Abstract
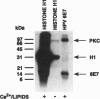
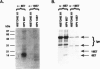
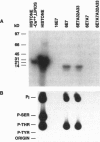
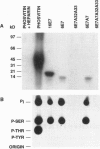
The E7 proteins of 'low-risk' and 'high-risk' human papillomaviruses (HPV) are phosphorylated by casein kinase II. In this study, we report that the 'low-risk' HPV 6 E7 protein, but not the 'high-risk' HPV 16 E7 protein, can be phosphorylated in vitro on threonine at amino acid position 7 by protein kinase C. This is the first example of a qualitative biochemical difference between the HPV 6 E7 and HPV 16 E7 proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Roman A. Mutagenesis of human papillomavirus types 6 and 16 E7 open reading frames alters the electrophoretic mobility of the expressed proteins. J Gen Virol. 1992 May;73(Pt 5):1275–1279. doi: 10.1099/0022-1317-73-5-1275. [DOI] [PubMed] [Google Scholar]
- Armstrong D. J., Roman A. The anomalous electrophoretic behavior of the human papillomavirus type 16 E7 protein is due to the high content of acidic amino acid residues. Biochem Biophys Res Commun. 1993 May 14;192(3):1380–1387. doi: 10.1006/bbrc.1993.1569. [DOI] [PubMed] [Google Scholar]
- Arroyo M., Bagchi S., Raychaudhuri P. Association of the human papillomavirus type 16 E7 protein with the S-phase-specific E2F-cyclin A complex. Mol Cell Biol. 1993 Oct;13(10):6537–6546. doi: 10.1128/mcb.13.10.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Hannun Y., Loomis C. Mixed micelle assay of protein kinase C. Methods Enzymol. 1986;124:353–359. doi: 10.1016/0076-6879(86)24027-6. [DOI] [PubMed] [Google Scholar]
- Bousset K., Henriksson M., Lüscher-Firzlaff J. M., Litchfield D. W., Lüscher B. Identification of casein kinase II phosphorylation sites in Max: effects on DNA-binding kinetics of Max homo- and Myc/Max heterodimers. Oncogene. 1993 Dec;8(12):3211–3220. [PubMed] [Google Scholar]
- Ciccolini F., Di Pasquale G., Carlotti F., Crawford L., Tommasino M. Functional studies of E7 proteins from different HPV types. Oncogene. 1994 Sep;9(9):2633–2638. [PubMed] [Google Scholar]
- Davies R., Hicks R., Crook T., Morris J., Vousden K. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J Virol. 1993 May;67(5):2521–2528. doi: 10.1128/jvi.67.5.2521-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A. A., Yuspa S. H. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993 Jan;120(1):217–225. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Długosz A. A., Yuspa S. H. Protein kinase C regulates keratinocyte transglutaminase (TGK) gene expression in cultured primary mouse epidermal keratinocytes induced to terminally differentiate by calcium. J Invest Dermatol. 1994 Apr;102(4):409–414. doi: 10.1111/1523-1747.ep12372171. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Eggert M., Radomski N., Tripier D., Traub P., Jost E. Identification of phosphorylation sites on murine nuclear lamin C by RP-HPLC and microsequencing. FEBS Lett. 1991 Nov 4;292(1-2):205–209. doi: 10.1016/0014-5793(91)80868-4. [DOI] [PubMed] [Google Scholar]
- Farr A., Wang H., Kasher M. S., Roman A. Relative enhancer activity and transforming potential of authentic human papillomavirus type 6 genomes from benign and malignant lesions. J Gen Virol. 1991 Mar;72(Pt 3):519–526. doi: 10.1099/0022-1317-72-3-519. [DOI] [PubMed] [Google Scholar]
- Fiol C. J., Mahrenholz A. M., Wang Y., Roeske R. W., Roach P. J. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987 Oct 15;262(29):14042–14048. [PubMed] [Google Scholar]
