Abstract
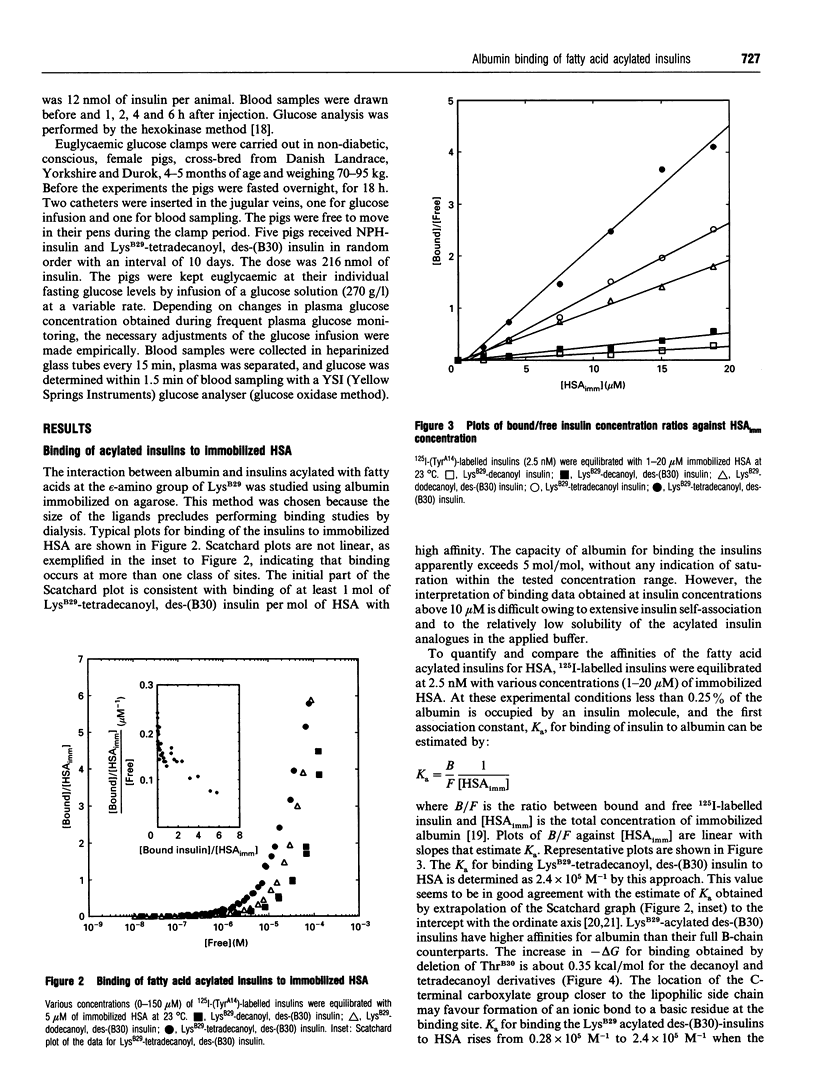
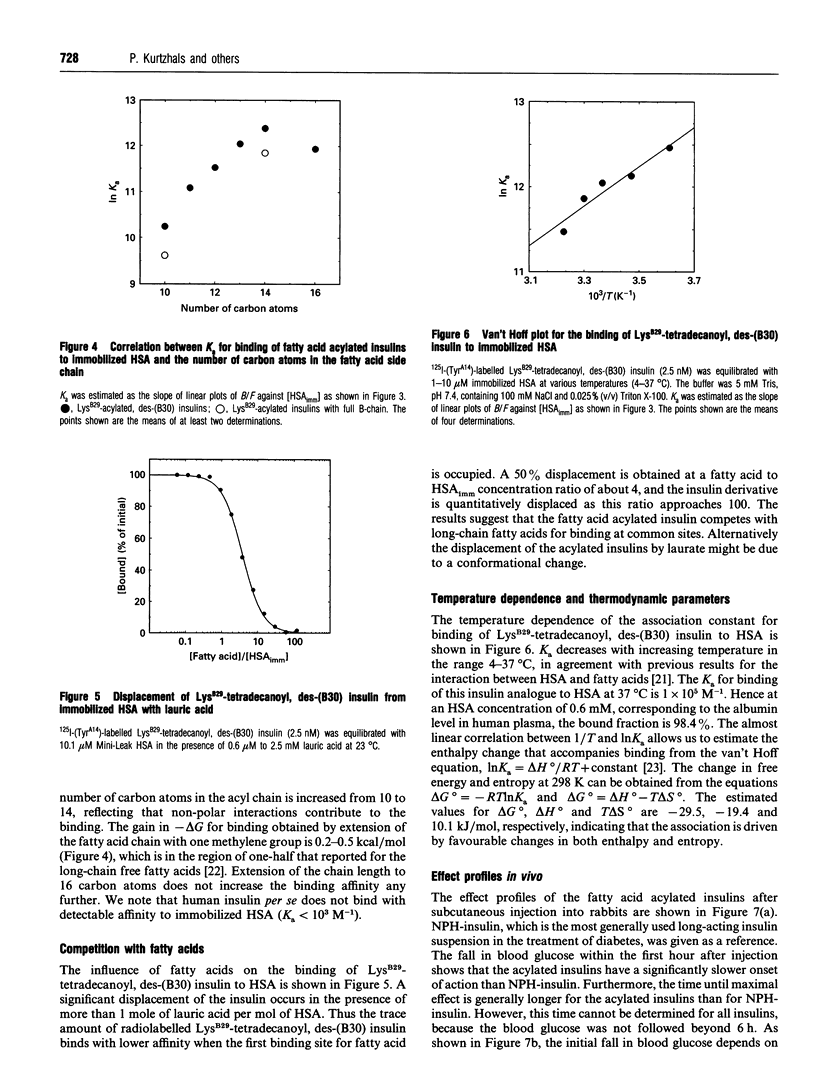
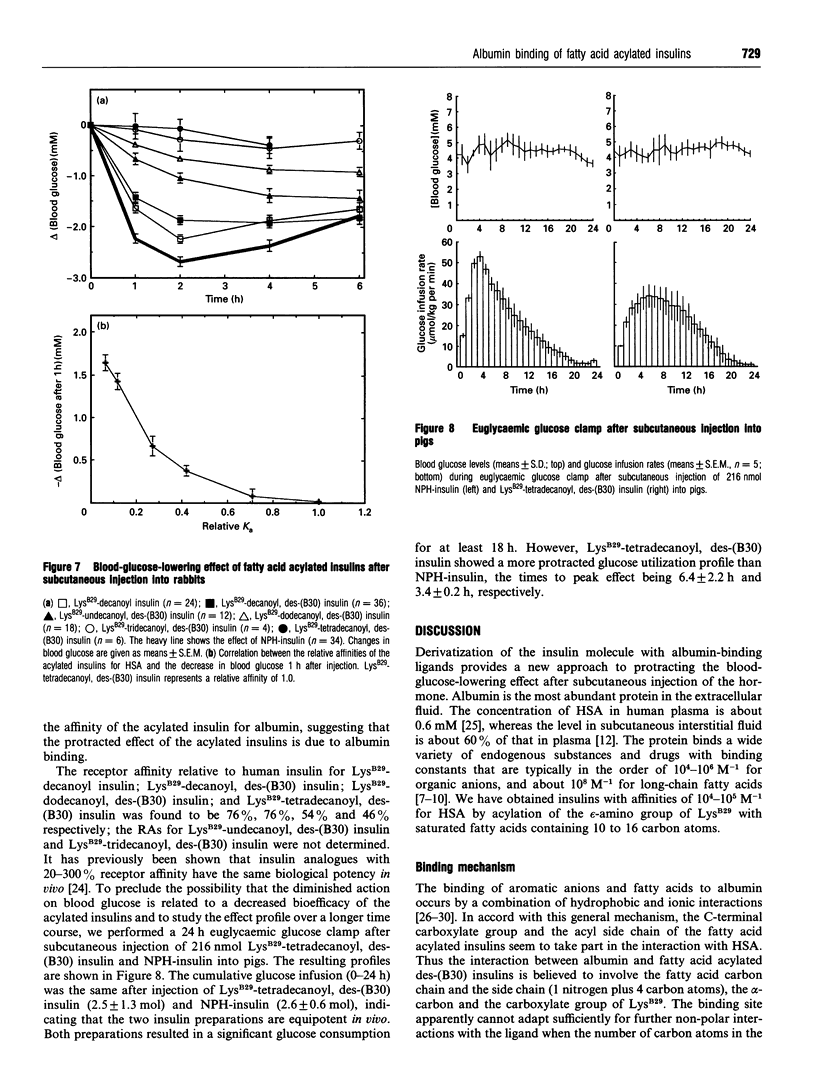
Albumin is a multifunctional transport protein that binds a wide variety of endogenous substances and drugs. Insulins with affinity for albumin were engineered by acylation of the epsilon-amino group of LysB29 with saturated fatty acids containing 10-16 carbon atoms. The association constants for binding of the fatty acid acylated insulins to human albumin are in the order of 10(4)-10(5) M-1. The binding apparently involves both non-polar and ionic interactions with the protein. The acylated insulins bind at the long-chain fatty acid binding sites, but the binding affinity is lower than that of the free fatty acids and depends to a relatively small degree on the number of carbon atoms in the fatty acid chain. Differences in affinity of the acylated insulins for albumin are reflected in the relative timing of the blood-glucose-lowering effect after subcutaneous injection into rabbits. The acylated insulins provide a breakthrough in the search for soluble, prolonged-action insulin preparations for basal delivery of the hormone to the diabetic patient. We conclude that the biochemical concept of albumin binding can be applied to protract the effect of insulin, and suggest that derivatization with albumin-binding ligands could be generally applicable to prolong the action profile of peptide drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashbrook J. D., Spector A. A., Santos E. C., Fletcher J. E. Long chain fatty acid binding to human plasma albumin. J Biol Chem. 1975 Mar 25;250(6):2333–2338. [PubMed] [Google Scholar]
