Abstract
Nucleophosmin 1 (NPM1) is commonly mutated in myelodysplastic syndrome (MDS) and acute myeloid leukemia. Concurrent inflammatory bowel diseases (IBD) and MDS are common, indicating a close relationship between IBD and MDS. Here we examined the function of NPM1 in IBD and colitis-associated colorectal cancer (CAC). NPM1 expression was reduced in patients with IBD. Npm1+/− mice were more susceptible to acute colitis and experimentally induced CAC than littermate controls. Npm1 deficiency impaired the function of interleukin-22 (IL-22)-producing group three innate lymphoid cells (ILC3s). Mice lacking Npm1 in ILC3s exhibited decreased IL-22 production and accelerated development of colitis. NPM1 was important for mitochondrial biogenesis and metabolism by oxidative phosphorylation in ILC3s. Further experiments revealed that NPM1 cooperates with p65 to promote mitochondrial transcription factor A (TFAM) transcription in ILC3s. Overexpression of Npm1 in mice enhanced ILC3 function and reduced the severity of dextran sulfate sodium-induced colitis. Thus, our findings indicate that NPM1 in ILC3s protects against IBD by regulating mitochondrial metabolism through a p65-TFAM axis.
Subject terms: Innate lymphoid cells, Tumour immunology, Ulcerative colitis
Given associations between colitis and myelodysplastic syndrome (in which nucleophosmin 1 is often mutated), the authors here look at the contribution of nucleophosmin 1 to colitis, showing that it is important for protection mediated by ILC3s owing to effects on mitochondrial metabolism.
Main
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are characterized as chronic and recurring ailments of the gastrointestinal tract1, which is considered a high risk of colitis-associated colorectal cancer (CAC)2–4. The precise pathogenesis of IBD remains unknown, but hypotheses include immune response disorders, alterations in intestinal microbiota, genetic susceptibility and environmental factors2,5.
Myelodysplastic syndrome (MDS) is a hematopoietic stem cell disorder characterized by deficient hematopoiesis, cytopenia of peripheral blood and a predisposition to acute myeloid leukemia (AML)6–8. The cause of MDS is linked to the presence of acquired chromosomal abnormalities and genetic mutations that alter oncogene and tumor suppressor gene function9. Since the first report of seven patients with both IBD and MDS in 1997, numerous cases of concurrent IBD and MDS have been documented9–16. Case studies of patients with IBD indicate a high incidence of AML/MDS in patients with IBD16. A high prevalence of IBD was also found in a large cohort of patients with MDS, suggesting a close association between IBD and MDS15.
Mutations in nucleophosmin 1 (NPM1, also known as B23, numatrin 1 or NO38) are associated with a high risk of MDS and AML6,17. NPM1 was identified as a nucleolar phosphoprotein with multiple functions and binding partners18. NPM1 interacts with many partners in distinct cellular compartments, including nucleolar factors, transcription factors and histones. NPM1 is the most frequently mutated gene in patients with AML19,20, accounting for ~60% of patients with a normal karyotype and 35% of total cases21,22. However, whether NPM1 regulates IBDs remains unknown.
Innate lymphoid cells (ILCs) were characterized as a family of heterogeneous lymphocytes that originate from common lymphoid progenitors in the bone marrow but with the absence of variable antigen receptors23. Group three ILCs (ILC3s) are the most abundant subgroup of ILCs in the gut and are the primary source of interleukin-22 (IL-22). ILC3s expressing the transcription factors retinoid-related orphan receptor gamma t (RORγt)24 and aryl hydrocarbon receptor25,26 produce IL-22, which triggers the synthesis of antimicrobial peptides, such as RegIIIβ and RegIIIγ, by epithelial cells27,28. Thus, ILC3s are at the beginning of a pathway that promotes immunity to infection. In a colon cancer model, Il22−/− mice were observed to undergo accelerated tumorigenesis compared to wild-type (WT) mice29, suggesting a potential protective role for ILC3s in gut homeostasis.
