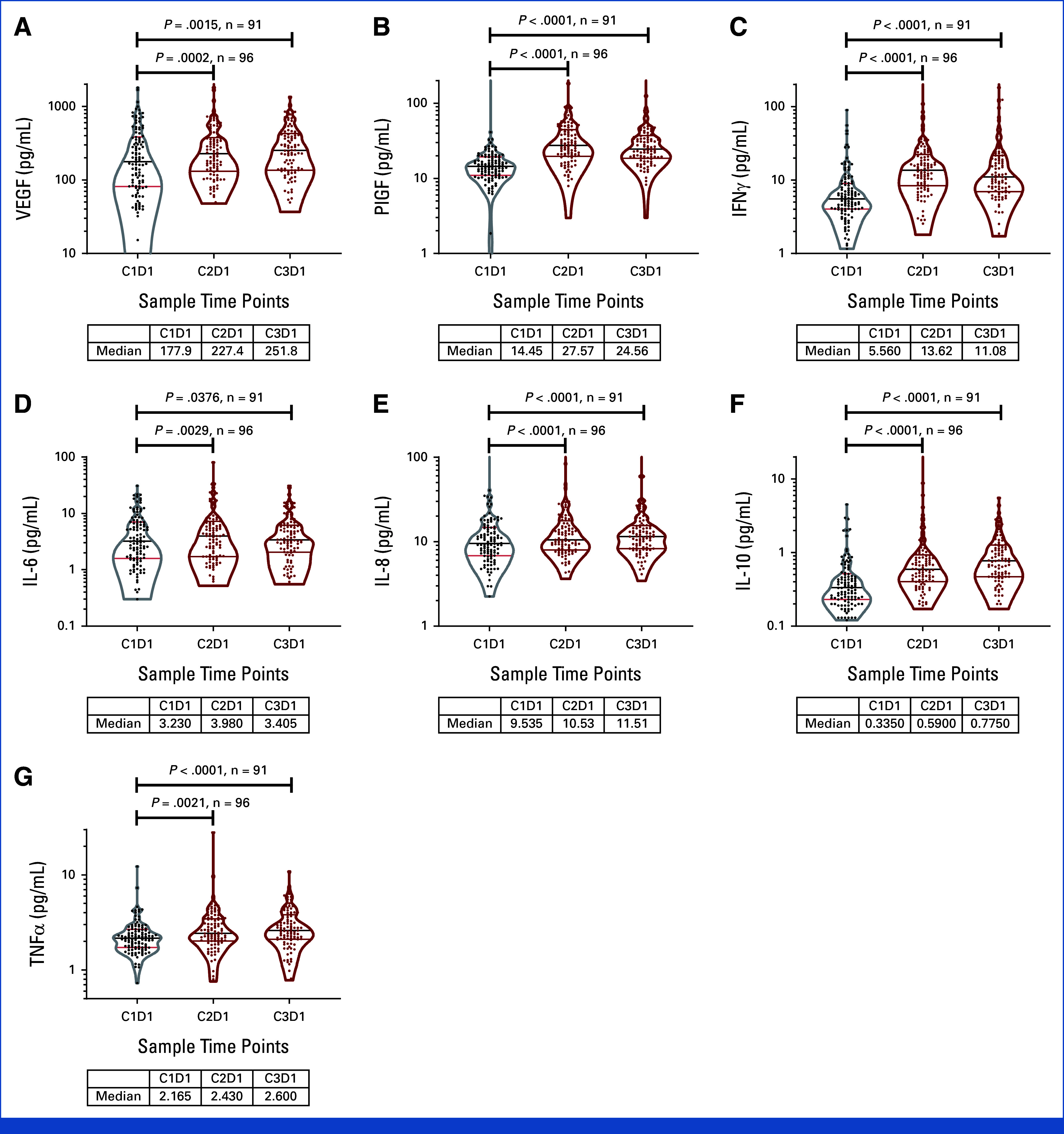
Cytokine changes in response to CaboNivo or CaboNivoIpi at C2D1 and C3D1 relative to baseline. n = 110 for C1D1, n = 96 for C2D1, and n = 91 for C3D1. Black bar represents median; upper red bar represents 75th percentile; lower red bar represents 25th percentile. Table below each plot lists median value at C1D1, C2D1, and C3D1. IFN, interferon; IL, interleukin; PlGF, placental growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
FIG A10.
