Abstract
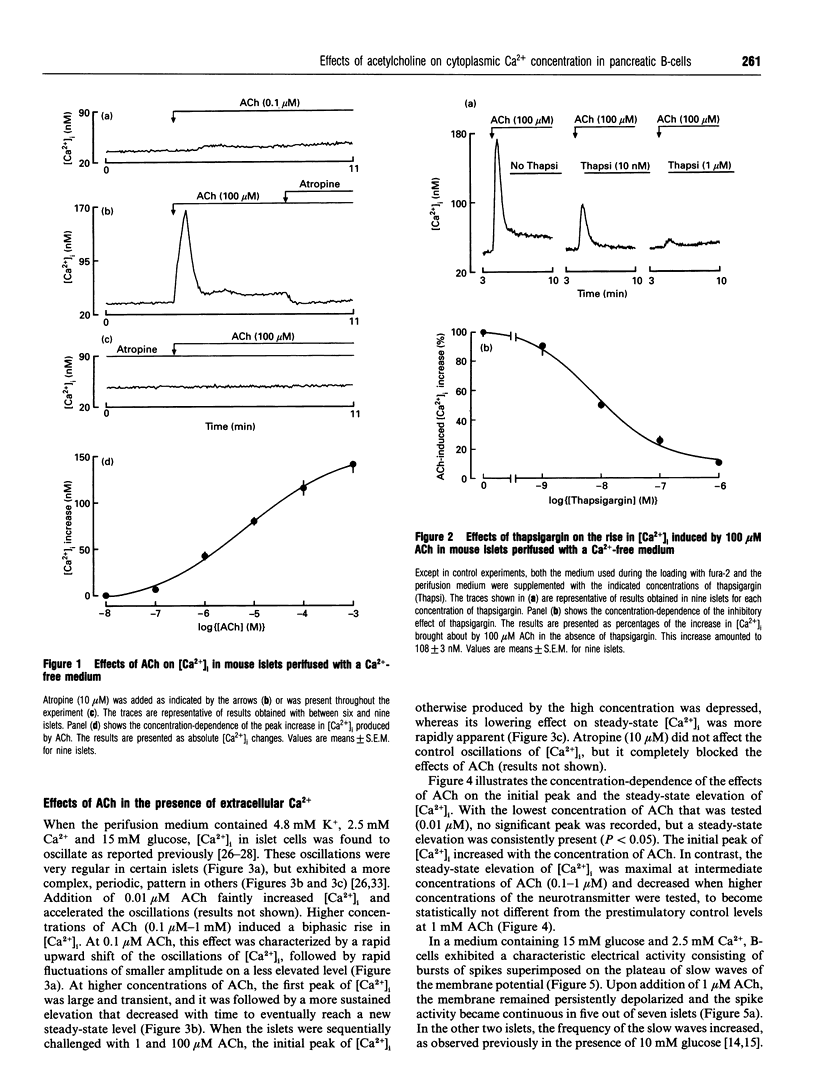
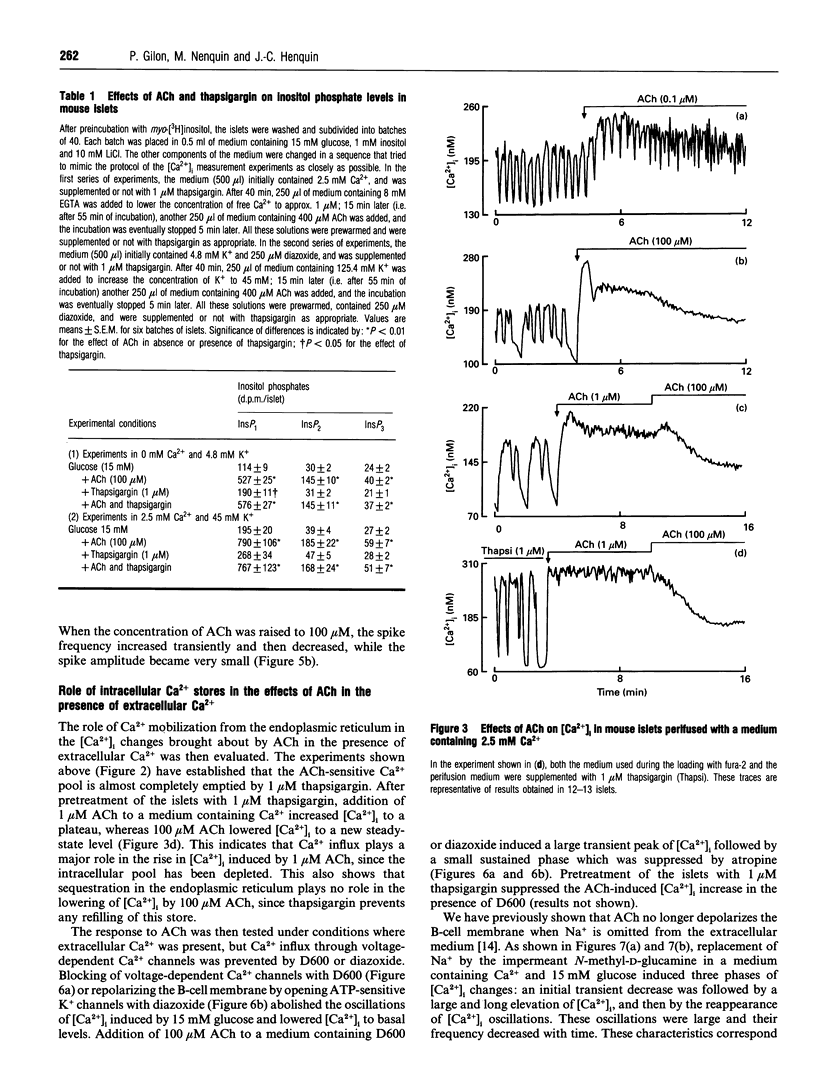
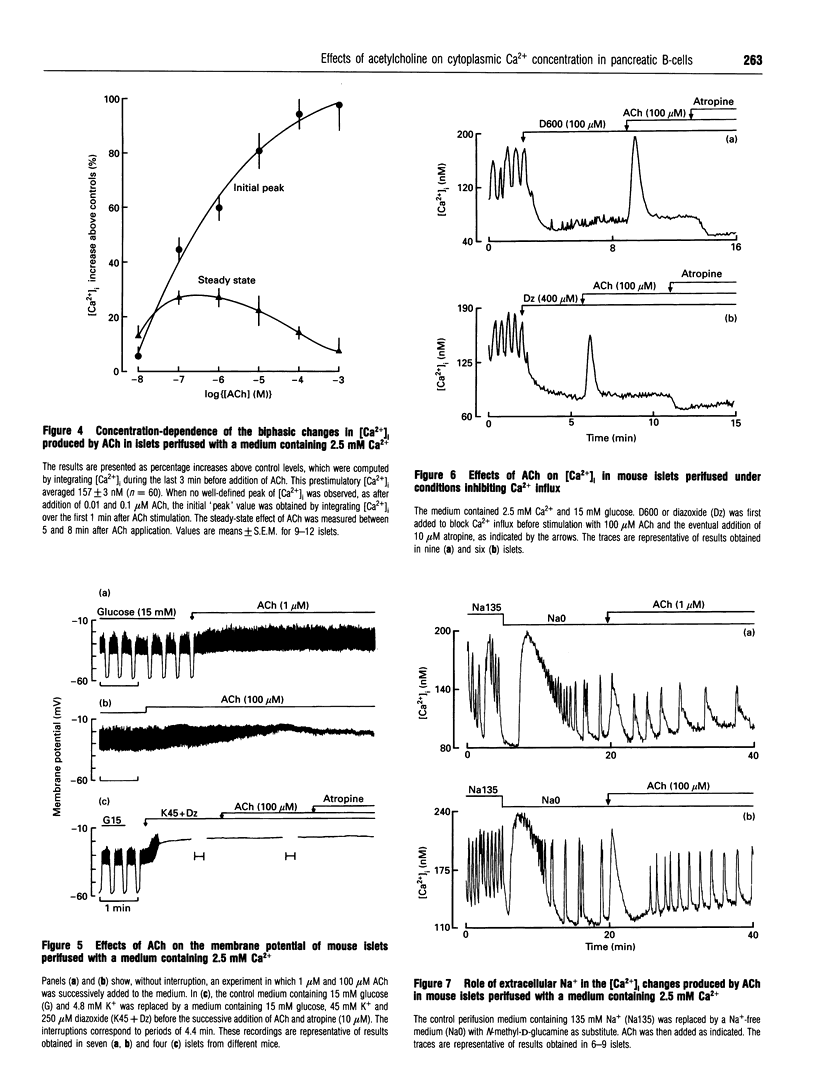
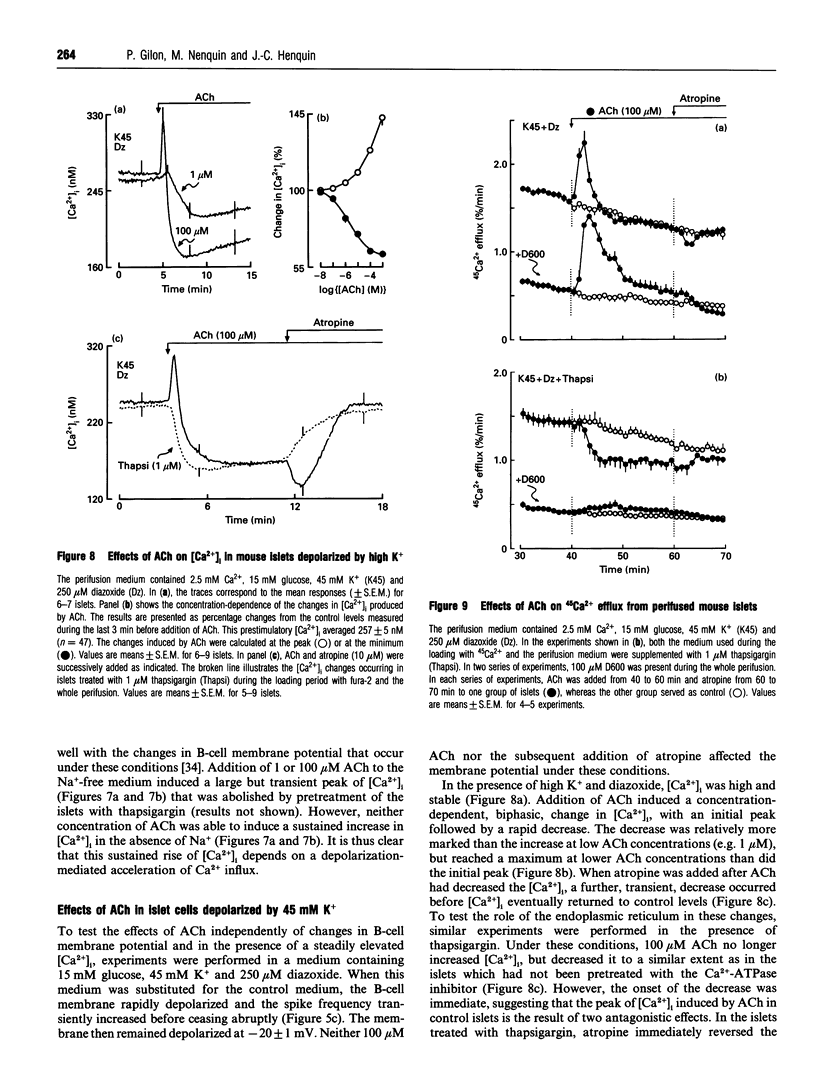
Mouse pancreatic islets were used to investigate how muscarinic stimulation influences the cytoplasmic Ca2+ concentration ([Ca2+]i) in insulin-secreting B-cells. In the absence of extracellular Ca2+, acetylcholine (ACh) triggered a transient, concentration-dependent and thapsigargin-inhibited increase in [Ca2+]i. In the presence of extracellular Ca2+ and 15 mM glucose, ACh induced a biphasic rise in [Ca2+]i. The initial, transient phase increased with the concentration of ACh, whereas the second, sustained, phase was higher at low (0.1-1 microM) than at high (> or = 10 microM) concentrations of ACh. Thapsigargin attenuated (did not suppress) the first phase of the [Ca2+]i rise and did not affect the sustained response. This sustained rise was inhibited by omission of extracellular Na+ (which prevents the depolarizing action of ACh) and by D600 or diazoxide (which prevent activation of voltage-dependent Ca2+ channels). During steady-state stimulation, the Ca2+ action potentials in B-cells were stimulated by 1 microM ACh but inhibited by 100 microM ACh. When B-cells were depolarized by 45 mM K+, ACh induced a concentration-dependent, biphasic change in [Ca2+]i, consisting of a first peak rapidly followed by a decrease. Thapsigargin suppressed the peak without affecting the drop in [Ca2+]i. Measurements of 45Ca2+ efflux under similar conditions indicated that ACh decreases Ca2+ influx and slightly increases the efflux. All effects of ACh were blocked by atropine. In conclusion, three mechanisms at least are involved in the biphasic change in [Ca2+]i that muscarinic stimulation exerts in excitable pancreatic B-cells. A mobilization of Ca2+ from the endoplasmic reticulum contributes significantly to the first peak, but little to the steady-state rise in [Ca2+]i. This second phase results from an influx of Ca2+ through voltage-dependent Ca2+ channels activated by a Na(+)-dependent depolarization. However, when high concentrations of ACh are used, Ca2+ influx is