Abstract
Background: Patterns of psoriasis characteristics by sex are not fully understood. Objective: Evaluate patient characteristics by sex at enrollment in the Psoriasis Longitudinal Assessment and Registry (PSOLAR). Methods: Two PSOLAR cohorts were evaluated by sex: patients who were biologic-naïve (n = 3329) and patients who were systemic therapy-naïve (n = 1290) at entry. Baseline demographic and disease characteristics, medical history, social activity, and lifestyle risk factors were collected for all patients and were compared between males and females using an independent samples t-test for continuous variables and chi-square tests for categorical variables. Results: In both cohorts, disease duration was similar for males and females; however, disease severity based on baseline Physician Global Assessment and body surface area of psoriasis was greater in males versus females (P < .05). Baseline Dermatology Life Quality Index scores were higher for biologic-naïve females than for males (P = .008). In both cohorts, females were significantly more likely than males to have a history of anxiety, depression, and cancer excluding nonmelanoma skin cancer, to have received systemic steroid therapy, and to have health insurance; males were significantly more likely than females to have a history of cardiovascular disease, smoking, and alcohol consumption, and to work full time. Conclusions: Based on patient data obtained at entry into PSOLAR, significant differences in psoriasis disease characteristics, and medical, family, and social history-related variables were observed between males and females. Among systemic therapy-naïve patients, there was a greater negative impact on quality of life for females compared with males, despite generally lower objective disease severity for females.
Keywords: psoriasis, gender, sex, PSOLAR, biologic, systemic, quality of life, social history
Introduction
Sex is an important factor that affects health and susceptibility to illness throughout a person’s lifetime. 1 Sex-related differences in physiological characteristics, such as anatomy, reproductive function, hormones, and genetics, as well as environmental, lifestyle, behavioral, and socioeconomic factors, are known to affect life expectancy and risk for developing cardiovascular, neurodegenerative, autoimmune, metabolic, and infectious diseases.1-3
In psoriasis and other immune-mediated diseases, levels of proinflammatory cytokines associated with disease pathogenesis have been shown to differ between males and females and to fluctuate with sex hormone levels.2,4 Additionally, studies have identified differences between males and females in skin thickness, sebum content, hydration, pH, vasculature, and pigmentation, which may affect manifestations of psoriasis.3,5
Psoriasis affects an estimated 2% to 4% of adults worldwide, and overall prevalence estimates are roughly the same for males and females.6-10 However, results of numerous studies suggest that there are sex-based differences in disease severity and in the prevalence of comorbidities including diabetes, metabolic syndrome, obesity, pruritus, depression, psychological distress, smoking, alcohol consumption, psoriatic arthritis, and other clinical and psychosocial characteristics among patients with psoriasis.11-21
Most sex-related psoriasis studies published to date have been small and/or limited to a single center or geographic region;12-21 therefore, significant gaps remain in our understanding of potential differences between males and females with psoriasis. This study analyzes patterns of psoriasis characteristics by sex using data collected at the time of enrollment in the Psoriasis Longitudinal Assessment and Registry (PSOLAR), a large, international, disease-based registry that collects data on patients receiving or eligible to receive systemic or biologic treatment for psoriasis.22,23
Patients and Methods
Data Source and Study Population
PSOLAR is a longitudinal, prospective, observational registry that was established in 2007 and consists of a cohort of adult patients (≥18 years of age) with moderate to severe psoriasis who were receiving or were candidates to receive systemic therapy at clinics in North America, South America, and Europe.22,23 Demographic and disease characteristics and psoriasis treatment history were collected at baseline (registry entry). Registry enrollment began in 2007 and was complete in 2013. Efficacy and safety data are collected every 6 months. This analysis is based on data reported at baseline.
The registry protocol was approved for all study sites by appropriate institutional review boards or ethics committees. All patients provided written informed consent at the start of the registry.
Assessments
Two subpopulations of patients were evaluated by sex in this cross-sectional study: patients who were biologic-naïve (n = 3329) and patients who were systemic therapy-naïve (n = 1290) at enrollment. Systemic therapy-naïve patients were defined as those with no prior or current use of oral immunosuppressive medications, phototherapy, or biologic therapy. Demographic and disease characteristics, medical history, social activity history, and lifestyle risk factors were collected at registry entry for all patients. Patients who had exposure to biologic or systemic therapies at baseline were excluded from this analysis because these treatments have the potential to confound results (e.g., by changing psoriasis severity or affecting comorbid conditions that were measured in this analysis).
