Abstract
Background
Achieving ≥90% improvement in Psoriasis Area and Severity Index (PASI90) is achievable with newer biologic therapies, such as ixekizumab. Standard of care payment systems such as the Merit-based Incentive Payment System (MIPS) responder criteria could lead to under treatment and lower quality of life (QoL) outcomes compared with PASI90.
Objective
Show PASI90 is a higher standard than MIPS and is associated with greater improvements in QoL and other PRO outcomes.
Methods
Patients with moderate-to-severe psoriasis meeting PASI90 and MIPS criteria were compared in 3 phase 3 clinical trials of the interleukin-17A inhibitor ixekizumab (pooled UNCOVER-2/3 and IXORA-S). Patients satisfying MIPS criteria met either static Physician Global Assessment score ≤2, body surface area <3%, PASI <3, or Dermatology Life Quality Index ≤5. Improvements in QoL were compared between patients meeting PASI90 and MIPS criteria.
Results
All PASI90 responders were also MIPS responders (PASI90 responders). Not all MIPS responders met PASI90 (MIPS-only responders). Significantly larger change from baseline improvements for all health (skin pain, Itch NRS, DLQI, PtGA, WPAI-PsO work productivity loss, and WPAI-PsO activity impairment) and quality of life (EQ-5D 5L VAS and acute SF-36 PCS/MCS) outcome measures were observed in the PASI90 responders vs the MIPS-only responders.
Conclusion
PASI90 is a higher standard of response than MIPS and is associated with greater improvements in health and quality of life outcome measures.
Keywords: DLQI, ixekizumab, merit-based incentive payment system, PASI90, psoriasis, psoriasis area and severity index, quality of life, treatment goal
Introduction
Psoriasis is a common inflammatory dermatologic condition in which activated T lymphocytes and other immune cells produce inflammatory cytokines such as interleukin-17, resulting in increased proinflammatory mediators, the hyperproliferation of keratinocytes, and systemic inflammation. As a result, skin cells differentiate to form lesions characterized by thick, scaly, well-demarcated, erythematous plaques that arise from a complex genetic environment. The severity of psoriasis (i.e., mild vs moderate-to-severe) is determined clinically and most commonly based on the degree of skin involvement (body surface area or BSA) and the patient’s health-related quality of life (QoL) or other associated clinical symptoms.1,2,3-6 Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets IL-17A and has demonstrated high efficacy in the treatment of patients with moderate-to-severe plaque psoriasis and psoriatic arthritis.7-12
One of the accepted tools to measure the severity of plaque psoriasis is the Psoriasis Area and Severity Index (PASI). Elements of the PASI describe the severity of the various aspects of a psoriasis plaque (redness, thickness, and scale) and affected BSA (head, trunk, arms, and legs). This information is then converted into a score that ranges from 0 (no disease) to 72 (maximum disease). 13 Patient improvement is measured as the percent reduction from the patient’s baseline PASI. Several organizations have developed target guidelines for patient outcomes in psoriasis treatment; these include the National Psoriasis Foundation, the American Academy of Dermatology, European Association of Dermatology and Venology (EADV), and the European Dermatology Forum (EDF).13,14 Overall these organizations recommend a treatment that gives the patient at least a 75% improvement (PASI75) from their baseline PASI score, with some organizations recommending improvement moving toward PASI90 (EDF).9,13
The Centers for Medicare and Medicaid Services (CMS) is required by law to implement the Quality Payment Program for patients receiving systemic or biologic therapy who meet minimum physician or patient-reported disease activity levels.15-17 The Merit-based Incentive Payment System (MIPS) is the value system component of the CMS Quality Payment Program, designed to link physician/clinician payments to the quality and cost efficiency of patient care.15-17 The goals are to promote improvement in care and health outcomes and increase the use of healthcare.17,18
Clinicians are included if they are an eligible clinician type and meet the minimum volume of services and associated charges a clinician/physician participating in MIPS must exceed to qualify for MIPS. Performance is measured through the data clinicians report in 4 categories (quality of care delivered, promoting interoperability, improvement activities, and cost of care).15,17,18 Recent reports suggest that the percentage of patients with psoriasis achieving MIPS performance criteria in a clinical setting can be as high as 89.5%. 19
The primary objective of this post hoc analysis is to compare MIPS performance criteria and PASI90 as treatment targets in patients with moderate-to-severe plaque psoriasis treated with biologics, evaluate the impact of those different treatment targets on patient-reported outcomes, and to provide evidence for raising the treatment goal to PASI90 or greater for patients with psoriasis.
Methods
Population, Study Design, and Treatment
Three clinical trials, 2 phase 3 (UNCOVER-2 [NCT01597245] and UNCOVER-3 [NCT01646177]) and 1 phase 3b (IXORA-S [NCT02561806]) multicenter, randomized trials with active comparators were used in this analysis. UNCOVER-2/3 were double-blind, placebo-controlled trials, with active comparators and parallel groups. IXORA-S was an investigator- and patient-blinded, double-dummy trial. At baseline patients from all 3 trials were aged 18 years or older and had confirmed plaque psoriasis for 6 months or more prior to baseline. UNCOVER-2/3 patients were included if ≥ 10% BSA was affected, static physician global assessment (sPGA) ≥ 3, PASI ≥ 12, and were candidates for phototherapy and/or systemic therapy. Patients from IXORA-S were included if they had a PASI score ≥ 10.