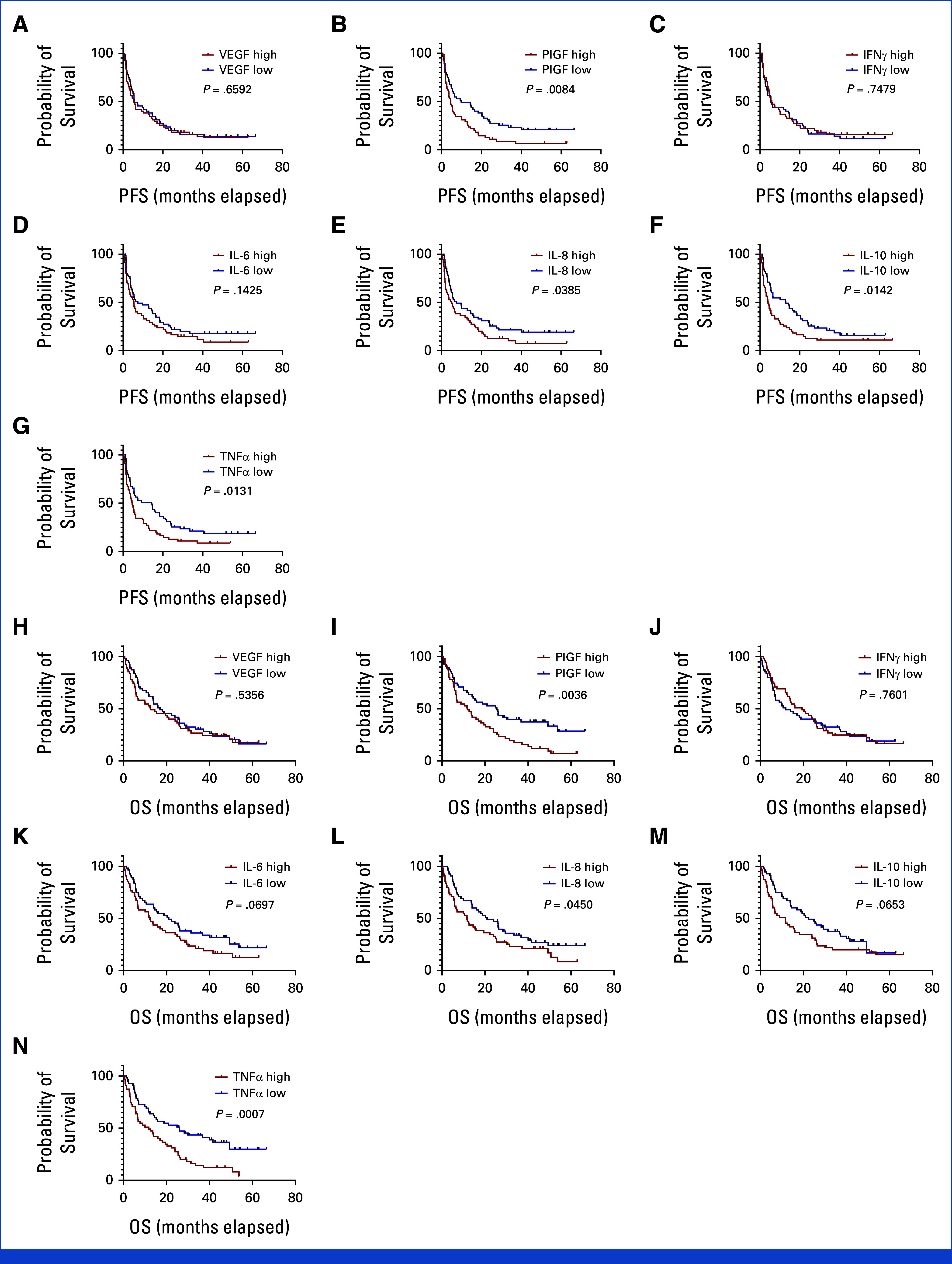
Cytokine levels at baseline and association with PFS and OS with CaboNivo and CaboNivoIpi: n = 110. PFS: (A) VEGF. (B) PIGF. (C) IFN-γ. (D) IL-6. (E) IL-8. (F) IL-10. (G) TNF-α. OS: (H) VEGF. (I) PIGF. (J) IFN-γ. (K) IL-6. (L) IL-8. (M) IL-10. (N) TNF-α. OS, overall survival; PFS, progression-free survival. IFN, interferon; IL, interleukin; PlGF, placental growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
FIG A11.