Analyses
Prespecified covariates for analysis included age, race, and body mass index (BMI). Within each subpopulation (biologic- and systemic therapy-naïve), males and females were compared using an independent samples t-test (expressed as mean ± standard deviation) for continuous variables and chi-square tests for categorical variables. P-values of ≤.05 were considered statistically significant. Multivariate regression analysis was performed to determine if differences in variables were due to sex or other covariates, such as age.
Results
Psoriasis Disease Characteristics by Sex
Psoriasis disease characteristics by sex for the biologic-naïve and systemic therapy-naïve cohorts are shown in Table 1. In both cohorts, disease duration (years since psoriasis diagnosis) was similar for males and females. Measures of disease severity, including baseline Physician Global Assessment (PGA) score, historical peak PGA score, baseline body surface area (BSA) of psoriasis, and historical peak BSA, were all numerically higher for males than for females in both cohorts; in most cases, the difference was statistically significant (P < .05). Baseline Dermatology Life Quality Index (DLQI) scores were higher for females than for males in both cohorts. The difference between females and males was statistically significant (P = .008) only for the biologic-naïve cohort. Taken together, these results suggest that, on average, severity of psoriasis is greater for males than for females, but that the impact of disease on quality of life (i.e., DLQI) is greater for females.
Table 1.
Demographic and psoriasis disease characteristics in males and females.
| Characteristic | Biologic-naïve cohort (n = 3329) | Systemic therapy-naïve cohort (n = 1290) | ||||
|---|---|---|---|---|---|---|
| Males (n = 1726) | Females (n = 1603) | P-value a | Males (n = 682) | Females (n = 608) | P-value a | |
| Age, mean (SD), years | 49.5 (14.9) | 49.7 (15.6) | .623 | 46.7 (15.0) | 48.1 (15.4) | .096 |
| Race/ethnicity, n (%) | <.001 | <.001 | ||||
| White | 1401 (81.2) | 1360 (84.8) | 535 (78.4) | 501 (82.4) | ||
| Black | 56 (3.2) | 78 (4.9) | 22 (3.2) | 41 (6.7) | ||
| Asian | 75 (4.3) | 42 (2.6) | 28 (4.1) | 12 (2.0) | ||
| Hispanic or Latino | 144 (8.3) | 95 (5.9) | 80 (11.7) | 42 (6.9) | ||
| Other | 50 (2.9) | 28 (1.7) | 17 (2.5) | 12 (2.0) | ||
| Geographic region, n (%) | .003 | .128 | ||||
| North America | 1469 (85.1) | 1428 (89.1) | 612 (89.7) | 562 (92.4) | ||
| Europe | 226 (13.1) | 153 (9.5) | 55 (8.1) | 40 (6.6) | ||
| Latin America | 31 (1.8) | 22 (1.4) | 15 (2.2) | 6 (1.0) | ||
| Disease duration, years b | 11.51 | 11.61 | .836 | 7.58 | 7.87 | .678 |
| Baseline DLQI score (0-30) b | 9.16 | 9.82 | .008 | 10.91 | 11.45 | .173 |
| Baseline PGA score (0-5) b | 2.56 | 2.39 | <.001 | 2.65 | 2.51 | .018 |
| Historical peak PGA score (0-5) b | 2.95 | 2.87 | .084 | 2.88 | 2.69 | .007 |
| Baseline BSA, m2 b | 19.89 | 16.62 | <.001 | 20.01 | 15.97 | <.001 |
| Historical peak BSA, m2 b | 27.02 | 23.78 | .003 | 24.14 | 19.61 | .006 |
BSA, body surface area; DLQI, Dermatology Life Quality Index; PGA, Physician Global Assessment; SD, standard deviation.
aP-values for comparison of continuous variables between sexes were based on an independent sample t-test; P-values for categorical variables were obtained from exact Pearson chi-square tests.
bResults are reported as least-square mean values, adjusted for age, race, and body mass index.