- Ashbrook J. D., Spectro A. A., Fletcher J. E. Medium chain fatty acid binding to human plasma albumin. J Biol Chem. 1972 Nov 10;247(21):7038–7042. [PubMed] [Google Scholar]
- Brange J., Ribel U., Hansen J. F., Dodson G., Hansen M. T., Havelund S., Melberg S. G., Norris F., Norris K., Snel L. Monomeric insulins obtained by protein engineering and their medical implications. Nature. 1988 Jun 16;333(6174):679–682. doi: 10.1038/333679a0. [DOI] [PubMed] [Google Scholar]
- Carter D. C., Ho J. X. Structure of serum albumin. Adv Protein Chem. 1994;45:153–203. doi: 10.1016/s0065-3233(08)60640-3. [DOI] [PubMed] [Google Scholar]
- Dahlquist F. W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978;48:270–299. doi: 10.1016/s0076-6879(78)48015-2. [DOI] [PubMed] [Google Scholar]
- Fraze E., Donner C. C., Swislocki A. L., Chiou Y. A., Chen Y. D., Reaven G. M. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1985 Nov;61(5):807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Era S., Bhamidipati S. P., Reed R. G. Locations of the three primary binding sites for long-chain fatty acids on bovine serum albumin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2051–2054. doi: 10.1073/pnas.88.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X. M., Carter D. C. Atomic structure and chemistry of human serum albumin. Nature. 1992 Jul 16;358(6383):209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Hildebrandt P. Subcutaneous absorption of insulin in insulin-dependent diabetic patients. Influence of species, physico-chemical properties of insulin and physiological factors. Dan Med Bull. 1991 Aug;38(4):337–346. [PubMed] [Google Scholar]
- Honoré B., Brodersen R. Albumin binding of anti-inflammatory drugs. Utility of a site-oriented versus a stoichiometric analysis. Mol Pharmacol. 1984 Jan;25(1):137–150. [PubMed] [Google Scholar]