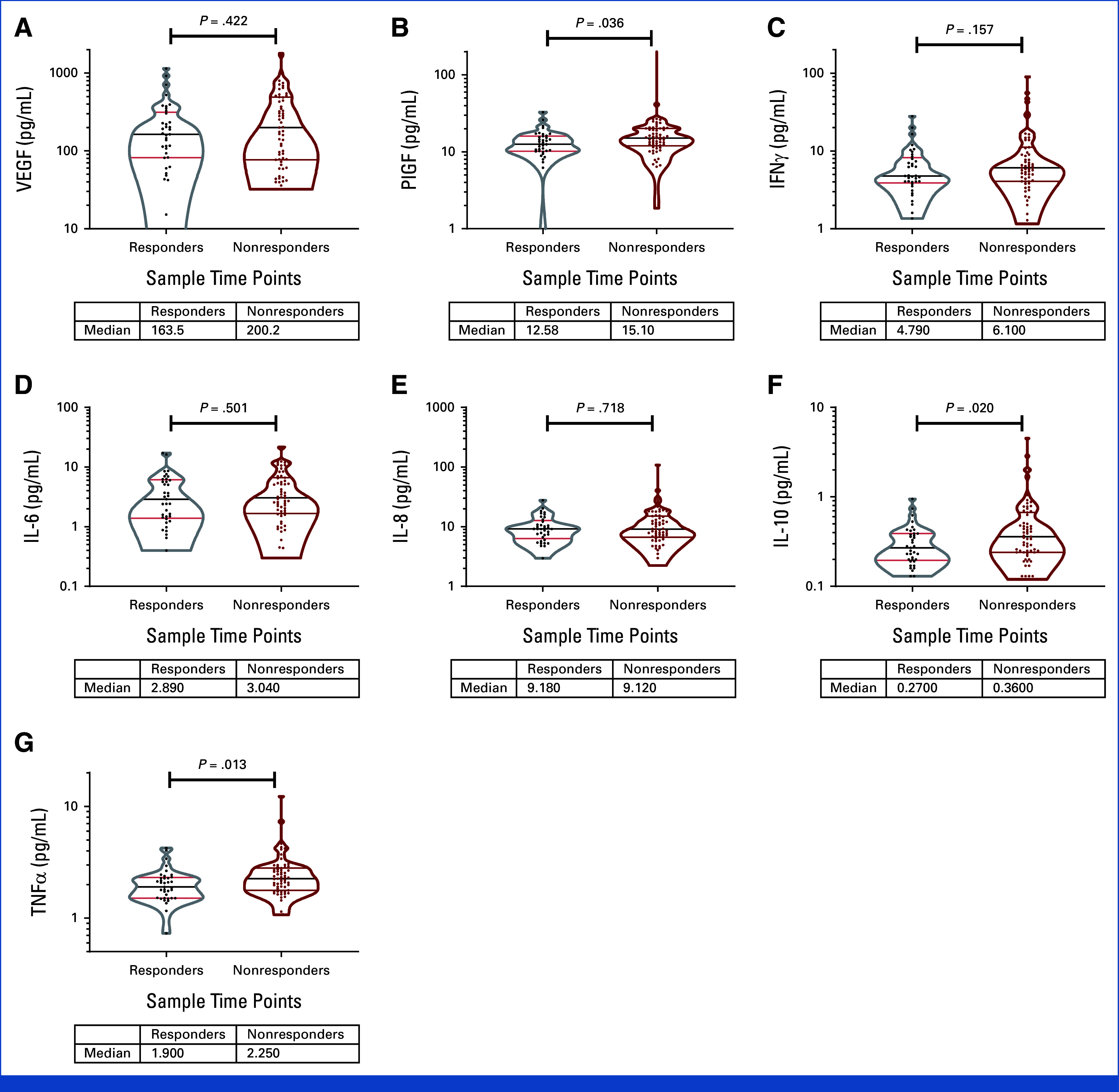
Cytokine changes in response to CaboNivo or CaboNivoIpi at C2D1 and C3D1 relative to baseline. (A) VEGF. (B) PlGF. (C) IFNγ. (D) IL-6. (E) IL-8. (F) IL-10. (G) TNFα. n = 110 for C1D1, n = 96 for C2D1, and n = 91 for C3D1. Violin plots for cytokine level at baseline, separated between responders and nonresponders. Black bar represents median; upper red bar represents 75th percentile; lower red bar represents 25th percentile. IFN, interferon; IL, interleukin; PlGF, placental growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
TABLE A1.
Patient Characteristics—Enrolled Patients With mUC
| Characteristic | All (n = 39) |
|---|---|
| Median age, years (range) | 61 (42-82) |
| Sex, No. (%) | |
| Male | 30 (76.1) |
| Female | 9 (23.1) |
| Race, No. (%) | |
| White | 35 (89.7) |
| Black or African American | 1 (2.6) |
| Asian | 3 (7.7) |
| Tumor location, No. (%) | |
| Lower tract (bladder, urethra) | 28 (71.8) |
| Upper tract (ureter/renal pelvis) | 11 (28.2) |
| Karnofsky performance status, %, No. (%) | |
| 90 | 20 (51.3) |
| 80 | 13 (33.3) |
| 70 | 6 (15.4) |
| Bajorin risk score, No. (%) | |
| 0 | 12 (30.8) |
| 1 | 21 (53.8) |
| 2 | 5 (12.8) |
| Previous treatments, No. (%)a | |
| Cisplatin | 33 (84.6) |
| Carboplatin | 9 (23.1) |
Abbreviation: mUC, metastatic urothelial carcinoma.
All patients with mUC had received platinum-based chemotherapy before enrollment; three patients had received both cisplatin and carboplatin treatments before enrollment.
TABLE A2.