In this study, we investigate the protective role of NPM1 in gut homeostasis and in the prevention of colitis. Using Npm1-haploinsufficient (Npm1+/−) mice, we observed increased susceptibility to colitis and colitis-associated colorectal cancer. NPM1 was abundant in ILC3s and was essential for IL-22 production in response to dextran sulfate sodium (DSS)-induced colitis. Conditional deletion of Npm1 in the ILC3 lineage exacerbated colitis and decreased protective IL-22 secretion. Additionally, heterozygous deletion of Npm1 in ILC3 dysregulated mitochondrial homeostasis, including decreased mitochondrial biogenesis and oxidative phosphorylation (OXPHOS). Mechanistically, we found that NPM1 acted as a transcription cofactor that bound p65 and stimulated mitochondrial transcription factor A (Tfam) transcription in DSS-induced colitis. Thus, our findings demonstrated that NPM1 regulates mitochondrial function and IL-22 production in ILC3s through the p65-TFAM axis, promoting gut homeostasis and protection against IBD.
Results
NPM1 deficiency leads to increased susceptibility to colitis
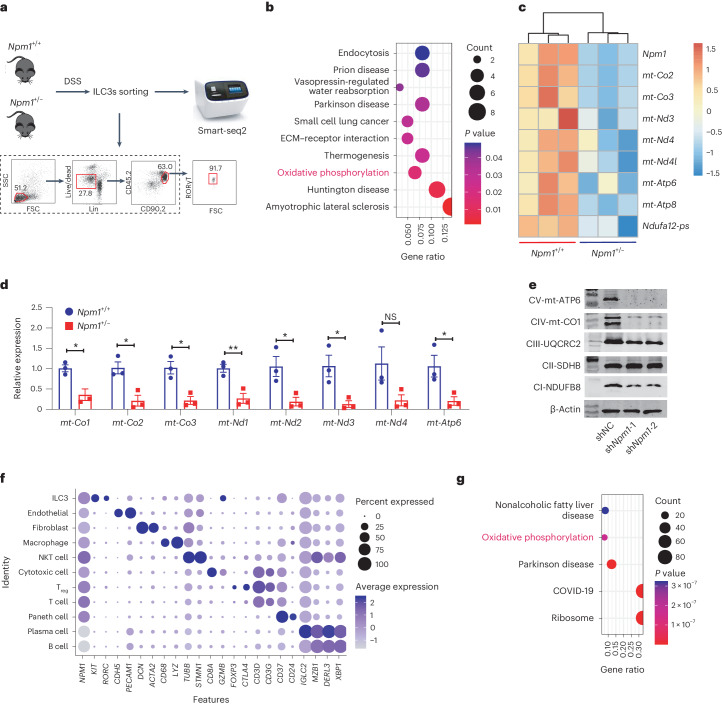
Patients with UC exhibited a decreased abundance of NPM1 in the colon compared to controls (Fig. 1a and Supplementary Table 1). We also observed a trend in reduced NPM1 in patients with CD; however, the reduction compared to controls was not significant (Fig. 1a). Further single-cell RNA-sequencing (scRNA-seq) analysis (GSE182270) on colonic biopsies of patients with UC and healthy control (HC)30 indicated that the expression of NPM1 decreased mainly in ILC3s, macrophages, natural killer T cells (NKT), cytotoxic T cells, regulatory T cells (Treg) and Paneth cells in patients with UC (Extended Data Fig. 1a,b). NPM1 mRNA abundance was also significantly reduced in patients with high-grade colon adenocarcinoma (COAD; stages III and IV), compared to those with low-grade COAD (stages I and II; Extended Data Fig. 1c). Analysis of The Cancer Genome Atlas database revealed that lower NPM1 mRNA correlated with worse overall survival in patients with either COAD or rectum adenocarcinoma (READ; Extended Data Fig. 1d,e). These findings suggested that NPM1 may be involved in the pathology of IBD, especially UC, and may contribute to tumorigenesis.