Medical History by Sex
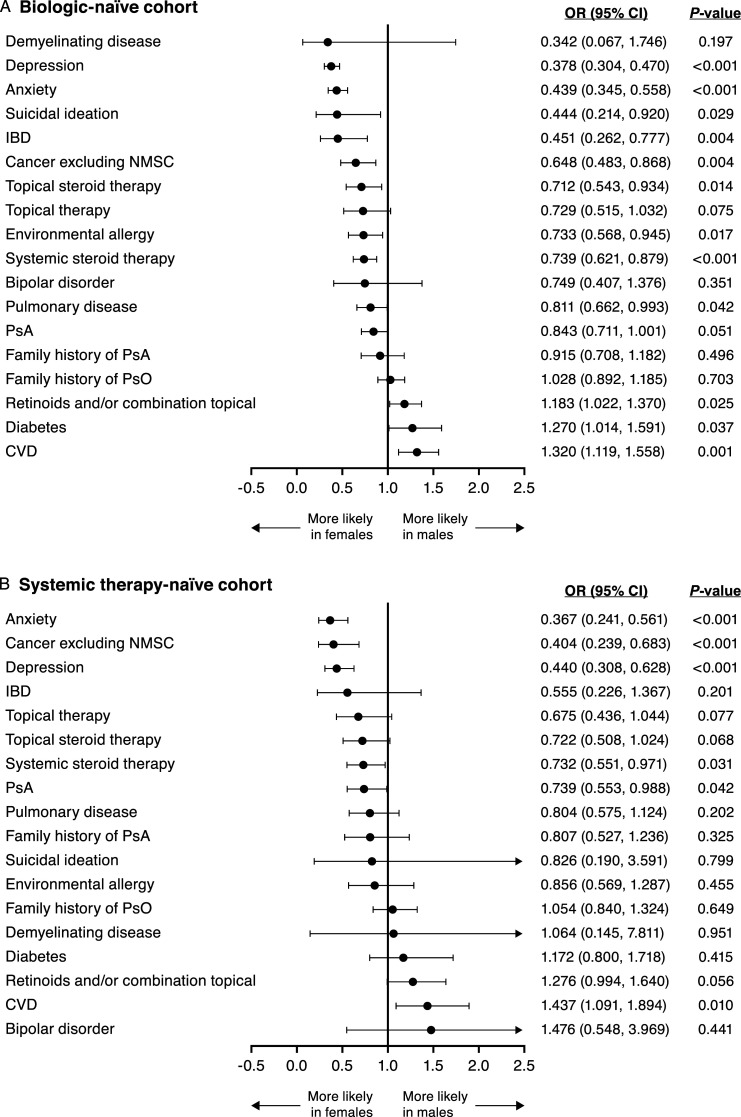
After adjusting for the covariates of age, race, and BMI, sex was observed to be an independent predictor of several specific medical and family history variables (Figure 1). In both the biologic- and the systemic therapy-naïve cohorts, females were significantly more likely than males to have a history of anxiety, depression, and cancer excluding nonmelanoma skin cancer. Females were also significantly more likely than males to have received systemic steroid therapy.
Figure 1.
Adjusted OR (95% CI) for medical and family history variables in (A) the biologic-naïve cohort and (B) the systemic therapy-naïve cohort. CI, confidence interval; CVD, cardiovascular disease; IBD, inflammatory bowel disease; NMSC, nonmelanoma skin cancer; OR, odds ratio; PsA, psoriatic arthritis; PsO, psoriasis.
In the biologic-naïve cohort, females were also significantly more likely than males to have a history of environmental allergies, inflammatory bowel disease, pulmonary disease, and suicidal ideation. Additionally, biologic-naïve females were more likely to have received topical steroid therapy. Similar trends were observed for these variables in the systemic therapy-naïve cohort, but results did not reach statistical significance (P > .05). Furthermore, in the systemic therapy-naïve cohort, females were significantly more likely than males to have a history of psoriatic arthritis (P = .042), and differences in psoriatic arthritis history approached significance in the biologic-naïve cohort (P = .051).
Conversely, in both the biologic- and systemic therapy-naïve cohorts, males were significantly more likely than females to have a history of cardiovascular disease. In the biologic-naïve cohort, males were significantly more likely to have a history of diabetes and to have received treatment with retinoids and/or combination topical therapies. Similar trends were observed for these variables in the systemic therapy-naïve cohort, but results did not reach statistical significance (P > .05).