Immune-Related AEs
| Preferred Term AE | CaboNivo (n = 64) | CaboNivoIpi (n = 56) | ||
|---|---|---|---|---|
| G1/G2, No. (%) | G3/G4, No. (%) | G1/G2, No. (%) | G3/G4, No. (%) | |
| Aseptic meningitis | 0 | 1 (2) | 0 | 0 |
| Hypogonadism | 1 (2) | 0 | 0 | 0 |
| Pneumonitis | 0 | 2 (3) | 3 (5) | 0 |
| Hepatitis | 0 | 0 | 0 | 5 (9) |
| Bullous dermatitis | 0 | 0 | 0 | 1 (2) |
| Colitis | 2 (3) | 1 (2) | 0 | 4 (7) |
| Adrenal insufficiency | 1 (2) | 1 (2) | 1 (2) | 0 |
| Uveitis | 1 (2) | 0 | 0 | 0 |
| Rash | 0 | 2 (3) | 0 | 0 |
| Pericarditis | 1 (1.5) | 0 | 0 | 0 |
| Total | 6 (9) | 7 (11) | 4 (7) | 10 (18) |
Abbreviation: AEs, adverse events.
TABLE A3.
Induction of Cytokines in Response to CaboNivo and CaboNivoIpi
| Biomarker | Data Stats | C1D1 (N = 110) | C2D1 (N = 99) | C3D1 (N = 97) | C2D1, P (N = 96) | C3D1, P (N = 91) |
|---|---|---|---|---|---|---|
| VEGF, pg/mL | 25% percentile | 81.6 | 130.7 | 136.4 | ||
| Median | 176.1 | 227.4 | 253.6 | .0002 | .0015 | |
| 75% percentile | 382.0 | 378.3 | 431.0 | |||
| PlGF, pg/mL | 25% percentile | 11.0 | 19.7 | 18.6 | ||
| Median | 14.5 | 27.6 | 24.6 | <.0001 | <.0001 | |
| 75% percentile | 19.3 | 44.7 | 37.3 | |||
| IFN-γ, pg/mL | 25% percentile | 4.0 | 8.4 | 7.0 | ||
| Median | 5.6 | 13.6 | 11.1 | <.0001 | <.0001 | |
| 75% percentile | 9.0 | 22.4 | 21.6 | |||
| IL-6, pg/mL | 25% percentile | 1.6 | 1.7 | 2.0 | ||
| Median | 3.2 | 4.0 | 3.4 | .0029 | .0376 | |
| 75% percentile | 7.0 | 7.2 | 6.2 | |||
| IL-8, pg/mL | 25% percentile | 6.8 | 8.0 | 8.3 | ||
| Median | 9.5 | 10.5 | 11.5 | <.0001 | <.0001 | |
| 75% percentile | 14.7 | 17.6 | 15.5 | |||
| IL-10, pg/mL | 25% percentile | 0.2 | 0.4 | 0.5 | ||
| Median | 0.3 | 0.6 | 0.8 | <.0001 | <.0001 | |
| 75% percentile | 0.5 | 0.9 | 1.3 | |||
| TNF-α, pg/mL | 25% percentile | 1.7 | 2.0 | 2.1 | ||
| Median | 2.2 | 2.4 | 2.6 | .0021 | <.0001 | |
| 75% percentile | 2.7 | 3.5 | 3.8 |
Abbreviations: IFN, interferon; IL, interleukin; PlGF, placental growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Daniel M. Girardi
Consulting or Advisory Role: BMS Brazil, Janssen Oncology, Ipsen
Speakers' Bureau: Pfizer, Merck, BMS Brazil, Bayer, Janssen, Ipsen, Adium Pharma
Travel, Accommodations, Expenses: Pfizer, Adium Pharma, Ipsen
Bernadette Redd
Consulting or Advisory Role: AbbVie, 2020DX
Hadi Bagheri
Employment: Genentech, Georgiamune
Stock and Other Ownership Interests: Genentech, Georgiamune
Travel, Accommodations, Expenses: Genentech
Piyush K. Agarwal
Employment: Pfizer
Stock and Other Ownership Interests: Amgen, Lilly
Honoraria: Aura Biosciences
Consulting or Advisory Role: AstraZeneca, Aura Biosciences, Janssen, Urogen pharma
Speakers' Bureau: PeerView
Heather J. Chalfin
Consulting or Advisory Role: Bayer
Research Funding: Elephas (Inst)
Elad Sharon
Consulting or Advisory Role: Mallinckrodt/Therakos, D.E. Shaw Research
William D. Figg
Research Funding: Celgene (Inst), Astellas Pharma (Inst), Nerviano Medical Sciences (Inst), Pfizer (Inst), NovaRX (Inst), TRACON Pharma (Inst), Biocompatibles (Inst), Propella Therapeutics (Inst)
Howard L. Parnes
Stock and Other Ownership Interests: Illumina, Health Care Select Sector, Gilead Sciences, Roper Technologies, Intuitive Surgical, Schlumberger, IQvia
James L. Gulley
Research Funding: EMD Serono (Inst), Bavarian Nordic (Inst), Astellas Medivation (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Merck (Inst), ImmunityBio (Inst), Janssen Oncology (Inst), Incyte (Inst), Marengo Therapeutics (Inst), Precigen (Inst), PDS Biotechnology (Inst), Kite, a Gilead company (Inst), NextCure (Inst), Syntrix Biosystems (Inst), Syndax (Inst)
Patents, Royalties, Other Intellectual Property: COMBINATION PDL1 AND TGF-BETA BLOCKADE IN PATIENTS WITH HPV+ MALIGNANCIES Publication number: 20200062849 Abstract: The invention provides a method of inhibiting a malignancy associated with human papilloma-virus (HPV) comprising administering to a subject an agent that blocks PD-L1 and TGF-beta pathways, thereby inhibiting a malignancy associated with HPV in the subject. No money is associated with this.