Patients were excluded if they had other forms of psoriasis, such as guttate, pustular, or erythrodermic subtypes. Patients were excluded from UNCOVER-2/3 if they were previously treated with etanercept (ETN), had concurrent or recent use of any biologic agent, or had a serious infection within 2 months of screening. Patients from IXORA-S were excluded if they had prior use of ustekinumab (UST) or received concurrent or recent biologic agents (including ETN) within agent-specific wash-out periods.
Patients were randomly assigned to 1 of 4 treatment arms. During the induction period (weeks 0–12) patients randomized to IXE received 160 mg of IXE by subcutaneous injection at Week 0 followed by 1 subcutaneous IXE injection every 2 weeks (Q2W) or every 4 weeks (Q4W). Patients randomized to the placebo (PBO) treatment arm received it every 2 weeks during the induction period, and active comparator treatment arms (ETN in UNCOVER-2/3; UST in IXORA-S) were administered in accordance with the specific labels. The study designs of UNCOVER-2/3 and IXORA-S are detailed in the online supplement (Supplementary Figure 1).
Ethics
The study protocols for UNCOVER−2/3 8 and IXORA-S 11 were approved by investigational review boards and all patients were provided with and signed written informed consent prior to trial procedures and treatments.
Psoriasis Area and Severity Index
PASI is a research tool used to measure the severity and affected BSA of plaque psoriasis, rather than a clinical tool. The body is divided into 4 sections, with each section representing a specific percentage of the overall body (head 10%, arms 20%, trunk 30%, and legs 20%). The severity index is estimated based on the amount of erythema, thickness, and scaling in each body area. The total of these 3 severity parameters is determined and then multiplied by the percentage assigned to each section. These 4 section scores are then combined to comprise the PASI final score. The percent improvement from baseline gives the PASI50, PASI75, and PASI90 scores, representing ≥50%, ≥75%, and ≥90% improvement, respectively. Herein, we focus on PASI90, defined as a ≥90% improvement in a patient’s PASI score when compared to their score at baseline, as a treatment target for patients with moderate-to-severe psoriasis treated with biologics. IXE has previously demonstrated a significantly higher PASI90 response vs PBO and active comparators (ETN and UST) in patients with moderate-to-severe psoriasis.11,20
Merit-based Incentive Payment System
MIPS is 1 of 2 ways for health care providers to participate in the CMS Quality Payment Program. The goal of the MIPS is to reward value and patient outcomes under the theory that increased patient satisfaction will result in patient adherence to treatment.15,17 To satisfy the MIPS requirements, data from clinicians must show that patients receiving systemic or biologic treatment for psoriasis meet “minimal disease activity levels” with establishment and maintenance of these minimal level of disease control measures. 17 MIPS requires at least 1 of the following criteria be met to satisfy the “minimal disease activity level” requirement: an sPGA score ≤2 (clear to mild disease on a 6-point scale), affected BSA <3% (mild disease), PASI <3 (no or minimal disease), or a score of ≤5 on the Dermatology Life Quality Index (DLQI; disease has no or little effect on patient’s quality of life). Per MIPS, treatment is successful if at least 1 of these criteria are met.15,17,18 It should be noted that the National Psoriasis Foundation (NPF) criteria for clearance has defined a BSA of 3% or less as an acceptable response 3 months after treatment initiation, and a BSA of 1% or less as the target BSA response 3 months after treatment initiation 21 ; thus, the NPF criteria for both acceptable response and target response are included in the MIPS criteria described above.
Additionally, MIPS recommends the following treatment goals: PASI75 (75% improvement in PASI from baseline), sPGA of clear or almost clear, or DLQI score of 0 or 1 after 10 to 16 weeks of treatment, and continued therapy if the treatment response is PASI75 or PASI50 (50% improvement from baseline) plus DLQI ≤5. Therefore, under MIPS criteria, treatment response is considered adequately met if the patient achieves PASI75 or reaches PASI50 plus DLQI ≤5. 17
Patient-Reported Outcomes and QoL Measures
Patient-reported outcomes and QoL measures were recorded for UNCOVER-2/3. Change from baseline in patient-reported health outcome and QoL measures included 9 outcomes. These included the skin pain visual analog scale (VAS), a patient-administered scale assessment of the patient’s skin pain from psoriasis using a horizontal scale of 0 mm (no skin pain) to 100 mm (severe skin pain); Itch Numeric Rating Scale (NRS), an 11-point scale from 0 (no itch) to 10 (worst itch imaginable); DLQI, a 10-question QoL questionnaire scoring from 0–1 (disease has no effect on patient’s life) to 21–30 (disease has an extremely large effect on patient’s life); Patients Global Assessment Disease Severity (PtGA) score from 0 (clear) to 5 (severe); Work Productivity and Activity Impairment Questionnaire-Psoriasis (WPAI-PsO), a 6-item instrument in which higher scores indicate greater impairment in productivity; the 5-level European Quality of Life–5 Dimensions (EQ-5D 5L), which uses a scale of 100 mm (best health imaginable) to 0 mm (worst health imaginable); and the acute version of the Short Form Health Survey Physical and Mental components summary (SF-36 PCS/MCS), which has a 1-week recall period and uses a summary score of 0 to 100 with higher scores indicating better function/health.
