Abstract
Background:
Urethritis associated with non-viral sexually transmitted infections (STI) increases the risk of HIV acquisition and transmission in those living with HIV (LWH) without viral load suppression (VLS). Compared to women, men typically have lower rates of HIV VLS. We assessed the prevalence of VLS and drug resistance mutations in men LWH and urethral discharge syndrome (UDS) in Kampala, Uganda.
Methods:
Men with UDS were recruited in Kampala October 2019-November 2020. Medical, demographic, and behavioural data were collected with biological samples. All reactive HIV results (rapid, sequential algorithm) underwent confirmatory HIV antibody- and HIV incidence-testing, and viral load (VL) measurement. The pol and gp41 regions were sequenced on samples with VLs > 1000 cpm, phylogenetic trees were generated, and resistance mutations were investigated.
Results:
50 of 250 participants (20%) had reactive HIV rapid tests and 48/50 (96%) were aware of their HIV status and using antiretroviral therapy (ART). The median age was 38 years (IQR 32-45), 27/50 (54%) had engaged in transactional sex, and 30/50 (60%) reported alcohol before sex. VLS was present in 46/50 (92%). There were no major resistance mutations present in any samples analyzed.
Conclusions:
The prevalence of HIV and VLS was greater in these men than in the general Ugandan adult population. Most men LWH were on ART and thus less likely to transmit HIV despite demonstrating sexual behaviours associated with high-risk of STIs. These data emphasize that high levels of ART coverage and VLS are achievable among men with UDS in urban Kampala.
Keywords: HIV viral suppression, urethral discharge syndrome, sexually transmitted infections, antiretroviral therapy resistance, factors associated with HIV transmission, Uganda, men, risk behaviours
Introduction
HIV and other sexually transmitted infections (STIs) are synergistic; the presence of urethritis caused by non-viral STIs elevates the risk of HIV transmission in those living with HIV (LWH) and increases the potential for HIV acquisition.1 Regions with a high prevalence of both HIV and other STIs create ideal conditions for HIV transmission when people LWH are not on suppressive antiretroviral therapy (ART).2 Therefore, treatment and prevention of both HIV and non-HIV STI are required to end the HIV epidemic.
Historically, heterosexual men have not received the same degree of attention as other priority populations with respect to HIV prevention. In 2017, Ugandan President Yoweri Museveni launched the Presidential Fast Track Initiative on Ending HIV & AIDS in Uganda. A key facet of the strategy is to “engage men in HIV prevention.”3 Compared to women, men typically have lower awareness of their HIV status, particularly among younger individuals.4 Multiple factors contribute to low rates of HIV awareness in men, including stigma, health care barriers, and low self-perceived risk of HIV acquisition.5,6 Additionally, men LWH have lower rates of viral load suppression (VLS) in sub-Saharan Africa (SSA), where HIV prevalence and incidence is highest globally,7 and where one in five people LWH initiating ART may have drug resistance.8 In Uganda in 2022, an estimated 1.4 million people were LWH (an adult prevalence of 5.1%) with 79% (74%–87%) achieving VLS.9 Furthermore, in 2022, 88% (82%–98%) of Ugandan women LWH aged 15 and over were receiving ART, compared to only 80% (75%–89%) of men.9 VLS in Ugandan men aged 15 years or older was estimated to be 83.8% in 2020.10 World Health Organization guidelines recommend the use of viral load as the preferred method for monitoring treatment response and defines virologic failure with a threshold of 1000 copies/ml.11 Uganda, like most countries in SSA, uses the same threshold.12 We assessed the prevalence of VLS and drug resistance in men with a positive HIV rapid test and urethral discharge syndrome (UDS) in Kampala, Uganda. Additionally, we conducted laboratory-based HIV testing on all available samples from the parent study (N=250).