Biren Saraiya
Research Funding: Regeneron (Inst), Merck (Inst), Hookipa Pharma (Inst), OncoC4 (Inst)
Sumanta K. Pal
Travel, Accommodations, Expenses: CRISPR Therapeutics, Ipsen, Exelixis
(OPTIONAL) Open Payments Link: https://openpaymentsdata.cms.gov/physician/259259
David Quinn
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Mark N. Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Exelixis, Xencor, Janssen Oncology, Vaccitech, Bristol Myers Squibb/Medarex
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Lilly (Inst), Nektar (Inst), Seagen (Inst), Xencor (Inst), Tmunity Therapeutics, Inc (Inst), Exelixis (Inst), Bellicum Pharmaceuticals, Regeneron (Inst), Bicycle Therapeutics (Inst), AstraZeneca (Inst)
Primo N. Lara
Research Funding: Taiho Pharmaceutical (Inst), AstraZeneca (Inst)
Donald P. Bottaro
Honoraria: Janssen Oncology
Patents, Royalties, Other Intellectual Property: Bottaro DP, Petryshyn R. US Patent No. 6,326,466; December 4, 2001: Double Stranded RNA Dependent Protein Kinase Derived Peptides to Promote Proliferation of Cells and Tissues in a Controlled Manner. Related International Publication No. WO/1998/004717, Chan AML, Rubin JS, Bottaro DP, Aaronson SA. US Patent No. 6,566,098; May 20, 2003: DNA Encoding Truncated Hepatocyte Growth Factor Variants. Related International Publication No. WO/1992/005184, Bottaro DP, Soriano JV, Atabey SN, Breckenridge DE, Gao Y, Yao Z-J, Burke TR Jr. US Patent No. 7,132,392; November 7, 2006: Inhibition of Cell Motility and Angiogenesis by Inhibitors of Grb2-SH2-Domain. Related International Publications No. WO/2001/028577 and No. WO/2008/036565, Chan AML, Rubin JS, Bottaro DP, Aaronson SA, Stahl SJ, Wingfield PT, Cioce V. US Patent No. 7,605,127; October 20, 2009: Truncated Hepatocyte Growth Factor Variant Protein HGF/NK2. Related International Publication No. WO/1996/040914, Bottaro DP, Giubellino A, Atabey N, Soriano JV, Breckenridge DE, Burke TR Jr. US Patent No. 7,871,981; January 18, 2011: Inhibition of Cell Motility, Angiogenesis and Metastasis. Related International Publication No. WO/2001/028577, Bottaro DP, Athauda G, Burgess TL. US Patent No. 7,964,365; June 21, 2011: Methods for Diagnosing and Monitoring the Progression of Cancer. Related International Publication No. WO/2007/056523, Bottaro DP, Athauda G, Burgess TL. US Patent No. 8,304,199; November 6, 2012: Methods for Diagnosing and Monitoring the Progression of Cancer by Measuring Soluble c-Met Ectodomain, Bottaro DP, Peach M, Nicklaus M, Tan N. US Patent No. 8,569,360; October 29, 2013: Compositions and Methods for Inhibition of Hepatocyte Growth Factor Receptor c-Met Signaling. Related International Publications No. WO/2009/124024 and WO/2009/124013, Bottaro DP, Athauda G, Burgess TL. US Patent No. 8,617,831; December 31, 2013: Methods for Diagnosing and Monitoring the Progression of Cancer by Measuring Soluble c-Met Ectodomain, Bottaro DP, Peach M, Nicklaus M, Burke TR Jr, Athauda G, Choyke S, Giubellino A, Tan N, Shi Z-D. US Patent No. 8,754,081; June 17, 2014: Compositions and methods for inhibition of hepatocyte growth factor receptor c-Met signaling. Related International Publication No. WO/124013, Bottaro DP, Cecchi F. US Patent No. 9,550,818, January 24, 2017: Methods for Use of Vascular Endothelial Growth Factor Antagonists. Related International Publication No. WO/2013/163606, Bottaro DP, Cecchi F. US Patent No. 10,035,833, July 31, 2018: Vascular Endothelial Growth Factor Antagonists and Methods of Making
Amir Mortazavi
Consulting or Advisory Role: Targeted Oncology
Research Funding: Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Seagen (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst), GlaxoSmithKline (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Cancer Institute Intramural Program and the Cancer Therapy Evaluation Program.
