Abstract
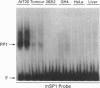
Corticotrophs are the first fully differentiated cells to appear in the anterior pituitary during organogenesis and are distinguished by pro-opiomelanocortin (POMC) gene expression. Earlier studies in our laboratory defined three DNA regions (sites 1, 2 and 3) within promoter sequences at the 5'-end of the rat POMC gene (-323/-34) that cooperatively targeted cell-specific gene expression to corticotrophs and melanotrophs in transgenic mice. In this study we analysed the DNA-nuclear protein interactions underlying this functional activity. We demonstrated that the transcriptional activator SP1 interacts with GC-rich regions in sites 1 (-146/-136) and 2 (-201/-192) and an unidentified protein, which we call PP1 (putative pituitary POMC1), interacts with AT-rich regions in sites 2 (-202/-193) and 3 (-262/-253). The PP1-binding activity appears to be specific to cells that express the POMC gene because it was detected in nuclear extracts prepared from AtT20 corticotroph cells and mouse melanotroph tumours but not from GH4 pituitary tumour cells, HeLa cells or liver. Site-directed mutagenesis of core binding sequences demonstrated that PP1 is required for the correct cell-specific expression of the POMC gene in the pituitary gland of transgenic mice and SP1 appears to support such an expression. The best core binding sequence for PP1 is TAAT, a possible transcription factor homeodomain contact site. However, PP1 is distinct from Brn 3.0, a POU protein that also binds to site 3. We conclude that PP1 is a transcriptional activator for pituitary-specific POMC gene expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begeot M., Dubois M. P., Dubois P. M. Comparative study in vivo and in vitro of the differentiation of immunoreactive corticotropic cells in fetal rat anterior pituitary. Neuroendocrinology. 1982 Oct;35(4):255–264. doi: 10.1159/000123391. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Cochet M., Chang A. C., Cohen S. N. Characterization of the structural gene and putative 5'-regulatory sequences for human proopiomelanocortin. Nature. 1982 May 27;297(5864):335–339. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- Dobrenski A. F., Zeft A. S., Wellstein A., Riegel A. T. DNA binding of the transcription factor PO-B is regulated during differentiation of HL-60 cells. Cell Growth Differ. 1993 Aug;4(8):647–656. [PubMed] [Google Scholar]
- Drouin J., Chamberland M., Charron J., Jeannotte L., Nemer M. Structure of the rat pro-opiomelanocortin (POMC) gene. FEBS Lett. 1985 Nov 25;193(1):54–58. doi: 10.1016/0014-5793(85)80078-8. [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Qian Y. Q., Billeter M., Furukubo-Tokunaga K., Schier A. F., Resendez-Perez D., Affolter M., Otting G., Wüthrich K. Homeodomain-DNA recognition. Cell. 1994 Jul 29;78(2):211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Gerrero M. R., McEvilly R. J., Turner E., Lin C. R., O'Connell S., Jenne K. J., Hobbs M. V., Rosenfeld M. G. Brn-3.0: a POU-domain protein expressed in the sensory, immune, and endocrine systems that functions on elements distinct from known octamer motifs. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10841–10845. doi: 10.1073/pnas.90.22.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Japón M. A., Rubinstein M., Low M. J. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994 Aug;42(8):1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Karpen G. H. Position-effect variegation and the new biology of heterochromatin. Curr Opin Genet Dev. 1994 Apr;4(2):281–291. doi: 10.1016/s0959-437x(05)80055-3. [DOI] [PubMed] [Google Scholar]
- Li P., He X., Gerrero M. R., Mok M., Aggarwal A., Rosenfeld M. G. Spacing and orientation of bipartite DNA-binding motifs as potential functional determinants for POU domain factors. Genes Dev. 1993 Dec;7(12B):2483–2496. doi: 10.1101/gad.7.12b.2483. [DOI] [PubMed] [Google Scholar]
- Lin S. C., Li S., Drolet D. W., Rosenfeld M. G. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development. 1994 Mar;120(3):515–522. doi: 10.1242/dev.120.3.515. [DOI] [PubMed] [Google Scholar]
- Lipkin S. M., När A. M., Kalla K. A., Sack R. A., Rosenfeld M. G. Identification of a novel zinc finger protein binding a conserved element critical for Pit-1-dependent growth hormone gene expression. Genes Dev. 1993 Sep;7(9):1674–1687. doi: 10.1101/gad.7.9.1674. [DOI] [PubMed] [Google Scholar]
- Lira S. A., Kalla K. A., Glass C. K., Drolet D. W., Rosenfeld M. G. Synergistic interactions between Pit-1 and other elements are required for effective somatotroph rat growth hormone gene expression in transgenic mice. Mol Endocrinol. 1993 May;7(5):694–701. doi: 10.1210/mend.7.5.8316253. [DOI] [PubMed] [Google Scholar]
- Liu B., Hammer G. D., Rubinstein M., Mortrud M., Low M. J. Identification of DNA elements cooperatively activating proopiomelanocortin gene expression in the pituitary glands of transgenic mice. Mol Cell Biol. 1992 Sep;12(9):3978–3990. doi: 10.1128/mcb.12.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. J., Liu B., Hammer G. D., Rubinstein M., Allen R. G. Post-translational processing of proopiomelanocortin (POMC) in mouse pituitary melanotroph tumors induced by a POMC-simian virus 40 large T antigen transgene. J Biol Chem. 1993 Nov 25;268(33):24967–24975. [PubMed] [Google Scholar]
- Nakanishi S., Teranishi Y., Watanabe Y., Notake M., Noda M., Kakidani H., Jingami H., Numa S. Isolation and characterization of the bovine corticotropin/beta-lipotropin precursor gene. Eur J Biochem. 1981 Apr;115(3):429–438. doi: 10.1111/j.1432-1033.1981.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Notake M., Tobimatsu T., Watanabe Y., Takahashi H., Mishina M., Numa S. Isolation and characterization of the mouse corticotropin-beta-lipotropin precursor gene and a related pseudogene. FEBS Lett. 1983 May 30;156(1):67–71. doi: 10.1016/0014-5793(83)80250-6. [DOI] [PubMed] [Google Scholar]
- Riegel A. T., Remenick J., Wolford R. G., Berard D. S., Hager G. L. A novel transcriptional activator (PO-B) binds between the TATA box and cap site of the pro-opiomelanocortin gene. Nucleic Acids Res. 1990 Aug 11;18(15):4513–4521. doi: 10.1093/nar/18.15.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M., Mortrud M., Liu B., Low M. J. Rat and mouse proopiomelanocortin gene sequences target tissue-specific expression to the pituitary gland but not to the hypothalamus of transgenic mice. Neuroendocrinology. 1993 Oct;58(4):373–380. doi: 10.1159/000126566. [DOI] [PubMed] [Google Scholar]
- Theill L. E., Karin M. Transcriptional control of GH expression and anterior pituitary development. Endocr Rev. 1993 Dec;14(6):670–689. doi: 10.1210/edrv-14-6-670. [DOI] [PubMed] [Google Scholar]
- Therrien M., Drouin J. Cell-specific helix-loop-helix factor required for pituitary expression of the pro-opiomelanocortin gene. Mol Cell Biol. 1993 Apr;13(4):2342–2353. doi: 10.1128/mcb.13.4.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Drolet D. W., Rosenfeld M. G. POU-domain proteins: structure and function of developmental regulators. Curr Opin Cell Biol. 1993 Jun;5(3):488–498. doi: 10.1016/0955-0674(93)90015-i. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]