Materials and methods
The data presented focus on a subset of participants LWH from a previously reported study of men with UDS attending government health centres in Kampala.13 Only participants with two reactive HIV point-of-care (POC) tests (POCT) were evaluated. This analysis describes the proportion of participants LWH demonstrating VLS and the presence of ART-associated resistance mutations. The parent observational study, nested within an enhanced gonococcal antimicrobial surveillance program (EGASP),14 has been previously published.13 In brief, 250 symptomatic male participants with UDS were recruited at six urban clinics in Kampala between October 2019 and November 2020. HIV/ART-specific medical information and demographic and behavioural data were collected with biological samples in participants LWH. Penile meatal swabs were tested for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium using Aptima (Hologic Inc., Marlborough, MA, US) nucleic acid amplification tests. In Kampala, onsite POCT for HIV followed the Ugandan rapid sequential testing algorithm using Determine (Alere, Waltham, MA, US), Stat-Pak (Chembio, Medford, NY, US), and SD-Bioline (Standard Diagnostics, Giheung-gu, Republic of Korea). Discordant HIV results were classified as those having two reactive POCTs and a negative laboratory-based antibody and HIV RNA result. Serum samples were stored at −80°C at the Infectious Disease Institute, Makerere University, Kampala prior to shipping to the International STD Reference laboratory at Johns Hopkins University (JHU), Baltimore, MD, USA for further testing. After arrival at JHU, available serum samples from the parent study underwent additional laboratory-based testing for HIV. Descriptive statistical analysis was conducted.
Ethical oversight was provided by the Ugandan National Council for Science and Technology (study number HS455ES), and the Joint Clinical Research Centre (protocol reference number JC0919). The Johns Hopkins Institutional Review Board (IRB number 00215298) also approved the study. No procedures were completed until signed informed consent was obtained. All participants received standard-of-care antimicrobial treatment at the recruiting clinic prior to study enrollment.
At JHU, each sample with a reactive HIV POC test underwent confirmatory HIV testing using the Bio-Rad GS HIV-1/HIV-2 PLUS O EIA tests (Bio-Rad Laboratories, Redmond, WA, US). In addition, to evaluate concordance between POC and lab-based values, samples that were available for analysis were tested on the Roche Elecsys HIV combi PT 4th Generation test (Roche, Indianapolis, IN, US). Reactive tests on the Roche Elecsys assay were tested again to confirm. The HIV viral load (VL) of each sample was then determined using the Abbott m2000 RealTime HIV-1 assay per the manufacturer’s instructions (Abbott Molecular Inc., Des Plaines, IL, US). Samples were diluted by a factor of eight with phosphate-buffered saline to enable a limit of detection of 320 copies per milliliter (cpm). Incidence testing for HIV was performed using the Asante HIV-1 rapid recency assay (Sedia Biosciences, Beaverton, OR, US) on all HIV-positive samples. The absence of a long-term band on samples with a VL >1000 cpm would be classified as a “recent infection”, indicating that infection occurred in the previous 180 days.
The pol and gp41 regions of the HIV genome were subsequently sequenced on samples with VLs >1000 cpm. Sequencing was completed in the Genomics Unit at Rocky Mountain Laboratories, US using a MiSeq next generation sequencing platform with NEXTERA index primer sets (Illumina, San Diego, CA, US) as previously described.15 After sequencing, neighbor joining phylogenetic trees were generated using Geneious Prime 2021.0.1 and FigTree v1.4.4 to determine HIV-1 subtype. Major and accessory resistance mutations were identified using the Genotypic Resistance Interpretation Algorithm within the Stanford HIV Drug Resistance Database.16
Results
The median age of study participants LWH was 38 years (IQR 32-45), compared to a median age in the parent study of 24 years (IQR 22-32), p<.001. The median number of reported sexual partners in the previous 2 months was one (IQR 1-2). Within the previous 6 months, 27/50 (54%) of participants LWH had engaged in transactional sex, and 29/50 (58%) indicated that they had used alcohol before sex with 8/50 (16%) reporting intoxication prior to sex. Less than 2% reported “always” condom use, whereas 32% had participated in condomless sex since the onset of UDS symptoms and prior to treatment, compared to 18.5% of participants not LWH.