attenuated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén B., Taborsky G. J., Jr, Porte D., Jr Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986 Dec;29(12):827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Welsh M., Welsh N., Berggren P. O. Effects of protein kinase C activation on the regulation of the stimulus-secretion coupling in pancreatic beta-cells. Biochem J. 1989 Nov 15;264(1):207–215. doi: 10.1042/bj2640207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Proks P., Smith P. A., Ammälä C., Bokvist K., Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. J Cell Biochem. 1994;55 (Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Nutrient and hormone-neurotransmitter stimuli induce hydrolysis of polyphosphoinositides in rat pancreatic islets. Endocrinology. 1984 Nov;115(5):1814–1820. doi: 10.1210/endo-115-5-1814. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prugue M. L., Davison A. G. Evidence for phosphatidylinositol hydrolysis in pancreatic islets stimulated with carbamoylcholine. Kinetic analysis of inositol polyphosphate metabolism. Biochem J. 1992 Jul 15;285(Pt 2):541–549. doi: 10.1042/bj2850541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozem M., Nenquin M., Henquin J. C. The ionic, electrical, and secretory effects of protein kinase C activation in mouse pancreatic B-cells: studies with a phorbol ester. Endocrinology. 1987 Sep;121(3):1025–1033. doi: 10.1210/endo-121-3-1025. [DOI] [PubMed] [Google Scholar]
- Caulfield M. P. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993 Jun;58(3):319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Gagerman E., Sehlin J., Täljedal I. B. Effects of acetylcholine on ion fluxes and chlorotetracycline fluorescence in pancreatic islets. J Physiol. 1980 Mar;300:505–513. doi: 10.1113/jphysiol.1980.sp013175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z. Y., Drews G., Nenquin M., Plant T. D., Henquin J. C. Mechanisms of the stimulation of insulin release by arginine-vasopressin in normal mouse islets. J Biol Chem. 1990 Sep 15;265(26):15724–15730. [PubMed] [Google Scholar]