Schizophrenia was significantly more common among males than females in the biologic-naïve cohort, and a similar trend was seen in the systemic therapy-naïve cohort. However, results for schizophrenia are not shown graphically in Figure 1 because the adjusted odds ratio (OR) in the biologic-naïve cohort was much larger than that for any other measured variable, and 95% confidence intervals (CIs) were very wide for both cohorts (biologic-naïve cohort, adjusted OR = 8.519 [95% CI: 1.047, 69.307]; P = .045; systemic therapy-naïve cohort, adjusted OR = 1.727 [95% CI: .143, 20.885]; P = .667).
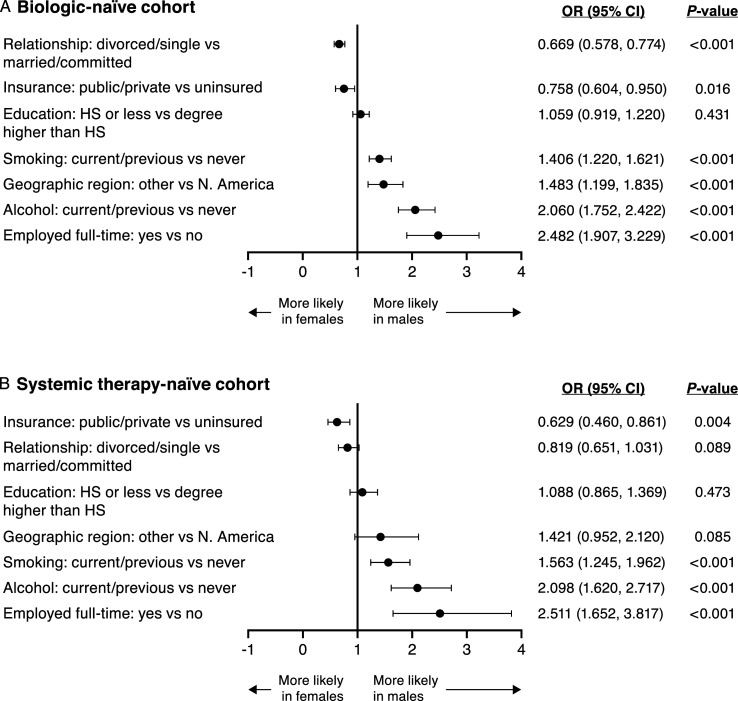
Social History by Sex
Sex was an independent predictor of several social history-related variables after adjusting for the covariates of age, race, and BMI (Figure 2). In both the biologic- and the systemic therapy-naïve cohorts, females were more likely than males to be divorced or single than married or in a committed relationship (P < .001 for the biologic-naïve cohort; P = .089 for the systemic therapy-naïve cohort). Conversely, in both cohorts, males were significantly more likely than females to be a current or past smoker, to report current or previous alcohol consumption, and to work full time (all P < .05). In both cohorts, females were significantly more likely than males to have public or private health insurance versus no insurance (both P < .05).
Figure 2.
Adjusted OR (95% CI) for social history variables in (A) the biologic-naïve cohort and (B) the systemic therapy-naïve cohort. CI, confidence interval; HS, high school; N, North; OR, odds ratio.
Males in both cohorts were more likely than females to live outside of North America (P < .001 in the biologic-naïve cohort; P = .085 in the systemic therapy-naïve cohort). This observed difference in geographic location by sex could be a potential source of bias in these results.
Discussion
Results of this analysis of PSOLAR data identified several significant differences between male and female patients with psoriasis related to disease severity and medical, family, and social history recorded at the time they entered the registry. Results were generally consistent across the biologic and systemic therapy-naïve cohorts; differences between cohorts regarding achievement of statistical significance for some comparisons may reflect differences in sample sizes between cohorts.
Findings from this study are generally consistent with results reported by other researchers. In PSOLAR, males were observed to have greater disease severity at registry entry based on baseline and historical peak PGA scores and BSA of psoriasis. These results are consistent with findings from the Swedish PsoReg database and the Italian Integrated Research Center for Psoriasis indicating that males are more likely than females to have higher Psoriasis Area and Severity Index scores.11,13,24 Similarly, a relatively small United Kingdom study (n = 294) found that male sex was associated with significantly greater area of skin involvement. 20 Additionally, while overall psoriasis prevalence is similar between sexes,6-10 studies evaluating the prevalence of moderate to severe disease have reported male:female ratios as high as 2:1,21,25 suggesting that males may be more likely to have more extensive disease and/or to participate in psoriasis research studies.