Following completion of the HIV POC testing algorithm, 50/250 (20%) tests were reactive; 82% of these individuals had at least one other curable STI. By self-report, 48/50 individuals (96%) were known to be LWH; of these, all reported taking ART. Of these 48, the most recent self-reported VL was available in 26/48 (54.2%); 22/26 (84.6%) reported an undetectable VL. Agreement between HIV POC and lab-based antibody testing was noted in 49/50 (98%) (Figure 1(a)). One participant (MN123) had two reactive POC tests, but negative confirmatory Bio-Rad antibody and undetectable HIV VL.
Figure 1.
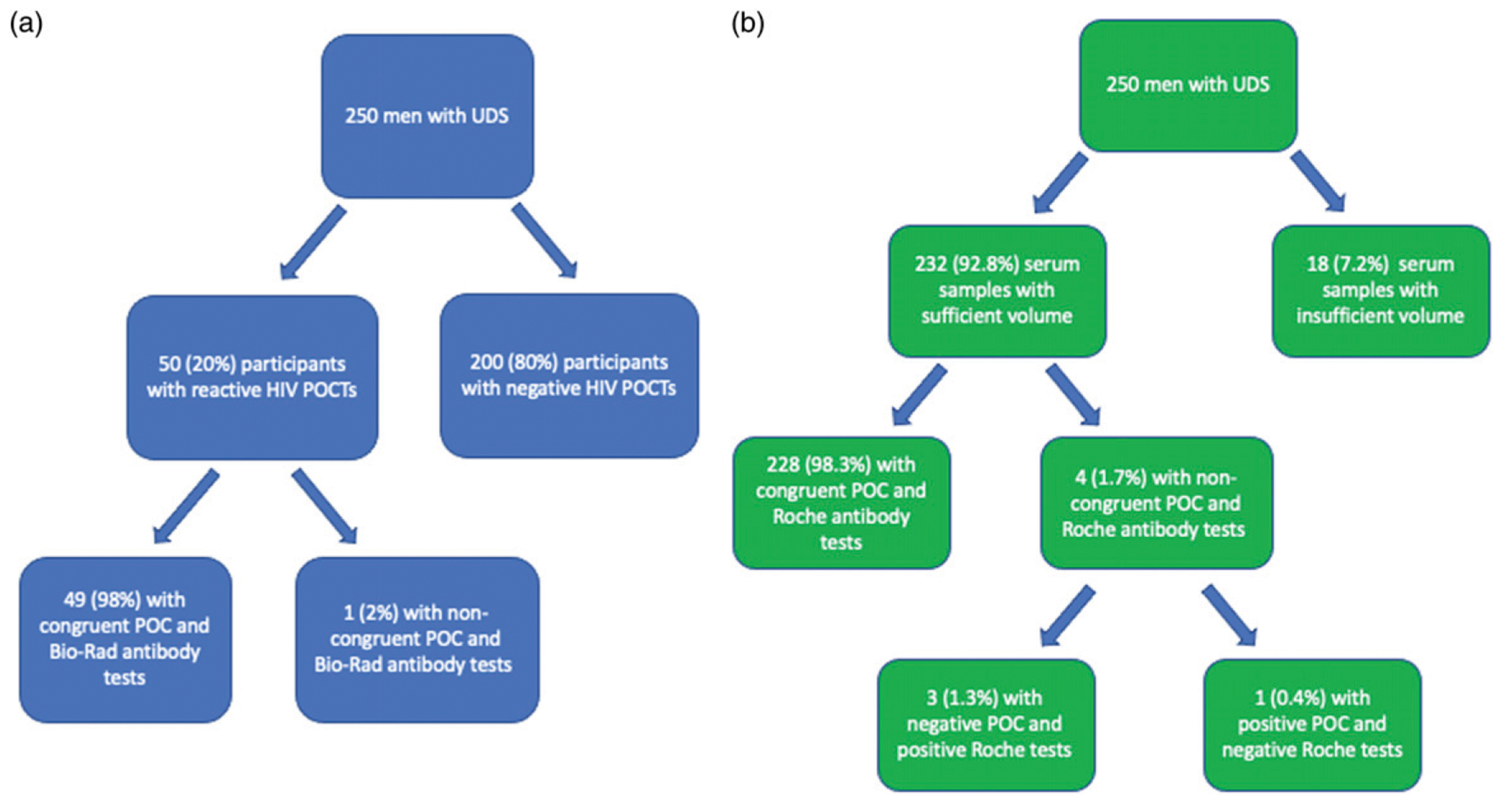
Agreement between point-of-care and laboratory-based tests for HIV. (a) (left). Congruence between HIV point-of-care-tests (POCT) and lab-based Bio-Rad antibody test. (b) (right). Congruence between HIV point-of-care-tests (POCT) and lab-based Roche antibody test.