- Gao Z. Y., Gilon P., Henquin J. C. The role of protein kinase-C in signal transduction through vasopressin and acetylcholine receptors in pancreatic B-cells from normal mouse. Endocrinology. 1994 Jul;135(1):191–199. doi: 10.1210/endo.135.1.8013353. [DOI] [PubMed] [Google Scholar]
- Garcia M. C., Hermans M. P., Henquin J. C. Glucose-, calcium- and concentration-dependence of acetylcholine stimulation of insulin release and ionic fluxes in mouse islets. Biochem J. 1988 Aug 15;254(1):211–218. doi: 10.1042/bj2540211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind J. F., Hiriart M., Glennon M. C., Najafi H., Corkey B. E., Matschinsky F. M., Prentki M. Selective activation of Ca2+ influx by extracellular ATP in a pancreatic beta-cell line (HIT). Biochim Biophys Acta. 1989 Jun 15;1012(1):107–115. doi: 10.1016/0167-4889(89)90018-9. [DOI] [PubMed] [Google Scholar]
- Gilon P., Henquin J. C. Activation of muscarinic receptors increases the concentration of free Na+ in mouse pancreatic B-cells. FEBS Lett. 1993 Jan 11;315(3):353–356. doi: 10.1016/0014-5793(93)81193-4. [DOI] [PubMed] [Google Scholar]
- Gilon P., Henquin J. C. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem. 1992 Oct 15;267(29):20713–20720. [PubMed] [Google Scholar]
- Gilon P., Jonas J. C., Henquin J. C. Culture duration and conditions affect the oscillations of cytoplasmic calcium concentration induced by glucose in mouse pancreatic islets. Diabetologia. 1994 Oct;37(10):1007–1014. doi: 10.1007/BF00400464. [DOI] [PubMed] [Google Scholar]
- Gilon P., Shepherd R. M., Henquin J. C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem. 1993 Oct 25;268(30):22265–22268. [PubMed] [Google Scholar]
- Glennon M. C., Bird G. S., Kwan C. Y., Putney J. W., Jr Actions of vasopressin and the Ca(2+)-ATPase inhibitor, thapsigargin, on Ca2+ signaling in hepatocytes. J Biol Chem. 1992 Apr 25;267(12):8230–8233. [PubMed] [Google Scholar]
- Grapengiesser E., Gylfe E., Hellman B. Three types of cytoplasmic Ca2+ oscillations in stimulated pancreatic beta-cells. Arch Biochem Biophys. 1989 Jan;268(1):404–407. doi: 10.1016/0003-9861(89)90602-4. [DOI] [PubMed] [Google Scholar]