Although females may tend to have less severe skin involvement, results of this and other studies indicate that quality of life and health status are more negatively affected among females than males with psoriasis based on DLQI, Psoriasis Disability Index, Skindex-17 and -29, and 12-item Short Form Health Survey outcomes.11,13,26-28 The literature supports that psychological distress associated with negative body image and social stigmatization is often greater among females with psoriasis;12,14,25,29,30 in turn, this may contribute to the difference in impact of psoriasis on quality of life, as well as the greater likelihood of anxiety, depression, and suicidal ideation among females versus males observed in our study. Additionally, some of the observed sex-specific negative effects of psoriasis among females may be associated with higher levels of treatment dissatisfaction and/or lack of response compared with males.31,32 However, patients in the current analysis were biologic or systemic therapy-naïve, suggesting that factors unrelated to systemic treatment also contribute to these sex differences.
This analysis of PSOLAR data also found that males with psoriasis were more likely than females to have a history of cardiovascular disease, including coronary artery disease, myocardial infarction, atherosclerotic cardiovascular disease, transient ischemic attack, stroke, and hypertension. A recent Italian study also found that males with psoriasis had higher risks of myocardial infarction and ischemic events than females. 11 Notably, other studies have identified an increased risk of metabolic syndrome for females compared with males with psoriasis, in part due to increased rates of obesity and dyslipidemia among females, although sex-based risk of metabolic syndrome may change with age.15,16,18,19
Relatively little has been published on sex-based differences related to treatment and social history among patients with psoriasis. Results of the current analysis identified statistically significant differences between males and females with psoriasis in geographic location, alcohol and smoking history, employment status, and insurance coverage, all of which need to be explored in more detail. Results of this analysis also showed that female patients who were eligible to receive systemic or biologic treatment, but were naïve to biologic therapy, were more likely to receive systemic steroid therapy, whereas males were more likely to receive retinoids and/or a combination of topical therapies. This observation likely relates to the contraindication to the use of systemic retinoids in women of childbearing age due to their teratogenic potential and association with certain common adverse events, such as alopecia, that may be more burdensome for females than for males. 33 The higher rate of systemic steroid use among females may also be related to the higher prevalence of history of inflammatory bowel disease and psoriatic arthritis observed for females versus males; symptoms of both of these conditions are often managed with systemic steroids. Future analyses are planned to investigate whether biologic or systemic treatment outcomes differ between males and females.
A strength of this study is that PSOLAR is the largest single intercontinental disease-based longitudinal registry for patients with psoriasis. 22 Therefore, results of this analysis may be more robust than previous studies with smaller sample sizes and/or geographic restrictions. However, results of the current analysis are limited to baseline demographic data collected between 2007 and 2013, which may not reflect the most current sex differences in patients with psoriasis. Furthermore, although adjustments were made for age, race, and BMI as covariates, a limitation of this study is that other variables not related to sex may have introduced bias. For example, one possible source of bias is the result showing that males were more likely than females to live in regions outside North America. Additionally, potential sex-related bias may occur within PSOLAR based on dermatologists’ treatment practices. Specifically, PSOLAR consists of patients who are considered by their treating dermatologist to be eligible for systemic therapy. Any sex biases in determining eligibility for such therapies could affect the composition of patients enrolled in PSOLAR. Another limitation of this analysis is that data on smoking status and alcohol consumption in PSOLAR were limited to patient responses at study entry and lacked quantifiable measures (e.g., number of packs smoked per day or drinks consumed per week). Finally, the observational nature of PSOLAR data may be limited by reporting inconsistencies.
In conclusion, based on patient data obtained at entry into PSOLAR, significant differences related to psoriasis disease characteristics, as well as medical, family, and social history-related variables were observed between males and females. Among systemic treatment-naïve patients, there was a greater negative impact on quality of life for females compared with males, despite generally lower objective disease severity for females. These findings are generally consistent with those from prior studies and lay the groundwork for future investigations to improve the understanding of sex-related differences among patients with psoriasis, including analysis of treatment choices, response to treatment, and patient-reported outcomes.