Though 7/50 individuals (14%) did not have a long-term band on the Asante assay, all seven individuals reported taking ART and had VLs <320 cpm. Of the two newly diagnosed HIV infections at POC, one (MN050) had a VL of 6355 cpm and the other (MN163) had a VL <320 cpm but positive Bio-Rad and Roche assays, raising the possibility that participant MN163 was an elite controller or taking undisclosed ART. Using a threshold of 1000 cpm, 46/50 (92%) of those LWH were virally suppressed; one individual had a VL of 374 cpm. Four individuals were not virally suppressed, with VLs of 1524, 6355, 6355, and 17812 cpm respectively. Of these, 1/4 reported transactional sex and 1/4 indicated alcohol use before sex; 3/4 did not answer that question. None of the four participants with viremia reported “always” condom use nor had they been sexually active since UDS symptom onset.
Sequencing analysis indicated that two individuals’ samples were HIV subtype A and two were subtype D which is consistent with predominate circulating subtypes in Uganda.17 Neither of the samples sequenced had major resistance mutations present. Table 1 describes the prevalence of curable STIs in men LWH. Among the 41/50 (82%) individuals with one or more curable STIs, 25/50 (50%) had one STI coinfection while 15/50 (30%) had two or more STIs.
Table 1.
Prevalence of urethritis-associated STIs in men with reactive HIV POC test in Kampala, Uganda.
| Total population | ||
|---|---|---|
| N= 50 | # Positive | Prevalence (95% CI) |
| HIV only (no coinfection) | 9 | 18% (8.5, 31.4%) |
| Chlamydia trachomatis | 6 | 12% (4.5%, 24.3%) |
| Mycoplasma genitalium | 7 | 14% (5.8%, 26.7%) |
| Neisseria gonorrhoeae | 31 | 62% (47.2%, 75.3%) |
| Trichomonas vaginalis | 2 | 4% (0.5%, 13.7%) |
Table 2 describes STI coinfections in four participants with VL >1000 cpm.
Table 2.
STI coinfections in participants with HIV VLs >1000 cpm
| Study ID | HIV VL (cpm) | STI coinfection(s) |
|---|---|---|
| MN017 | 1524 | Neisseria gonorrhoeae |
| MN050 | 6355 | Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, syphilis |
| MN098 | 6355 | N/A |
| MN238 | 17912 | Neisseria gonorrhoeae |
Additionally, 232/250 (92.8%) of available serum samples from the parent study underwent additional testing with the Roche Elecsys HIV combi PT 4th Generation test. Overall, concordance between rapid sequence POC testing and lab-based Roche testing was 228/232 (98.3%) (Figure 1(b)). Three samples that were negative with POC testing were subsequently twice reactive on the Roche Elecsys assay and one sample that was positive by POC testing was negative on the Roche Elecsys assay. The VL measurements on these four samples were undetectable. The sensitivity and specificity of POC HIV tests were 92.5% (95% CI: 79.6%–98.4%) and 99.5% (95% CI: 97.2%–100%), respectively.
Discussion
Among unselected high-risk Ugandan men with UDS attending non-specialist government clinics in Kampala, the prevalence of HIV and VLS was greater than the general Ugandan adult population and other Ugandan men. Men LWH were older than those at risk for HIV infection, which may represent cumulative exposure or secular changes in Uganda’s HIV epidemic. Notably, the test for recent HIV acquisition was not congruent with other laboratory tests for HIV and therefore may be of limited utility in estimating recent seroconversion in this population.