- Firzlaff J. M., Galloway D. A., Eisenman R. N., Lüscher B. The E7 protein of human papillomavirus type 16 is phosphorylated by casein kinase II. New Biol. 1989 Oct;1(1):44–53. [PubMed] [Google Scholar]
- Frankel S., Sohn R., Leinwand L. The use of sarkosyl in generating soluble protein after bacterial expression. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1192–1196. doi: 10.1073/pnas.88.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage J. R., Meyers C., Wettstein F. O. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990 Feb;64(2):723–730. doi: 10.1128/jvi.64.2.723-730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litchfield D. W., Lüscher B., Lozeman F. J., Eisenman R. N., Krebs E. G. Phosphorylation of casein kinase II by p34cdc2 in vitro and at mitosis. J Biol Chem. 1992 Jul 15;267(20):13943–13951. [PubMed] [Google Scholar]
- Marin O., Meggio F., Draetta G., Pinna L. A. The consensus sequences for cdc2 kinase and for casein kinase-2 are mutually incompatible. A study with peptides derived from the beta-subunit of casein kinase-2. FEBS Lett. 1992 Apr 13;301(1):111–114. doi: 10.1016/0014-5793(92)80221-2. [DOI] [PubMed] [Google Scholar]
- Meisner H., Czech M. P. Phosphorylation of transcriptional factors and cell-cycle-dependent proteins by casein kinase II. Curr Opin Cell Biol. 1991 Jun;3(3):474–483. doi: 10.1016/0955-0674(91)90076-b. [DOI] [PubMed] [Google Scholar]
- Muchardt C., Li C., Kornuc M., Gaynor R. CREB regulation of cellular cyclic AMP-responsive and adenovirus early promoters. J Virol. 1990 Sep;64(9):4296–4305. doi: 10.1128/jvi.64.9.4296-4305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Yee C. L., Phelps W. C., Pietenpol J. A., Moses H. L., Howley P. M. Biochemical and biological differences between E7 oncoproteins of the high- and low-risk human papillomavirus types are determined by amino-terminal sequences. J Virol. 1991 Jul;65(7):3943–3948. doi: 10.1128/jvi.65.7.3943-3948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. C., Koshland D. E., Jr High cooperativity, specificity, and multiplicity in the protein kinase C-lipid interaction. J Biol Chem. 1989 Sep 5;264(25):14909–14915. [PubMed] [Google Scholar]
- Osada S., Hashimoto Y., Nomura S., Kohno Y., Chida K., Tajima O., Kubo K., Akimoto K., Koizumi H., Kitamura Y. Predominant expression of nPKC eta, a Ca(2+)-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 1993 Mar;4(3):167–175. [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Peeper D. S., Parker L. L., Ewen M. E., Toebes M., Hall F. L., Xu M., Zantema A., van der Eb A. J., Piwnica-Worms H. A- and B-type cyclins differentially modulate substrate specificity of cyclin-cdk complexes. EMBO J. 1993 May;12(5):1947–1954. doi: 10.1002/j.1460-2075.1993.tb05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok R., Lorenz P., Ansorge W., Pyerin W. Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. J Biol Chem. 1994 Mar 4;269(9):6986–6991. [PubMed] [Google Scholar]
- Sun H., Tang J., Hu M. Preparation of Tyr-C-peptide from genetically altered human insulin precursor. Appl Biochem Biotechnol. 1995 Dec;55(3):167–174. doi: 10.1007/BF02786858. [DOI] [PubMed] [Google Scholar]
- Tommasino M., Adamczewski J. P., Carlotti F., Barth C. F., Manetti R., Contorni M., Cavalieri F., Hunt T., Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993 Jan;8(1):195–202. [PubMed] [Google Scholar]
- Turman M. A., Douvas A. A casein kinase type II (CKII)-like nuclear protein kinase associates with, phosphorylates, and activates topoisomerase I. Biochem Med Metab Biol. 1993 Oct;50(2):210–225. doi: 10.1006/bmmb.1993.1063. [DOI] [PubMed] [Google Scholar]