Acknowledgments
The authors would like to thank the PSOLAR participants and the staff at all study sites. Medical writing support was provided by Cherie Koch, PhD and Cynthia Arnold, BSc, CMPP (Janssen Scientific Affairs, LLC) under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2015;163:461-4). Joel Gelfand, MD, MSCE (Hospital of the University of Pennsylvania, Philadelphia), and the PSOLAR Scientific Advisory Committee provided critical review of the analytical plan.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.G. has nothing to disclose. R.C. has nothing to disclose. W.L. is employed by Janssen Research & Development, LLC and owns stock in Johnson & Johnson, of which Janssen is a subsidiary. K.P.L. is employed by Janssen Scientific Affairs, LLC and owns stock in Johnson & Johnson, of which Janssen is a subsidiary. M.G. has been an investigator, speaker, consultant, or advisory board member for AbbVie, Actelion, Akros, Amgen, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Galderma, GSK, Eli Lilly, Incyte, Janssen, Kyowa Kirin, Leo Pharma, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, UCB, and Valeant/Bausch. E.M.G.J.dJ. has received research grants from AbbVie, Novartis, Janssen Pharmaceutica, Leo Pharma, and UCB, and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Almirall, Janssen Pharmaceutica, Novartis, Lilly, Celgene, Leo Pharma, Sanofi, UCB, and Galapagos. Funding received by E.M.G.J.dJ. is not personal but goes to the independent research fund of the Department of Dermatology of the Radboud University Medical Center, Nijmegen, the Netherlands. B.S. serves as a consultant for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corporation, Dermavant, Eli Lilly, GlaxoSmithKline, Immunic Therapeutics, Janssen, LEO Pharma, Maruho, Meiji Seika Pharma, Mindera, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi-Genzyme, Sun Pharma, and UCB Pharma; a paid speaker for AbbVie, Eli Lilly, Janssen, and Sanofi-Genzyme/Regeneron; receives a consulting fee as a co-scientific director for the CorEvitas Psoriasis Registry; and an investigator for AbbVie, Cara, CorEvitas Psoriasis Registry, Dermavant, Dermira and Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Janssen Scientific Affairs, LLC, Horsham, PA, USA
Data Availability: Datasets related to this article are not currently publicly available because the registry is still ongoing but will be available by request when the study concludes.
ORCID iD
Bruce Strober https://orcid.org/0000-0002-8394-2057
References
- 1.Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences. In: Wizemann TM, Pardue ML, eds. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: Institute of Medicine; 2001:doi: 10.17226/10028. [DOI] [PubMed] [Google Scholar]
- 2.Ostan R, Monti D, Gueresi P, Bussolotto M, Franceschi C, Baggio G. Gender, aging and longevity in humans: an update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin Sci (Lond). 2016;130:1711-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Mempel M, Traidl-Hofmann C, Al Khusaei S, Ring J. Gender aspects in skin diseases. J Eur Acad Dermatol Venereol. 2010;24:1378-1385. [DOI] [PubMed] [Google Scholar]
- 4.Arain FA, Kuniyoshi FH, Abdalrhim AD, Miller VM. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ J. 2009;73:1774-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahrovan S, Fanian F, Mehryan P, Humbert P, Firooz A. Male versus female skin: What dermatologists and cosmeticians should know. Int J Womens Dermatol. 2018;4:122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. on behalf of the Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. [DOI] [PubMed] [Google Scholar]
- 8.Richard MA, Corgibet F, Beylot-Barry M, et al. Sex- and age-adjusted prevalence estimates of five chronic inflammatory skin diseases in France: Results of the « OBJECTIFS PEAU » study. J Eur Acad Dermatol Venereol. 2018;32:1967-1971. [DOI] [PubMed] [Google Scholar]
- 9.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. [DOI] [PubMed] [Google Scholar]
- 10.Helmick CG, Lee-Han H, Hirsch SC, Baird TL, Bartlett CL. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano M, Mastroeni S, Fania L, et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin Exp Dermatol. 2020;45:705-711. [DOI] [PubMed] [Google Scholar]
- 12.Wojtyna E, Łakuta P, Marcinkiewicz K, Bergler-Czop B, Brzezińska-Wcisło L. Gender, body image and social support: Biopsychosocial determinants of depression among patients with psoriasis. Acta Derm Venereol. 2017;97:91-97. [DOI] [PubMed] [Google Scholar]