- Gylfe E. Carbachol induces sustained glucose-dependent oscillations of cytoplasmic Ca2+ in hyperpolarized pancreatic beta cells. Pflugers Arch. 1991 Dec;419(6):639–643. doi: 10.1007/BF00370308. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Garcia M. C., Bozem M., Hermans M. P., Nenquin M. Muscarinic control of pancreatic B cell function involves sodium-dependent depolarization and calcium influx. Endocrinology. 1988 May;122(5):2134–2142. doi: 10.1210/endo-122-5-2134. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Nenquin M. The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett. 1988 Aug 15;236(1):89–92. doi: 10.1016/0014-5793(88)80290-4. [DOI] [PubMed] [Google Scholar]
- Hermans M. P., Schmeer W., Henquin J. C. Modulation of the effect of acetylcholine on insulin release by the membrane potential of B cells. Endocrinology. 1987 May;120(5):1765–1773. doi: 10.1210/endo-120-5-1765. [DOI] [PubMed] [Google Scholar]
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992 Aug;9(2):187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Chalk J. G., Ashcroft S. J. The role of cytosolic free Ca2+ and protein kinase C in acetylcholine-induced insulin release in the clonal beta-cell line, HIT-T15. Biochem J. 1990 Apr 1;267(1):227–232. doi: 10.1042/bj2670227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Protein kinase C and the regulation of insulin secretion from pancreatic B cells. J Mol Endocrinol. 1991 Apr;6(2):121–127. doi: 10.1677/jme.0.0060121. [DOI] [PubMed] [Google Scholar]
- Jones S. V. Muscarinic receptor subtypes: modulation of ion channels. Life Sci. 1993;52(5-6):457–464. doi: 10.1016/0024-3205(93)90302-j. [DOI] [PubMed] [Google Scholar]
- Konrad R. J., Jolly Y. C., Major C., Wolf B. A. Carbachol stimulation of phospholipase A2 and insulin secretion in pancreatic islets. Biochem J. 1992 Oct 1;287(Pt 1):283–290. doi: 10.1042/bj2870283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P. Membrane potential measurements in pancreatic beta cells with intracellular microelectrodes. Methods Enzymol. 1990;192:235–246. doi: 10.1016/0076-6879(90)92073-m. [DOI] [PubMed] [Google Scholar]