CLINICAL TRIAL INFORMATION
PREPRINT VERSION
Preprint version available on https://www.researchsquare.com/article/rs-2924051/v1.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02233.
AUTHOR CONTRIBUTIONS
Conception and design: Andrea B. Apolo, Howard Streicher, Elad Sharon, William D. Figg, Donald P. Bottaro
Financial support: Andrea B. Apolo, Elad Sharon
Administrative support: Andrea B. Apolo, Howard Streicher, Elad Sharon, James L. Gulley
Provision of study materials or patients: Andrea B. Apolo, Piyush K. Agarwal, Elad Sharon, Biren Saraiya, David Quinn, Mark N. Stein, Primo N. Lara, Amir Mortazavi
Collection and assembly of data: Andrea B. Apolo, Daniel M. Girardi, Scot A. Niglio, Rosa Nadal, Andre R. Kydd, Nicholas Simon, Lisa Ley, Lisa M. Cordes, Elias Chandran, Min-Jung Lee, Shraddha Rastogi, Nahoko Sato, Liang Cao, Dilara Akbulut, Bernadette Redd, Hadi Bagheri, Rene Costello, Heather J. Chalfin, Elad Sharon, William D. Figg, Biren Saraiya, David Quinn, Mark N. Stein, Primo N. Lara, Amir Mortazavi
Data analysis and interpretation: Andrea B. Apolo, Daniel M. Girardi, Scot A. Niglio, Rosa Nadal, Andre R. Kydd, Nicholas Simon, Elias Chandran, Seth M. Steinberg, Sunmin Lee, Min-Jung Lee, Shraddha Rastogi, Nahoko Sato, Liang Cao, A. Rouf Banday, Salah Boudjadi, Maria J. Merino, Antoun Toubaji, Sandeep Gurram, Piyush K. Agarwal, Vladimir Valera, John Joseph Wright, Elad Sharon, William D. Figg, Howard L. Parnes, James L. Gulley, Sumanta K. Pal, David Quinn, Primo N. Lara, Donald P. Bottaro
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Final Results From a Phase I Trial and Expansion Cohorts of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced/Metastatic Genitourinary Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniel M. Girardi
Consulting or Advisory Role: BMS Brazil, Janssen Oncology, Ipsen
Speakers' Bureau: Pfizer, Merck, BMS Brazil, Bayer, Janssen, Ipsen, Adium Pharma
Travel, Accommodations, Expenses: Pfizer, Adium Pharma, Ipsen
Bernadette Redd
Consulting or Advisory Role: AbbVie, 2020DX
Hadi Bagheri
Employment: Genentech, Georgiamune
Stock and Other Ownership Interests: Genentech, Georgiamune
Travel, Accommodations, Expenses: Genentech
Piyush K. Agarwal
Employment: Pfizer
Stock and Other Ownership Interests: Amgen, Lilly
Honoraria: Aura Biosciences
Consulting or Advisory Role: AstraZeneca, Aura Biosciences, Janssen, Urogen pharma
Speakers' Bureau: PeerView
Heather J. Chalfin
Consulting or Advisory Role: Bayer
Research Funding: Elephas (Inst)
Elad Sharon
Consulting or Advisory Role: Mallinckrodt/Therakos, D.E. Shaw Research
William D. Figg
Research Funding: Celgene (Inst), Astellas Pharma (Inst), Nerviano Medical Sciences (Inst), Pfizer (Inst), NovaRX (Inst), TRACON Pharma (Inst), Biocompatibles (Inst), Propella Therapeutics (Inst)
Howard L. Parnes
Stock and Other Ownership Interests: Illumina, Health Care Select Sector, Gilead Sciences, Roper Technologies, Intuitive Surgical, Schlumberger, IQvia
James L. Gulley
Research Funding: EMD Serono (Inst), Bavarian Nordic (Inst), Astellas Medivation (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Merck (Inst), ImmunityBio (Inst), Janssen Oncology (Inst), Incyte (Inst), Marengo Therapeutics (Inst), Precigen (Inst), PDS Biotechnology (Inst), Kite, a Gilead company (Inst), NextCure (Inst), Syntrix Biosystems (Inst), Syndax (Inst)
Patents, Royalties, Other Intellectual Property: COMBINATION PDL1 AND TGF-BETA BLOCKADE IN PATIENTS WITH HPV+ MALIGNANCIES Publication number: 20200062849 Abstract: The invention provides a method of inhibiting a malignancy associated with human papilloma-virus (HPV) comprising administering to a subject an agent that blocks PD-L1 and TGF-beta pathways, thereby inhibiting a malignancy associated with HPV in the subject. No money is associated with this.