- Jørgensen K. H., Larsen U. D. Homogeneous mono-(125)i-insulins. Preparation and characterization of mono-(125)i-(tyr a14)-and mono-(125)i-(tyr a19)-insulin. Diabetologia. 1980;19(6):546–554. doi: 10.1007/BF00253183. [DOI] [PubMed] [Google Scholar]
- Kenyon M. A., Hamilton J. A. 13C NMR studies of the binding of medium-chain fatty acids to human serum albumin. J Lipid Res. 1994 Mar;35(3):458–467. [PubMed] [Google Scholar]
- Kragh-Hansen U. Structure and ligand binding properties of human serum albumin. Dan Med Bull. 1990 Feb;37(1):57–84. [PubMed] [Google Scholar]
- León L. P., Chu D. K., Snyder L. R., Horváth C. Continuous-flow analysis for glucose in serum, with use of hexokinase and glucose-6-phosphate dehydrogenase co-immobilized in tubular form. Clin Chem. 1980 Jan;26(1):123–129. [PubMed] [Google Scholar]
- Markussen J., Diers I., Engesgaard A., Hansen M. T., Hougaard P., Langkjaer L., Norris K., Ribel U., Sørensen A. R., Sørensen E. Soluble, prolonged-acting insulin derivatives. II. Degree of protraction and crystallizability of insulins substituted in positions A17, B8, B13, B27 and B30. Protein Eng. 1987 Jun;1(3):215–223. doi: 10.1093/protein/1.3.215. [DOI] [PubMed] [Google Scholar]
- Markussen J., Diers I., Hougaard P., Langkjaer L., Norris K., Snel L., Sørensen A. R., Sørensen E., Voigt H. O. Soluble, prolonged-acting insulin derivatives. III. Degree of protraction, crystallizability and chemical stability of insulins substituted in positions A21, B13, B23, B27 and B30. Protein Eng. 1988 Jul;2(2):157–166. doi: 10.1093/protein/2.2.157. [DOI] [PubMed] [Google Scholar]
- Markussen J., Halstrøm J., Wiberg F. C., Schäffer L. Immobilized insulin for high capacity affinity chromatography of insulin receptors. J Biol Chem. 1991 Oct 5;266(28):18814–18818. [PubMed] [Google Scholar]