- Metz S. A. The pancreatic islet as Rubik's Cube. Is phospholipid hydrolysis a piece of the puzzle? Diabetes. 1991 Dec;40(12):1565–1573. doi: 10.2337/diab.40.12.1565. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the role of inositol trisphosphate in the regulation of insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Jun 15;228(3):713–718. doi: 10.1042/bj2280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenquin M., Awouters P., Mathot F., Henquin J. C. Distinct effects of acetylcholine and glucose on 45calcium and 86rubidium efflux from mouse pancreatic islets. FEBS Lett. 1984 Oct 29;176(2):457–461. doi: 10.1016/0014-5793(84)81218-1. [DOI] [PubMed] [Google Scholar]
- Prentki M., Glennon M. C., Thomas A. P., Morris R. L., Matschinsky F. M., Corkey B. E. Cell-specific patterns of oscillating free Ca2+ in carbamylcholine-stimulated insulinoma cells. J Biol Chem. 1988 Aug 15;263(23):11044–11047. [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Ramanadham S., Gross R. W., Han X., Turk J. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta-cell cytosolic calcium ion concentration. Biochemistry. 1993 Jan 12;32(1):337–346. doi: 10.1021/bi00052a042. [DOI] [PubMed] [Google Scholar]
- Roe M. W., Lancaster M. E., Mertz R. J., Worley J. F., 3rd, Dukes I. D. Voltage-dependent intracellular calcium release from mouse islets stimulated by glucose. J Biol Chem. 1993 May 15;268(14):9953–9956. [PubMed] [Google Scholar]
- Saha S., Hellman B. Carbachol has opposite effects to glucose in raising the sodium content of pancreatic islets. Eur J Pharmacol. 1991 Nov 5;204(2):211–215. doi: 10.1016/0014-2999(91)90707-w. [DOI] [PubMed] [Google Scholar]
- Santos R. M., Rosario L. M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991 May;418(4):417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrés J. V., Ripoll C., Soria B. Evidence that muscarinic potentiation of insulin release is initiated by an early transient calcium entry. FEBS Lett. 1988 Apr 11;231(1):143–147. doi: 10.1016/0014-5793(88)80719-1. [DOI] [PubMed] [Google Scholar]