Statistical Analysis
Analyses were conducted on the intent-to-treat patients from 3 phase 3 trials (UNCOVER-2/3 and IXORA-S). In the integrated UNCOVER-2/3 studies, the number and percentage of patients who met PASI90 and MIPS, including the individual component, at each post-baseline up to Week 12 were summarized. Comparisons between treatments were made using the Cochran–Mantel–Haenszel test stratified by study. Missing data were imputed using nonresponder imputation. Similar analyses were conducted in the IXORA-S study up to Week 52. Comparisons between IXE and UST were made using a logistic model adjusting for geographic region and weight.
In addition, using the integrated UNCOVER-2/3 studies, PASI90 and MIPS responses at Week 12 were cross-tabulated by pooling all 4 treatment arms. Improvements in patient-reported outcomes were summarized by PASI90 and MIPS response status. Group comparisons were made using analysis of covariance adjusting for study and baseline patient-reported outcome value. Missing patient-reported outcomes were imputed by last observation carried forward.
Results
Patients
Data from 2872 patients in the intent-to-treat population are included in this report, 302 from IXORA-S (UST N = 166, IXE Q2W N = 136) and 2570 from pooled UNCOVER-2/3 (PBO N = 361, ETN N = 740, IXE Q4W N = 733 and IXE Q2W N = 736) (Table 1).
Table 1.
Baseline demographics and clinical characteristics for IXORA-S and pooled UNCOVER−2/3.
| IXORA-S | Pooled UNCOVER−2/3 | |||||
|---|---|---|---|---|---|---|
| UST Q12W N = 166 | IXE Q2W N = 136 | PBO N = 361 | ETN N = 740 | IXE Q4W N = 733 | IXE Q2W N = 736 | |
| Age, years | 44.0 (13.3) | 42.7 (12.7) | 45.9 (12.1) | 45.5 (13.3) | 45.3 (13.1) | 45.1 (13.2) |
| Male, n (%) | 112 (67.5) | 90 (66.2) | 257 (71.2) | 505 (68.2) | 502 (68.5) | 475 (64.5) |
| Race, White, n (%) | 157 (95.7) | 125 (93.3) | 325 (90.0) | 682 (92.7) | 675 (92.6) | 691 (94.0) |
| Weight, kg | 89.4 (24.8) | 85.8 (20.3) | 91.4 (21.6) | 92.5 (23.4) | 91.8 (23.3) | 89.8 (22.6) |
| Psoriasis duration, years | 18.2 (12.0) | 18.0 (11.1) | 18.6 (12.6) | 18.5 (12.1) | 18.5 (12.6) | 18.1 (12.2) |
| BSA, % | 27.5 (16.7) | 26.7 (16.5) | 28.0 (17.8) | 26.8 (16.6) | 27.7 (16.9) | 26.6 (16.7) |
| PASI | 19.8 (9.0) | 19.9 (8.2) | 20.9 (8.4) | 19.9 (7.5) | 20.6 (7.6) | 20.1 (7.8) |
| sPGA | 3.6 (0.6) | 3.6 (0.7) | 3.6 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) |
| Itch NRS | 6.2 (2.6) | 6.3 (2.7) | 6.4 (2.7) | 6.4 (2.6) | 6.4 (2.6) | 6.5 (2.6) |
| DLQI, total | 12.0 (7.3) | 11.1 (7.2) | 12.8 (7.1) | 12.0 (7.0) | 11.8 (6.8) | 12.4 (6.9) |
| WPAI-PsO | ||||||
| Work productivity loss | 22.8 (30.1) | 23.8 (29.8) | 25.7 (28.2) | 23.3(25.9) | 24.5 (27.9) | 26.1 (28.4) |
| Activity impairment | 29.9 (28.2) | 28.2 (30.6) | 30.6 (28.8) | 30.5 (28.4) | 30.3 (29.8) | 31.6 (29.4) |
| Acute SF-36 | ||||||
| PCS | 48.4 (9.8) | 47.3 (9.5) | 47.4 (9.5) | 48.1 (8.8) | 47.7 (9.2) | 47.8 (8.9) |
| MCS | 46.5 (11.9) | 47.1 (11.5) | 47.4 (11.2) | 48.5 (11.2) | 48.8 (11.1) | 48.0(11.5) |
Note: Unless otherwise noted, data is presented as mean (SD). Abbreviations BSA, body surface area; DLQI, Dermatology Life Quality Index; ETN, etanercept; IXE, ixekizumab; MCS, Mental Component Summary; NRS, numeric rating scale; PASI, psoriasis area and severity index; PBO, placebo; PCS, Physical Component Summary; Q2W, every 2 weeks; Q4W every 4 weeks; sPGA, static Physician Global Assessment; Acute SF-36, Acute version of the Short form (36-item) Health Survey; WPAI-PsO, Work Productivity Activity Impairment Questionnaire–Psoriasis; UST, ustekinumab.