These data re-demonstrate that high levels of ART coverage and viral suppression are achievable in urban Kampala. The high prevalence of HIV, curable STIs, and low condom use is typically associated with high risk of HIV transmission. These VLS data, however, suggest that the risk of onward HIV transmission among most participants was low, though 8% of participants were viremic with potentially transmissible virus, especially in the context of UDS. Almost all participants LWH were previously diagnosed and demonstrated VLS, suggesting that HIV testing and treatment opportunities were available to this group. However, 4% of participants were newly diagnosed with HIV by participating in the study, demonstrating the necessity of readily available rapid HIV testing to all with STI syndromes. The implications for HIV prevention are mixed, however. Most were likely noninfectious, though blood and the genital tract are distinct compartments and may contain different levels of virus 18; genital tract HIV VLs are often increased by genital STIs.19 Few studies have specifically reported the impact of STIs on genital shedding in men on ART. In a small study (N = 24) of men on ART who acquired urethritis, two (17%) had seminal VL of 1512 and 5928 cpm.20 In addition, a Malawian study of 111 men established on ART for more than 3 months described 87 episodes of acute urethritis. 15 of 87 episodes (17%) had seminal VLs of >400 cpm and a median seminal plasma VL of 7376 (IQR 1229–50 666). Paired plasma viral loads were undetectable.21 Modern integrase inhibitor-based ART regimens have been shown to rapidly decrease blood and seminal plasma to <40 cpm; this was achieved in 4 weeks in plasma compared with 12 weeks in seminal plasma.22 Assuming people with VL <1000 copies are relatively unlikely to transmit, these data suggest that a minority of participants were at greater risk for onward transmission.2 However, data from large clinical studies, upon which the concept of “Undetectable = Untransmissible” is based, examined transmission dynamics using a plasma VL of either 400 cpm or 200 cpm.19,23 STI coinfections were common in study participants.2 In the HPTN 052 study, a randomized controlled study that investigated the efficacy of HIV treatment as prevention, 97% of 1763 couples were heterosexual and 54% were from Africa. There were no genetically linked transmissions observed while the partner LWH was virally suppressed, defined as <400 cpm.19 Similarly, the PARTNER observational study of 1166 predominantly heterosexual couples did not show any linked transmissions when the person LWH was virally suppressed at <200 cpm.23 Other studies have demonstrated the potential for HIV transmission at VLs between 400 and 1000 cpm. In individuals not on ART, the rate of transmission per 100 person-years was 2.6 (95% CI 0.57-1.13) for VLs between 400 and 3499 cpm and 4.17 (95% CI 0.84-20.65) for VLs between 3500 and 9999 cpm24; in a Ugandan study there were no transmissions with VL <1500 cpm.25 Therefore, by these standards, at least 4/49 (8.2%) would be at risk for transmission and require additional support around ART adherence and other HIV prevention measures.
In 2020, Ugandan population-level data demonstrated 75.4% VLS, exceeding the national goal of population-level VLS of 73%.26 There were large differences in population VLS by age: only 43.5% of men aged 15 to 24 years had VLS compared with 91% VLS in men 55 to 64 years. The high prevalence of VLS in our study (median age 38 years), is comparable with data found in older Ugandan men.26 In contrast to the lack of resistance mutations in this sample, contemporary Ugandan data suggest that the prevalence of ART resistance mutations in people initiating ART was 18.2%.27
This study has limitations; the number LWH was small and only examined men with UDS. Alcohol use was not measured using a pre-validated tool such as AUDIT. One potential limitation is that a single VL measurement may mischaracterise the prevalence of viral suppression in a population.28 It is also possible that VLs with <1000 cpm harboured resistance mutations that were not detected in this analysis. In addition, we were unable to compare HIV VL or viability in blood and genital compartments. Any study of sexual behaviour is likely to be subject to social desirability bias which may overestimate self-reported condom use and adherence to ART.29 Recall and other, unmeasured, biases are likely to have also been present.