- 13.Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: Rheumatoid arthritis, inflammatory bowel disease and psoriasis: An observational study. BMC Med. 2012;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Łakuta P, Przybyła-Basista H. Toward a better understanding of social anxiety and depression in psoriasis patients: The role of determinants, mediators, and moderators. J Psychosom Res. 2017;94:32-38. [DOI] [PubMed] [Google Scholar]
- 15.Sondermann W, Djeudeu Deudjui DA, Körber A, et al. Psoriasis, cardiovascular risk factors and metabolic disorders: Sex-specific findings of a population-based study. J Eur Acad Dermatol Venereol. 2020;34:779-786. [DOI] [PubMed] [Google Scholar]
- 16.Danielsen K, Wilsgaard T, Olsen AO, et al. Elevated odds of metabolic syndrome in psoriasis: A population-based study of age and sex differences. Br J Dermatol. 2015;172:419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damiani G, Cazzaniga S, Conic RR, Naldi L, PsoCare Registry Network . Pruritus characteristics in a large Italian cohort of psoriatic patients. J Eur Acad Dermatol Venereol. 2019;33:1316-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aksu AEK, Saraçoğlu ZN, Metintaş S, Sabuncu I, Cetin Y. Age and gender differences in Framingham risk score and metabolic syndrome in psoriasis patients: A cross-sectional study in the Turkish population. Anatol J Cardiol. 2017;17:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Armenteros JM, Gómez-Arbonés X, Buti-Soler M, et al. Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J Eur Acad Dermatol Venereol. 2019;33:128-135. [DOI] [PubMed] [Google Scholar]
- 20.Osborne JE, Hutchinson PE. Demographic and clinical correlates of extent of psoriasis during stable disease and during flares in chronic plaque psoriasis. Br J Dermatol. 2008;158:721-726. [DOI] [PubMed] [Google Scholar]
- 21.Odorici G, Paganelli A, Peccerillo F, et al. Moderate to severe psoriasis: A single-centre analysis of gender prevalence. Ital J Dermatol Venereol. 2021;156:226-230. [DOI] [PubMed] [Google Scholar]
- 22.Papp KA, Strober B, Augustin M, et al. on behalf of the PSOLAR investigators and Steering Committee. PSOLAR: Design, utility, and preliminary results of a prospective, international, disease-based registry of patients with psoriasis who are receiving, or are candidates for, conventional systemic treatments or biologic agents. J Drugs Dermatol. 2012;11:1210-1217. [PubMed] [Google Scholar]
- 23.Kimball AB, Leonardi C, Stahle M, et al. Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR). Br J Dermatol. 2014;171:137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hägg D, Eriksson M, Sundström A, Schmitt-Egenolf M. The higher proportion of men with psoriasis treated with biologics may be explained by more severe disease in men. PLoS One. 2013;8:e63619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maul JT, Navarini AA, Sommer R, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis - a lesson for practice. J Eur Acad Dermatol Venereol. 2019;33:700-708. [DOI] [PubMed] [Google Scholar]
- 26.Daudén E, Pujol RM, Sánchez-Carazo JL, et al. Demographic characteristics and health-related quality of life of patients with moderate-to-severe psoriasis: The VACAP study. Actas Dermosifiliogr. 2013;104:807-814. [DOI] [PubMed] [Google Scholar]
- 27.Sampogna F, Chren MM, Melchi CF, et al. Age, gender, quality of life and psychological distress in patients hospitalized with psoriasis. Br J Dermatol. 2006;154:325-331. [DOI] [PubMed] [Google Scholar]
- 28.Sampogna F, Mastroeni S, Pallotta S, et al. Use of the SF-12 questionnaire to assess physical and mental health status in patients with psoriasis. J Dermatol. 2019;46:1153-1159. [DOI] [PubMed] [Google Scholar]
- 29.Wu KK, Armstrong AW. Suicidality among psoriasis patients: A critical evidence synthesis. G Ital Dermatol Venereol. 2019;154:56-63. [DOI] [PubMed] [Google Scholar]
- 30.Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426. [DOI] [PubMed] [Google Scholar]
- 31.Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to anti-tumor necrosis factor therapies in patients with moderate to severe psoriasis: Real-world data from the US Corrona Psoriasis Registry. J Dermatolog Treat. 2021;32:302-309. [DOI] [PubMed] [Google Scholar]
- 32.van der Schoot LS, van den Reek JMPA, Groenewoud JMM, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side-effects than male patients: Results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol. 2019;33:1913-1920. [DOI] [PubMed] [Google Scholar]
- 33.Balak DMW, Gerdes S, Parodi A, Salgado-Boquete L. Long-term safety of oral systemic therapies for psoriasis: A comprehensive review of the literature. Dermatol Ther (Heidelb). 2020;10:589-613. [DOI] [PMC free article] [PubMed] [Google Scholar]