Patients’ baseline demographic results (age, sex, race and weight) and clinical characteristics (psoriasis duration, BSA, PASI, sPGA, Itch NRS, DLQI, WPAI-PsO, and acute SF-36 PCS/MCS) were similar for IXORA-S and pooled UNCOVER-2/3 (Table 1). Individual IXORA-S and UNCOVER-2/3 baseline information was published previously.8,11
PASI90 and MIPS Week 12 Results
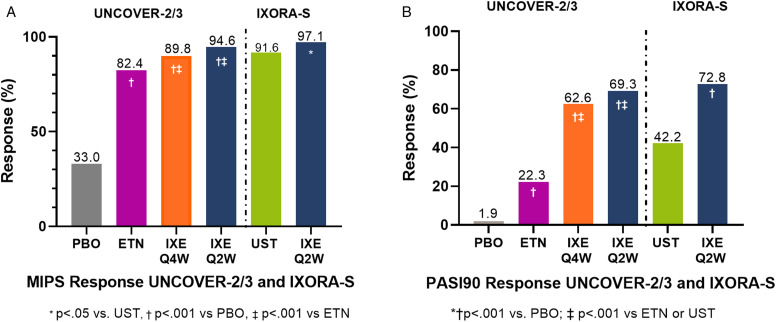
The percentage of patients at Week 12 treated with IXE who met the requirements for MIPS in the combined UNCOVER-2/3 population were significantly greater than those treated with PBO or ETN (Figure 1A). The percentage of patients treated with PBO or ETN that met the requirements for MIPS were 33.0% and 82.4%, respectively, vs 89.8% and 94.6% of patients in the IXE Q4W and IXE Q2W treatment groups, respectively (p < .001 vs. ETN, p < .001 vs. PBO). In the IXORA-S study, 97.1% of patients treated with IXE Q2W and 91.6% of those treated with UST (p < .05) met MIPS requirements.
Figure 1.
MIPS (A) and PASI90 (B) response rates at Week 12 in IXORA-S and UNCOVER-2/3 combined population. Abbreviations ETN, etanercept; IXE, ixekizumab; MIPS, Merit-based Incentive Payment System; PASI, Psoriasis Area and Severity Index; PASI90, ≥90% improvement in PASI score from baseline; PBO, placebo; Q2W, ixekizumab every 2 weeks; Q4W, ixekizumab every 4 weeks; UST, ustekinumab.
At Week 12, fewer patients reached PASI90 than met one of the MIPS eligibility requirements. Significantly more patients treated with IXE achieved PASI90 than those who received PBO, ETN, and UST. In the combined results of UNCOVER-2/3, 62.6%of patients treated with IXE Q4W and 69.3% of patients treated with IXE Q2W achieved PASI90, compared to 1.9% of PBO patients (p < .001 for both) and 22.3% of ETN-treated patients (p < .001 for both; Figure 1B). In IXORA-S, 72.8% of patients who received IXE Q2W achieved a PASI90 response compared to 42.2% of patients receiving UST (p < .001; Figure 1B). Among all treatment arms in which patients did not receive IXE Q2W or IXE Q4W, it was observed that more patients met MIPS criteria than reached PASI90. Of note, 33.0% of PBO patients, 82.4% of ETN-treated patients, and 91.6% of UST-treated patients met MIPS criteria vs 1.9%, 22.3%, and 42.2% who met PASI90 criteria, respectively (Figure 1B).
MIPS Response up to Week 12
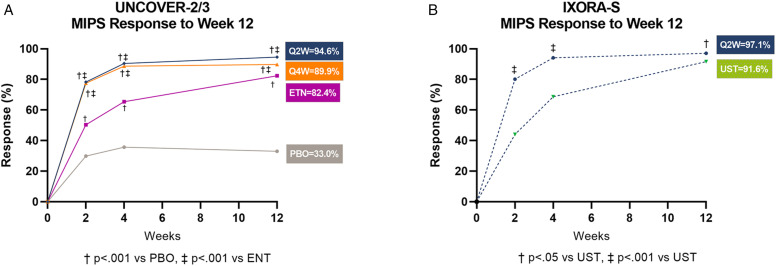
Patients’ MIPS responses were followed for 12 weeks with evaluations reported at Weeks 2, 4, and 12 (Figure 2A, B). In UNCOVER−2/3, patients at Week 2 were observed to have rapid MIPS response to at least 1 of either sPGA ≤2, BSA <3, PASI <3, or DLQI ≤5, with responder rates of 78.3% in patients treated with IXE Q2W and 77.5% in patients treated with IXE Q4W compared to 29.8% in PBO patients (p < .001 for both) and 50.3% in ETN-treated patients (p < .001 for both; Figure 2A). At Week 4, MIPS responder rates further increased to 90.4% in patients treated with IXE Q2W and 88.7% in patients treated with IXE Q4W, compared to 35.7% in PBO patients (p < .001 for both) and 65.4% in ETN-treated patients (p < .001 for both; Figure 2A). Similar results were observed in IXORA-S at Week 2 for patients treated with IXE Q2W (80.1%) vs UST-treated patients (44.0%; p<.001), increasing to 94.1% (IXE Q2W) and 68.7% (UST) at Week 4 (p < .001; Figure 2B). IXE-treated patients continued to increase their high rates of meeting 1 or more of the MIPS qualifying criteria through Week 12 (Figure 2A, B).
Figure 2.
Percentage of patients who achieved MIPS response at Weeks 2, 4, and 12 in pooled UNCOVER-2/3 (A) and IXORA-S (B). Abbreviations ETN, etanercept; IXE, ixekizumab; PBO, placebo; Q4W, every 4 weeks; Q2W every 2 weeks; UST, ustekinumab.