Despite these limitations, the level of VLS demonstrates the efficacy of ART interventions in urban Ugandan men LWH who present for STI services. The agreement between POC and laboratory-based testing highlights the excellent performance characteristics of sequential HIV POCTs in clinical settings in Kampala, Uganda as found in many other settings. In a 2016 meta-analysis, the pooled sensitivity of HIV POC tests in low-income settings was 97.7% (95% CI: 95.2%—98.9%) compared with gold standard laboratory testing.30
Curable STIs were common, likely contributing to adverse sexual and reproductive health outcomes at an individual and community level. These data suggest that most men LWH were on ART and thus less likely to transmit HIV despite engaging in high-risk sexual behaviours. The synergistic effect of UDS, however, may potentiate HIV transmission risk even in the presence of relatively low-level viremia.21 Therefore, efforts should focus on ensuring VLS in the minority of those with detectable plasma viral loads and developing targeted interventions to decrease STI coinfections. Further work is required to investigate predictors of VLS in Ugandan men and to examine the feasibility and added value of POC HIV RNA assays to increase diagnostic yield when screening men with UDS for HIV, particularly in those at risk with a negative POC antibody assay.
Acknowledgements
We thank participants and participating clinics, and Dr Lori Sokoll (Johns Hopkins School of Medicine) for sharing her expertise and coordination of testing.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the extramural support from the National Institute of Biomedical Imaging and Bioengineering [U54 EB007958 to Y.C.M.]. Other support was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fleming DT and Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999; 75: 3–17. DOI: 10.1136/sti.75.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Council OD and Chen JS. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: the biologic basis for epidemiologic synergy. J Int AIDS Soc 2019; 22(Suppl 6): 9. DOI: 10.1002/JIA2.25355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uganda AIDS Commission. Presidential Fast Track Initiative on Ending HIV & AIDS in Uganda: A Presidential Handbook. Kampala, Uganda: Uganda AIDS Commission. 2020. 1–20. https://uac.go.ug/index.php?option=com_content&view=article&id=14:hiv-prevention-5&catid=8&Itemid=101#:~:text=OnJune6th2017%2CPresidentthreatinUgandaby2030 [Google Scholar]
- 4.Nabukenya AM and Matovu JKB. Correlates of HIV status awareness among older adults in Uganda: results from a nationally representative survey 11 medical and health sciences 1117 public health and health services. BMC Publ Health 2018; 18(1): 1–8. DOI: 10.1186/S12889-018-6027-Z/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha JH, Van Lith LM, Mallalieu EC, et al. Gendered relationship between HIV stigma and HIV testing among men and women in Mozambique: a cross-sectional study to inform a stigma reduction and male-targeted HIV testing intervention. BMJ Open 2019; 9(10): 18. DOI: 10.1136/BMJOPEN-2019-029748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexovitz KA, Merchant RC, Clark MA, et al. Discordance of voluntary HIV testing with HIV sexual risk-taking and self-perceived HIV infection risk among social media-using black, Hispanic, and white young-men-who-have-sex-with-men (YMSM). AIDS Care 2018; 30(1): 81–85. DOI: 10.1080/09540121.2017.1381327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. HIV and women: viral suppression. Atlanta, USA: U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/group/gender/women/viral-suppression.html, Accessed 30 January 2022. [Google Scholar]
- 8.Kaleebu P, Kirungi W, Watera C, et al. Virological response and antiretroviral drug resistance emerging during antiretroviral therapy at three treatment centers in Uganda. PLoS One 2015; 10(12). DOI: 10.1371/JOURNAL.PONE.0145536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. Country factsheets: Uganda 2022. Geneva Switzerland: The Joint United Nations Programme on HIV and AIDS, Accessed July 27, 2023. https://www.unaids.org/en/regionscountries/countries/uganda [Google Scholar]
- 10.PEPFAR. Uganda Country Operational plan 2022: Strategic Direction summary. Washington, D.C., USA: U.S. Department of State, 2022, https://www.state.gov/wp-content/uploads/2022/09/Uganda-COP22-SDS.pdf. [Google Scholar]