- Markussen J., Hougaard P., Ribel U., Sørensen A. R., Sørensen E. Soluble, prolonged-acting insulin derivatives. I. Degree of protraction and crystallizability of insulins substituted in the termini of the B-chain. Protein Eng. 1987 Jun;1(3):205–213. doi: 10.1093/protein/1.3.205. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., Pownall H. J., Gotto A. M., Jr Bovine serum albumin. Study of the fatty acid and steroid binding sites using spin-labeled lipids. J Biol Chem. 1975 Apr 10;250(7):2487–2494. [PubMed] [Google Scholar]
- Parks J. S., Cistola D. P., Small D. M., Hamilton J. A. Interactions of the carboxyl group of oleic acid with bovine serum albumin: a 13C NMR study. J Biol Chem. 1983 Aug 10;258(15):9262–9269. [PubMed] [Google Scholar]
- Pedersen A. O., Honoré B., Brodersen R. Thermodynamic parameters for binding of fatty acids to human serum albumin. Eur J Biochem. 1990 Jul 5;190(3):497–502. doi: 10.1111/j.1432-1033.1990.tb15601.x. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Reed R. G., Gates T., Peters T. Albumin immobilized on agarose as a tool for measuring ligand binding of proteins or peptides. Anal Biochem. 1975 Dec;69(2):361–371. doi: 10.1016/0003-2697(75)90138-4. [DOI] [PubMed] [Google Scholar]
- Santos E. C., Spector A. A. Effects of fatty acids on the interaction of 1-anilino-8-naphthalenesulfonate with human plasma albumin. Mol Pharmacol. 1974 May;10(3):519–528. [PubMed] [Google Scholar]
- Schmitt E. W., Gattner H. G. Verbesserte Darstellung von Des-alanylB30-insulin. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):799–802. [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]