Individual MIPS Performance Criteria at Week 12 for UNCOVER-2/3 and IXORA-S and Weeks 24 and 52 for IXORA-S
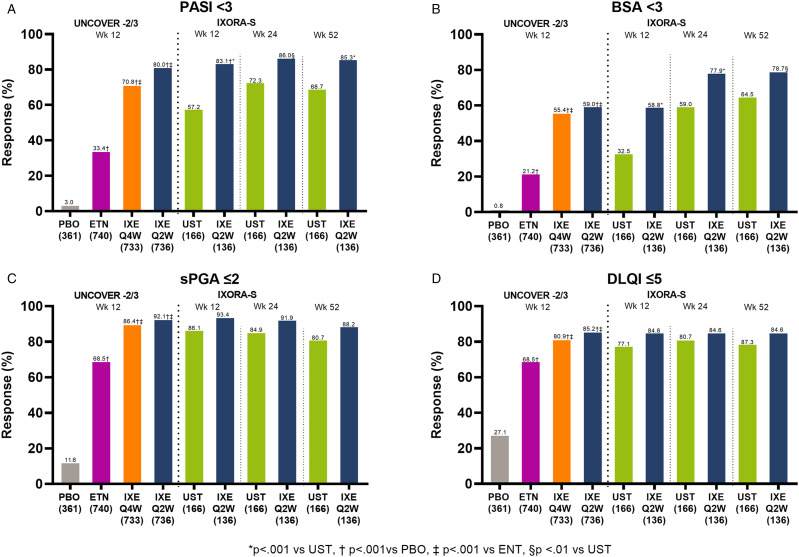
For clinicians to meet the performance standards set out for MIPS, patients needed to achieve only 1 of the required criteria: sPGA ≤ 2 or BSA<3 or PASI < 3 or DLQI ≤5. In the studies reported here, patients who received IXE Q4W or IXE Q2W had a higher MIPS response rate compared to those receiving placebo or 1 of the active control treatments.16,17 At Week 12, the criteria sPGA ≤2 had the highest response rates, with over 65% of patients receiving IXE or active control achieving this measure (Figure 3C). In the combined UNCOVER−2/3 studies, the percentage of patients who reached sPGA ≤2 was significantly greater for those treated with IXE Q4W (86.4%) and IXE Q2W (92.1%) compared to those treated with PBO (11.6%, p < .001) and ETN (68.5%, p < .001; Figure 3C). In the IXORA-S study, 93.4% of patients who received IXE Q2W achieved an sPGA≤2, compared to 86.1% of those receiving UST at Week 12 (p < .05) (Figure 3C).
Figure 3.
Individual criteria to meet MIPS requirements in UNCOVER-2/3 pooled and IXORA-S. Definitions of individual criteria levels required to meet the MIPS “minimal disease activity level” requirement: sPGA score ≤2 = clear to mild disease on a 6-point scale; affected BSA <3% = mild disease; PASI <3 = no or minimal disease; DLQI ≤5 = disease has no or little effect on patient’s quality of life.Abbreviations BSA, body surface area; DLQI, Dermatology Life Quality Index; ETN, etanercept; PBO, placebo; Q2W, ixekizumab every 2 weeks; Q4W, ixekizumab every 4 weeks; sPGA, static Physician Global Assessment; UST, ustekinumab.
At Week 12 the criteria DLQI ≤5 responses were slightly less than sPGA≤2 for the UNCOVER-2/3 pooled results. However, the percentage of patients treated with the IXE Q4W and IXE Q2W and who responded (80.9% and 85.2%, respectively) was still significantly greater than among those patients receiving PBO (27.1%; p < .001) or ETN (68.5%; p < .001) (Figure 3D). In the IXORA-S study, patients receiving IXE Q2W also had a higher response rate than those receiving UST, though the difference was not statistically significant (84.6% and 77.1%, respectively; p = NS) (Figure 3A-D).
The overall percentage of patients that achieved PASI <3 in the UNCOVER−2/3 and IXORA-S populations was somewhat less for all groups, and the IXE-treated patients had a higher response rate than those receiving PBO or active control (Figure 3A). The patients in the combined UNCOVER population PBO-, ETN-, IXE Q4W-, and IXE Q2W-treated groups had response rates of 3.0%, 33.4%, 70.8% (p < .001 vs PBO/ETN) and 80.0% (p < .001 vs PBO/ETN), respectively (Figure 3A). IXORA-S response rates in the IXE Q2W group were also significantly greater than the responses in the UST-treated group at Week 12 (83.1% and 57.2%, respectively; p < .001) (Figure 3A).
In the combined UNCOVER-2/3 studies, BSA <3 response rates were notably lower than the other MIPS criteria, with 0.8%, 21.2% 55.4%, and 59.0% of patients in the PBO, ETN, IXE Q4W, and IXE Q2W treatment groups at Week 12, respectively, meeting this criterion (Figure 3B). At Week 12, the IXORA-S response rate was significantly greater for IXE Q2W patients than for UST-treated patients (58.8% vs 32.5%, p < .001) (Figure 3B).
The rate of sPGA≤2 response was significantly higher for patients treated with IXE Q2W than for UST-treated patients at Week 24 (91.9% vs 84.9%, p < .05) and was generally maintained through Week 52 (Figure 3C). Although the rate of sPGA ≤ 2 response remained numerically higher for IXE Q2W-treated vs UST-treated patients at Week 52, the difference did not remain statistically significant (88.2% vs 80.7%, p=.06) (Figure 3C). Similar findings were observed with DLQI≤5 results at Week 24 (84.6% for IXE Q2W vs 80.7% for UST) and Week 52 (84.6% for IXE Q2W vs 78.3% for UST) although the differences were not statistically significant (Figure 3D).
PASI <3 response rates were numerically greater for IXE Q2W patients compared to UST-treated patients at Week 24 (86.0% vs 72.3%, p < .01) and significantly greater at Week 52 (85.3% vs 68.7%, p < .001) (Figure 3C).