- 11.Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. Geneva, Switzerland: World Health Organization. Accessed April 3, 2023.https://www.who.int/publications/i/item/9789240022232 [PubMed] [Google Scholar]
- 12.Consolidated guidelines for prevention and treatment of HIV in Uganda. Kampala, Uganda: Republic of Uganda Ministry of Health. 2016. [Google Scholar]
- 13.Hamill MM, Onzia A, Wang TH, et al. High burden of untreated syphilis, drug resistant neisseria gonorrhoeae, and other sexually transmitted infections in men with urethral discharge syndrome in Kampala, Uganda. BMC Infect Dis 2022; 22(1): 3. DOI: 10.1186/S12879-022-07431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakooza F, Musinguzi P, Workneh M, et al. Implementation of a standardised and quality-assured enhanced gonococcal antimicrobial surveillance programme in accordance with WHO protocols in Kampala, Uganda. Sex Transm Infect 2021; 97(4): 312–316. DOI: 10.1136/SEXTRANS-2020-054581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtney CR, Mayr L, Nanfack AJ, et al. Contrasting antibody responses to intrasubtype superinfection with CRF02_AG. PLoS One 2017; 12(3): e0173705. DOI: 10.1371/JOURNAL.PONE.0173705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee S, Gonzales M, Kantor R, et al. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Research 2003; 31(1): 298–303. DOI: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaleebu P, French N, Mahe C, et al. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large Cohort of HIV-1—positive persons in Uganda. J Infect Dis 2002; 185(9): 1244–1250. DOI: 10.1086/340130. [DOI] [PubMed] [Google Scholar]
- 18.Kariuki SM, Selhorst P, Anthony C, et al. Compartmentalization and clonal amplification of HIV-1 in the male genital tract characterized using next-generation sequencing. J Virol 2020; 94(12): 13. DOI: 10.1128/JVI.00229-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365(6): 493–505. DOI: 10.1056/NEJMOA1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadiq ST, Taylor S, Kaye S, et al. The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. AIDS 2002; 16(2): 219–225. DOI: 10.1097/00002030-200201250-00011. [DOI] [PubMed] [Google Scholar]
- 21.Chen JS, Matoga M, Massa C, et al. Effects of urethritis on human immunodeficiency virus (HIV) in semen: implications for HIV prevention and cure. Clin Infect Dis 2021; 73(7): e2000–e2004. DOI: 10.1093/CID/CIAA1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imaz A, Martinez-Picado J, Niubó J, et al. HIV-1-RNA decay and dolutegravir concentrations in semen of patients starting a first antiretroviral regimen. J Infect Dis 2016; 214(10): 1512–1519. DOI: 10.1093/INFDIS/JIW406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316(2): 171–181. DOI: 10.1001/JAMA.2016.5148. [DOI] [PubMed] [Google Scholar]
- 24.Attia S, Egger M, Müller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: Systematic review and meta-analysis. AIDS 2009; 23(11): 1397–1404. DOI: 10.1097/QAD.0B013E32832B7DCA. [DOI] [PubMed] [Google Scholar]
- 25.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai project study group. N Engl J Med 2000; 342(13): 921–929. DOI: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 26.Ocero JRA. Release of Preliminary results of the 2020 Uganda population-based HIV impact Assessment. Kampala, Uganda: Republic of Uganda Ministry of Health, 2020. [Google Scholar]
- 27.Watera C, Ssemwanga D, Namayanja G, et al. HIV drug resistance among adults initiating antiretroviral therapy in Uganda. J Antimicrob Chemother 2021; 76(9): 2407–2414. DOI: 10.1093/JAC/DKAB159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks G, Patel U, Stirratt M, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr 2016; 73(2): 205–212. DOI: 10.1097/QAI.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao A, Tobin K, Davey-Rothwell M, et al. Social desirability bias and prevalence of sexual HIV risk behaviors among people who use drugs in Baltimore, Maryland: implications for identifying individuals prone to underreporting sexual risk behaviors. AIDS Behav 2017; 21(7): 2207–2214. DOI: 10.1007/S10461-017-1792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan WS, Chow EPF, Fairley CK, et al. Sensitivity of HIV rapid tests compared with fourth-generation enzyme immunoassays or HIV RNA tests. AIDS 2016; 30(12): 1951–1960. DOI: 10.1097/QAD.0000000000001134. [DOI] [PubMed] [Google Scholar]