Biren Saraiya
Research Funding: Regeneron (Inst), Merck (Inst), Hookipa Pharma (Inst), OncoC4 (Inst)
Sumanta K. Pal
Travel, Accommodations, Expenses: CRISPR Therapeutics, Ipsen, Exelixis
(OPTIONAL) Open Payments Link: https://openpaymentsdata.cms.gov/physician/259259
David Quinn
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Mark N. Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Exelixis, Xencor, Janssen Oncology, Vaccitech, Bristol Myers Squibb/Medarex
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Lilly (Inst), Nektar (Inst), Seagen (Inst), Xencor (Inst), Tmunity Therapeutics, Inc (Inst), Exelixis (Inst), Bellicum Pharmaceuticals, Regeneron (Inst), Bicycle Therapeutics (Inst), AstraZeneca (Inst)
Primo N. Lara
Research Funding: Taiho Pharmaceutical (Inst), AstraZeneca (Inst)
Donald P. Bottaro
Honoraria: Janssen Oncology
Patents, Royalties, Other Intellectual Property: Bottaro DP, Petryshyn R. US Patent No. 6,326,466; December 4, 2001: Double Stranded RNA Dependent Protein Kinase Derived Peptides to Promote Proliferation of Cells and Tissues in a Controlled Manner. Related International Publication No. WO/1998/004717, Chan AML, Rubin JS, Bottaro DP, Aaronson SA. US Patent No. 6,566,098; May 20, 2003: DNA Encoding Truncated Hepatocyte Growth Factor Variants. Related International Publication No. WO/1992/005184, Bottaro DP, Soriano JV, Atabey SN, Breckenridge DE, Gao Y, Yao Z-J, Burke TR Jr. US Patent No. 7,132,392; November 7, 2006: Inhibition of Cell Motility and Angiogenesis by Inhibitors of Grb2-SH2-Domain. Related International Publications No. WO/2001/028577 and No. WO/2008/036565, Chan AML, Rubin JS, Bottaro DP, Aaronson SA, Stahl SJ, Wingfield PT, Cioce V. US Patent No. 7,605,127; October 20, 2009: Truncated Hepatocyte Growth Factor Variant Protein HGF/NK2. Related International Publication No. WO/1996/040914, Bottaro DP, Giubellino A, Atabey N, Soriano JV, Breckenridge DE, Burke TR Jr. US Patent No. 7,871,981; January 18, 2011: Inhibition of Cell Motility, Angiogenesis and Metastasis. Related International Publication No. WO/2001/028577, Bottaro DP, Athauda G, Burgess TL. US Patent No. 7,964,365; June 21, 2011: Methods for Diagnosing and Monitoring the Progression of Cancer. Related International Publication No. WO/2007/056523, Bottaro DP, Athauda G, Burgess TL. US Patent No. 8,304,199; November 6, 2012: Methods for Diagnosing and Monitoring the Progression of Cancer by Measuring Soluble c-Met Ectodomain, Bottaro DP, Peach M, Nicklaus M, Tan N. US Patent No. 8,569,360; October 29, 2013: Compositions and Methods for Inhibition of Hepatocyte Growth Factor Receptor c-Met Signaling. Related International Publications No. WO/2009/124024 and WO/2009/124013, Bottaro DP, Athauda G, Burgess TL. US Patent No. 8,617,831; December 31, 2013: Methods for Diagnosing and Monitoring the Progression of Cancer by Measuring Soluble c-Met Ectodomain, Bottaro DP, Peach M, Nicklaus M, Burke TR Jr, Athauda G, Choyke S, Giubellino A, Tan N, Shi Z-D. US Patent No. 8,754,081; June 17, 2014: Compositions and methods for inhibition of hepatocyte growth factor receptor c-Met signaling. Related International Publication No. WO/124013, Bottaro DP, Cecchi F. US Patent No. 9,550,818, January 24, 2017: Methods for Use of Vascular Endothelial Growth Factor Antagonists. Related International Publication No. WO/2013/163606, Bottaro DP, Cecchi F. US Patent No. 10,035,833, July 31, 2018: Vascular Endothelial Growth Factor Antagonists and Methods of Making
Amir Mortazavi
Consulting or Advisory Role: Targeted Oncology
Research Funding: Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Seagen (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst), GlaxoSmithKline (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Wagle NS, et al. : Cancer statistics, 2023. CA Cancer J Clin 73:17-48, 2023 [DOI] [PubMed] [Google Scholar]
- 2.Nadal R, Bellmunt J: Management of metastatic bladder cancer. Cancer Treat Rev 76:10-21, 2019 [DOI] [PubMed] [Google Scholar]
- 3.de Velasco G, Bex A, Albiges L, et al. : Sequencing and combination of systemic therapy in metastatic renal cell carcinoma. Eur Urol Oncol 2:505-514, 2019 [DOI] [PubMed] [Google Scholar]
- 4.U Gandhy S, Madan RA, Aragon-Ching JB: The immunotherapy revolution in genitourinary malignancies. Immunotherapy 12:819-831, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragon-Ching JB, Pagliaro LC: New developments and challenges in rare genitourinary tumors: Non-urothelial bladder cancers and squamous cell cancers of the penis. Am Soc Clin Oncol Educ Book 37:330-336, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Yi M, Jiao D, Qin S, et al. : Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 18:60, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apolo AB, Nadal R, Tomita Y, et al. : Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: An open-label, single-centre, phase 2 trial. Lancet Oncol 21:1099-1109, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apolo AB, Nadal R, Girardi DM, et al. : Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol 38:3672-3684, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girardi DM, Niglio SA, Mortazavi A, et al. : Cabozantinib plus nivolumab phase I expansion study in patients with metastatic urothelial carcinoma refractory to immune checkpoint inhibitor therapy. Clin Cancer Res 28:1353-1362, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T, Ballman K, Choudhury A, et al. : PDIGREE: An adaptive phase III trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). J Clin Oncol 39, 2021. (6_suppl; abstr TPS366) [Google Scholar]