BSA<3 remained the MIPS criteria achieved by the smallest percentages of patients. BSA <3 response rates were significantly greater for IXE Q2W patients compared to UST-treated patients at Week 24 (77.9% vs 59.0%, p < .001) and numerically greater at Week 52 (78.7% vs 64.5%, p < .01; Figure 3B).
Change from Baseline in Health and QoL Outcome Measures at Week 12 by MIPS and PASI90 Status in UNCOVER-2/3
In the combined UNCOVER−2/3, patients who met PASI90 also met MIPS (and so are defined here as PASI90 responders) across all treatment arms and time points. Approximately 37% of patients overall met MIPS but not PASI90 criteria (defined here as MIPS-only responders) at Week 12, indicating PASI90 is a more stringent threshold or measure of treatment response. A similar pattern was also observed in IXORA-S. Thus, the PASI90 response data shown in Figure 1 can be interpreted as response of simultaneous PASI90 and MIPS.
The benefit of meeting a high treatment response criteria was demonstrated by assessing the improvement in health outcome measures assessed across the following groups based on level of response: patients who failed to meet either MIPS criteria or PASI90 (nonresponders), patients who met MIPS criteria but did not meet PASI90 (MIPS-only responders), and patients who met both MIPS and PASI90 (PASI90 responders) in an integrated analysis of UNCOVER−2/3 with pooled data from the PBO and active treatment groups (IXE Q4W and Q2W, and ETN). Importantly, there were no patients who met PASI90 but did not respond to MIPS.
The observed results of the health (skin pain, Itch NRS, DLQI, PtGA, WPAI-PsO work productivity loss [WPL], and WPAI-PsO activity impairment [AI]) and QoL (EQ-5D 5L VAS, acute SF-36 PCS, and acute SF-36 MCS) outcomes demonstrated that changes from baseline were significantly larger in the PASI90 responders than in patients classified as MIPS-only responders for all outcome measures (Table 2).
Table 2.
Changes from baseline in health outcomes with a variety of scales at Week 12 by MIPS and PASI90 responder status.
| Least squares mean change from baseline (SE) | ||||
|---|---|---|---|---|
| Health outcomes | Scale | Nonresponders | MIPS-only responders | PASI90 responders |
| Skin pain | 0–100 mm (no skin pain to severe skin pain) | 2.6 (0.9) | −29.5 a (0.6) | −38.8a,b (0.5) |
| Itch | 0–10 (no itch to worst itch imaginable) | −0.4 (0.1) | −3.9 a (0.1) | −5.5a,b (0.1) |
| DLQI | 0–3 (not at all to very much) | −0.4 (0.2) | −8.6 a (0.1) | −10.7a,b (0.1) |
| PatGA | 0–5 (clear to severe) | −0.6 (0.0) | −2.3 a (0.0) | −3.4a,b (0.0) |
| WPAI | 6-Item instrument; higher scores indicate greater impairment in productivity | |||
| Work loss | 4.7 (1.0) | −17.2 a (0.7) | −20.5a,b (0.6) | |
| Impair | 4.5 (0.8) | −21.6 a (0.5) | −26.2a,b (0.5) | |
| EQ-5D 5L VAS | 100–0 mm (best health imaginable to worst health imaginable) | −2.6 (0.7) | 6.3 a (0.5) | 10.7a,b (0.4) |
| Acute SF-36 | Summary score: 0–100; higher scores indicate better function/health | |||
| PCS | −1.3 (0.3) | 3.0 a (0.2) | 4.8a,b (0.2) | |
| MCS | −0.6 (0.4) | 3.3 a (0.2) | 4.0a,c (0.2) | |
ap < .001 vs. nonresponders
bp < .001 vs. MIPS-only responders
cp < .05 vs. MIPS-only responders. Abbreviations DLQI, Dermatology Life Quality Index; EQ-5D 5L, 5-Level European Quality of Life–5 Dimensions; MCS, Mental Component Summary; PatGA, Patient Global Assessment; PCS, Physical Component Summary; Acute SF-36, Acute version of the Short form (36-item) Health Survey; VAS, visual analog scale; WPAI, Work Productivity Activity Impairment Questionnaire
Discussion
Psoriasis is a complex autoimmune disease that not only has an impact on physical health, but also affects a patient’s QoL. 22 According to the 2018 Options for Individual Measures published by the American Academy of Dermatology and the American Medical Association, the recommendation is to treat in order to achieve PASI75 or better. 17 Here, we report the results from 3 phase 3 studies (UNCOVER−2/3 pooled results and IXORA-S) that included over 2800 plaque psoriasis patients. We compared treatment responders vs nonresponders who met criteria for MIPS and PASI90 at Week 12 to determine whether meeting the PASI90 criteria is a higher treatment response standard than meeting 1 of 4 MIPS requirements and investigate the potential impact a higher level of response has on health and QoL measures.7-11 Patients were randomized to PBO, active controls (ETN or UST), or 1 of 2 doses of IXE (Q4W or Q2W).