- Tepel M., Kühnapfel S., Theilmeier G., Teupe C., Schlotmann R., Zidek W. Filling state of intracellular Ca2+ pools triggers trans plasma membrane Na+ and Ca2+ influx by a tyrosine kinase-dependent pathway. J Biol Chem. 1994 Oct 21;269(42):26239–26242. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theler J. M., Mollard P., Guérineau N., Vacher P., Pralong W. F., Schlegel W., Wollheim C. B. Video imaging of cytosolic Ca2+ in pancreatic beta-cells stimulated by glucose, carbachol, and ATP. J Biol Chem. 1992 Sep 5;267(25):18110–18117. [PubMed] [Google Scholar]
- Turk J., Gross R. W., Ramanadham S. Amplification of insulin secretion by lipid messengers. Diabetes. 1993 Mar;42(3):367–374. doi: 10.2337/diab.42.3.367. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M., Santos R. M., Contreras D., Soria B., Rosario L. M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett. 1989 Dec 18;259(1):19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Verspohl E. J., Tacke R., Mutschler E., Lambrecht G. Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol. 1990 Mar 27;178(3):303–311. doi: 10.1016/0014-2999(90)90109-j. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Wright L. C., Machan C. L., Allen B. G., Conigrave A. D., Roufogalis B. D. Protein kinase C phosphorylates the carboxyl terminus of the plasma membrane Ca(2+)-ATPase from human erythrocytes. J Biol Chem. 1991 May 15;266(14):9078–9085. [PubMed] [Google Scholar]
- Weng L., Davies M., Ashcroft S. J. Effects of cholinergic agonists on diacylglycerol and intracellular calcium levels in pancreatic beta-cells. Cell Signal. 1993 Nov;5(6):777–786. doi: 10.1016/0898-6568(93)90038-n. [DOI] [PubMed] [Google Scholar]
- Worley J. F., 3rd, McIntyre M. S., Spencer B., Dukes I. D. Depletion of intracellular Ca2+ stores activates a maitotoxin-sensitive nonselective cationic current in beta-cells. J Biol Chem. 1994 Dec 23;269(51):32055–32058. [PubMed] [Google Scholar]
- Zawalich W. S., Rasmussen H. Control of insulin secretion: a model involving Ca2+, cAMP and diacylglycerol. Mol Cell Endocrinol. 1990 Apr 17;70(2):119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]
- de Miguel R., Tamagawa T., Schmeer W., Nenquin M., Henquin J. C. Effects of acute sodium omission on insulin release, ionic flux and membrane potential in mouse pancreatic B-cells. Biochim Biophys Acta. 1988 Apr 25;969(2):198–207. doi: 10.1016/0167-4889(88)90076-6. [DOI] [PubMed] [Google Scholar]