- 12.Choueiri TK, Powles T, Albiges L, et al. : Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N Engl J Med 388:1767-1778, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A Study of XL092 as Single-Agent and Combination Therapy in Subjects with Solid Tumors (STELLAR-001). ClinicalTrials.Gov. https://clinicaltrials.gov/ct2/show/NCT03845166 [Google Scholar]
- 14.Testing the Effectiveness of Two Immunotherapy Drugs (Nivolumab and Ipilimumab) with One Anticancer Targeted Drug (Cabozantinib) for Rare Genitourinary Tumors. ClinicalTrials.Gov. https://clinicaltrials.gov/ct2/show/NCT03866382 [Google Scholar]
- 15.Choueiri T, Powles T, Albiges L, et al. : LBA8-Phase III study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313). Ann Oncol 33:S1430-S1431, 2022. (suppl 7) [Google Scholar]
- 16.Powles T, Valderrama BP, Gupta S, et al. : Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med 390:875-888, 2024 [DOI] [PubMed] [Google Scholar]
- 17.Hoimes CJ, Flaig TW, Milowsky MI, et al. : Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol 41:22-31, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGregor B, Mortazavi A, Cordes L, et al. : Management of adverse events associated with cabozantinib plus nivolumab in renal cell carcinoma: A review. Cancer Treat Rev 103:102333, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ready NE, Ott PA, Hellmann MD, et al. : Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: Results from the CheckMate 032 randomized cohort. J Thorac Oncol 15:426-435, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Grimm MO, Schmidinger M, Duran-Martinez I, et al. : Tailored immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Ann Oncol 30:v892, 2019. (suppl 5) [Google Scholar]
- 21.Grimm MO, Grün CB, Niegisch G, et al. : Tailored immunotherapy approach with nivolumab with or without ipilimumab in patients with advanced transitional cell carcinoma after platinum-based chemotherapy (TITAN-TCC): A multicentre, single-arm, phase 2 trial. Lancet Oncol 24:347-359, 2023 [DOI] [PubMed] [Google Scholar]
- 22.Apolo AB, Powles T, Escudier B, et al. : Nivolumab plus ipilimumab plus cabozantinib triplet combination for patients with previously untreated advanced renal cell carcinoma: Results from a discontinued arm of the phase III CheckMate 9ER trial. Eur J Cancer 177:63-71, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandran E, Simon N, Iannantuono G, et al. : Nivolumab plus ipilimumab plus cabozantinib triplet combination for patients with previously untreated advanced renal cell carcinoma: Results from a discontinued arm of the phase III CheckMate 9ER trial—Beyond the Abstract. UroToday. https://www.urotoday.com/recent-abstracts/urologic-oncology/renal-cancer/141968-nivolumab-plus-ipilimumab-plus-cabozantinib-triplet-combination-for-patients-with-previously-untreated-advanced-renal-cell-carcinoma-results-from-a-discontinued-arm-of-the-phase-iii-checkmate-9er-trial-beyond-the-abstract.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peranzoni E, Ingangi V, Masetto E, et al. : Myeloid cells as clinical biomarkers for immune checkpoint blockade. Front Immunol 11:1590, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu FF, Zhang K, Ma LL, et al. : The superior ability of human BDCA3(+) (CD141(+)) dendritic cells (DCs) to cross-present antigens derived from necrotic lung cancer cells. Front Immunol 11:1267, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wculek SK, Cueto FJ, Mujal AM, et al. : Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20:7-24, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Huang AC, Postow MA, Orlowski RJ, et al. : T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545:60-65, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamphorst AO, Pillai RN, Yang S, et al. : Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 114:4993-4998, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karzai F, VanderWeele D, Madan RA, et al. : Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer 6:141, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai CS, Huang JT, Lu X, et al. : Clonal deletion of tumor-specific T cells by interferon-γ confers therapeutic resistance to combination immune checkpoint blockade. Immunity 50:477-492.e8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebbé C, Meyer N, Mortier L, et al. : Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: Results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 37:867-875, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma P, Siefker-Radtke A, de Braud F, et al. : Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol 37:1608-1616, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammers HJ, Plimack ER, Infante JR, et al. : Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 study. J Clin Oncol 35:3851-3858, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascierto PA, Del Vecchio M, Robert C, et al. : Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 18:611-622, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02233.