To be included in the MIPS patient assessment, patients need to achieve 1 of the following “minimal disease activity levels”: sPGA ≤2, BSA <3, PASI <3, or DLQI ≤5.15,17 The 2018 Options for Individual Measures cited above also notes the rationale for MIPS is that patients being treated for psoriasis continue to be disappointed with their treatment for reasons such as lack of efficacy, troublesomeness, and undesirable side effects. 17 These obstacles to treatment can influence patient adherence to a treatment plan they and their physician have agreed upon. 23 Therefore, using the higher treatment response standard of PASI90 as a treatment goal would provide a better outcome with higher efficacy and decreased troublesomeness as assessed by health and QoL measures, and thus would potentially promote better treatment adherence. 23 At the same time, using the more stringent PASI90 instead of MIPS criteria as a therapeutic target may result in fewer patients achieving the treatment target; this has potential financial implications for clinicians, who will have to meet a higher standard for treatment response to be considered adequate. With MIPS theory positing that increased patient satisfaction results in patient adherence to treatment, the dependence to reward value and patient outcomes falls on the physician to work harder with patients to find the optimal and most effective therapeutics.
As previously observed at Week 12 for all treatment groups, more patients had at least 1 improved response to qualify for MIPS than patients achieving PASI90. This was especially notable in patients who achieved lower levels of objective skin clearance and efficacy compared to those who received IXE. The differences in percentage between MIPS-only and PASI90 responders at Week 12 were not the same with all the biologic drugs evaluated in this study, ranging from 60.1% in the ENT-treated group to 24.3% and 27.1% differences observed in the IXE Q2W- and IXE Q4W-treated patients, respectively. This suggests that MIPS presents a lower response hurdle than PASI90, but no association has been made between this trend according to the current novel biologic agents. More importantly, generally there are more MIPS-only responders than PASI90 responders, depending on the active drug treatment, and in this study, more patients responded to MIPS-only than to PASI90. It behooves us to identify just what population of patients taking a given therapeutic is being defined as having adequate treatment via MIPS when in fact response to the more stringent PASI90 criteria may suggest they can do better. PASI90 is more difficult to achieve than the MIPS requirement of meeting 1 of 4 outcome measures, suggesting that PASI90 is a higher threshold of treatment response, and its use affords a better patient efficacy outcome than the current criteria for MIPS alone (Figure 1). As previously reported, the average PASI score at baseline across UNCOVER clinical trials was approximately 20. 9 Therefore, to meet PASI90 patients would need to have a ≥90% improvement in their psoriasis when compared to their baseline measure. Patients who achieved PASI90 would thus have an average PASI score ≤2, which indicates lower disease activity than the MIPS criteria of PASI<3.
At Week 12 in UNCOVER−2/3, PASI90 responders had notable improvements in all Health-Related Quality of Life (HRQoL) measures. Improvement rates ranged from a 38.8% decrease in PASI90 responder rate for Skin Pain to a decrease of 3.4% in PtGA. It is also notable that rates were significantly less for MIPS-only responders than for PASI90 responders for all HRQoL measures (p < .05). Considering the impact on relevant patient-reported outcomes when comparing MIPS-only responders with PASI90 responders, PASI90 seems to have better patient-reported outcomes than the current MIPS criteria alone. Patients specifically receiving IXE Q4W and Q2W had significantly greater improvement at Week 12 in both MIPS criteria and PASI90.7-11 Those patients who achieved PASI90 achieved higher responses in health and QoL measures vs patients who met MIPS criteria alone.
It should be noted that efficacy and high-level safety data up to Week 12 for UNCOVER−2/3 (Gordon et al. 2016; Griffiths et al. 2015), up to Week 52 for IXORA-S (Paul et al. 2019), and up to 3 years for UNCOVER-3 (Blauvelt et al. 2017, Leonardi et al. 2017) have been reported previously.8,9,11,24,25 In addition, a literature review targeted articles published since 2016 relating to psoriasis treatment goals and treatment guidelines. 26 Following the literature review, a focus group discussion with psoriasis patients reported that these patients shared a deep desire for complete clearance so long as treatment had a favorable safety profile. 26
There are limitations to this study. Although PASI is not routinely used in clinical practice, this analysis clearly demonstrates the benefit to patients of reaching a higher level of clearance than those selected as MIPS criteria. Because the study results stem from a post hoc analysis, they should be viewed and interpreted with caution, and all statistically significant findings should be verified with subsequent studies. Additionally, these findings are based on data from trials designed around a single treatment (IXE) with limited comparisons. As such, the results may not be generalizable to other targeted biologic treatments for plaque psoriasis.
Conclusion
PASI90 is associated with greater improvement in the patient’s current health state in general and improvements in mobility, self-care, pain/discomfort, typical activities and mental state vs the individual MIPS measures. MIPS performance criteria were met with greater frequency than PASI90, emphasizing the “higher hurdle” of achieving a PASI90 response over the lower threshold of MIPS performance criteria in psoriasis. In addition, significantly greater improvements in QoL and PRO were observed in patients that achieved PASI90 response than among patients that achieved MIPS performance criteria. For moderate-to-severe psoriasis patients treated with biologic therapies, PASI90 seems to offer a higher bar as a treatment target than MIPS performance criteria and may improve the ability to discriminate between different biologics’ benefits.
Considering the overall improvement observed in patients’ psoriasis and QoL outcomes, using PASI90 as the treatment goal rather than MIPS performance criteria may provide better overall clinical and QoL-related outcomes for psoriasis patients who are disappointed with their treatment for reasons such as troublesomeness or lack of efficacy, warranting further investigation to improve patient satisfaction and adherence to therapy.
Supplemental Material
Supplemental Material, sj-pdf-1-jps-10.1177_24755303221082623 for Value of PASI90 Versus Merit-Based Incentive Payment System Efficacy Measures by Jason E. Hawkes, MD, MS, Paulo Reis, MD, Chen-Yen Lin, PhD, Talia Muram, MD, and William J. Eastman MD in Journal of Psoriasis and Psoriatic Arthritis
Acknowledgments
The authors thank the patients, their families, the study sites, and the study personnel who participated in these clinical trials. Medical writing support was provided by Debra Sherman and Regina E. Burris, PhD, editorial support was provided by Dana Schamberger, MA, and submission support was provided by Regina E. Burris, PhD, all of Syneos Health; writing, editorial, and submission support were funded by Eli Lilly and Company in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). All authors have authorized the submission of the manuscript via third party and approved all statements and declarations.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J. E. Hawkes has received personal consulting fees from Eli Lilly and Company for advisory board participation and is a current member of the Medical Board of the National Psoriasis Foundation (unpaid service). Dr. Hawkes was not paid to participate in the authorship of the manuscript. P. Reis, C.-Y. Lin, T. Muram, and W. J. Eastman are full-time employees of Eli Lilly and Company, Indianapolis, IN, USA, and minority holders of company stock.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Eli Lilly and Company.
Data Availability: Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: Recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9(6):461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girolomoni G, Mrowietz U, Paul C. Psoriasis: Rationale for targeting interleukin-17. Br J Dermatol. 2012;167(4):717-724. [DOI] [PubMed] [Google Scholar]
- 3.Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med. 2014;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langley RG, Papp K, Gooderham M, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). Br J Dermatol. 2018;178(6):1315-1323. [DOI] [PubMed] [Google Scholar]
- 6.Ryan C, Menter A, Guenther L, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179(4):844-852. [DOI] [PubMed] [Google Scholar]
- 7.Gordon KB, Leonardi CL, Lebwohl M, et al. A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2014;71(6):1176-1182. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541-551. [DOI] [PubMed] [Google Scholar]
- 9.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345-356. [DOI] [PubMed] [Google Scholar]
- 10.Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014-1023. [DOI] [PubMed] [Google Scholar]
- 11.Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70-79. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A, Papp K, Gottlieb A, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2019;182(6):1348-1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golbari NM, Porter ML, Kimball AB. Current guidelines for psoriasis treatment: a work in progress. Cutis. 2018;101(3S):10-12. [PubMed] [Google Scholar]
- 14.Nast A, Spuls PI, van der Kraaij G, et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris - Update Apremilast and Secukinumab - EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2017;31(12):1951-1963. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services Medicare Program; Merit-based Incentive Payment System (MIPS) and Alternative Payment Model (APM) Incentive under the physician fee schedule, and criteria for physician-focused payment models final rule with comment period. Fed Regist. 2016;81:77008-77831. [PubMed] [Google Scholar]
- 16.Nuckols TK. With the merit-based incentive payment system, pay for performance is now national policy. Ann Intern Med. 2017;166(5):368-369. https://annals.org. Accessed 7/11/2019. [DOI] [PubMed] [Google Scholar]
- 17.Quality Payment Program cms Measure ID #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications – National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes. [On-Line]. 2017; 2.0:2018 Options For Individual Measures. 2019, Available at: https://qpp.cms.gov/docs/QPP_quality_measure_specifications/Claims-Registry-Measures/2018_Measure_410_Registry.pdf. Accessed 8/9/2019. [Google Scholar]
- 18.MIPS Overview . Quality Payment Program 2019; https://qpp.cms.gov/mips/overview. Accessed 10/18/2019, 2019. [Google Scholar]
- 19.Taliercio VI, Langer A, Secrest AM, Duffin KC. Measuring quality in psoriasis: Early experience with the MIPS 410 quality measure e psoriasis: Clinical response to oral systemic or biologic medications. J Invest Dermatol. 2019;139(5, Supplemental):1. [Google Scholar]
- 20.Reich K, Leonardi C, Langley RG, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: A presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76(3):441-448 e442. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: Treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290-298. [DOI] [PubMed] [Google Scholar]
- 22.Eissing L, Radtke MA, Zander N, Augustin M. Barriers to guideline-compliant psoriasis care: analyses and concepts. J Eur Acad Dermatol Venereol. 2016;30(4):569-575. [DOI] [PubMed] [Google Scholar]
- 23.Augustin M, Holland B, Dartsch D, Langenbruch A, Radtke MA. Adherence in the treatment of psoriasis: a systematic review. Dermatology. 2011;222(4):363-374. [DOI] [PubMed] [Google Scholar]
- 24.Blauvelt A, Gooderham M, Iversen L, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3). J Am Acad Dermatol. 2017;77(5):855-862. [DOI] [PubMed] [Google Scholar]
- 25.Leonardi CL, Blauvelt A, Sofen HL, et al. Rapid improvements in health-related quality of life and itch with ixekizumab treatment in randomized phase 3 trials: Results from UNCOVER-2 and UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(9):1483-1490. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong A, Paul C, Puig L, et al. Safety of ixekizumab treatment for up to 5 years in adult patients with moderate-to-severe psoriasis: results from greater than 17,000 patient-years of exposure. Dermatol Ther (Heidelb) 2020;10(1):133-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-jps-10.1177_24755303221082623 for Value of PASI90 Versus Merit-Based Incentive Payment System Efficacy Measures by Jason E. Hawkes, MD, MS, Paulo Reis, MD, Chen-Yen Lin, PhD, Talia Muram, MD, and William J. Eastman MD in Journal of Psoriasis and Psoriatic Arthritis