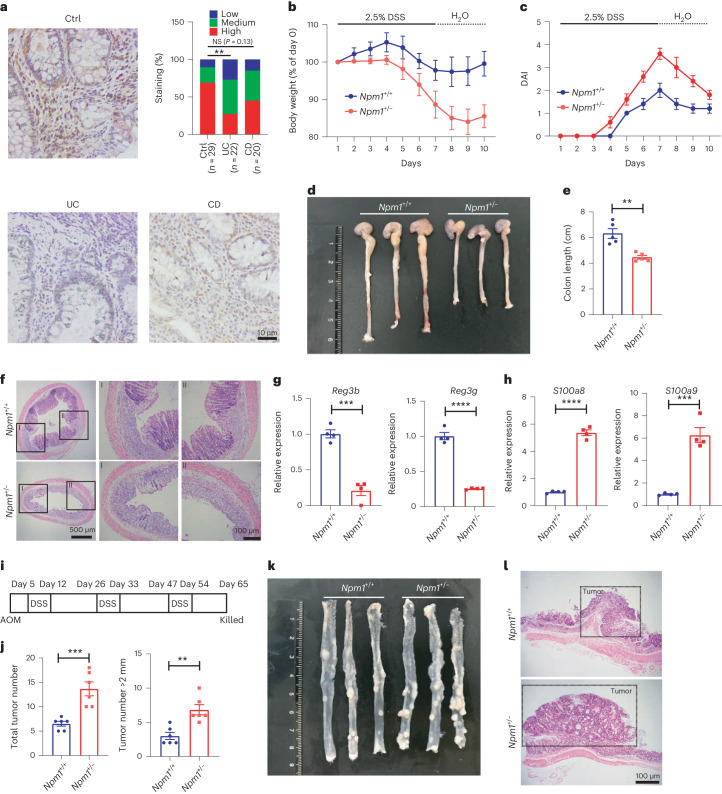
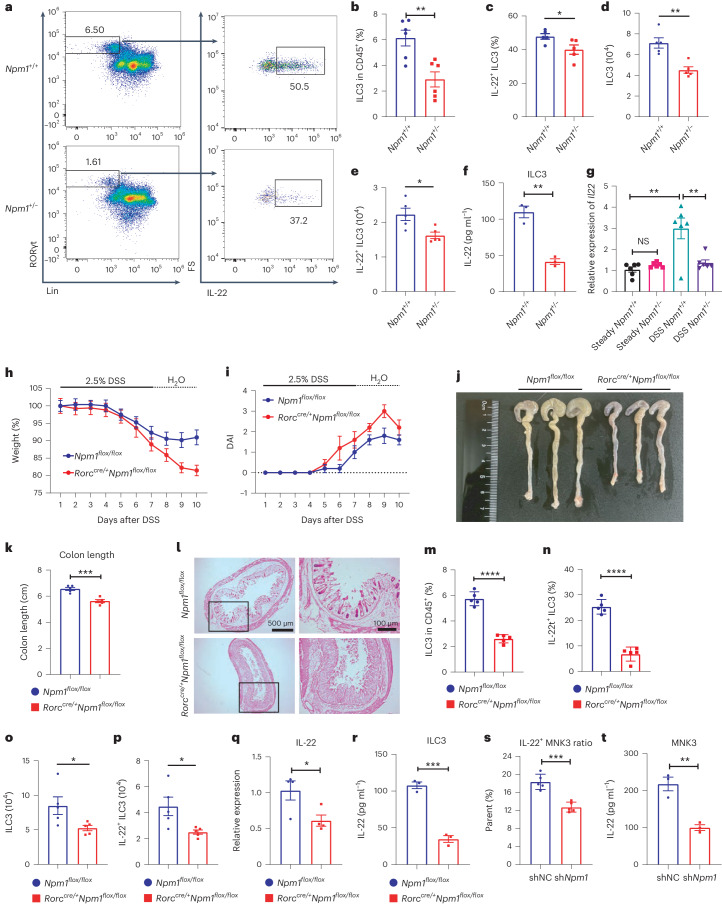
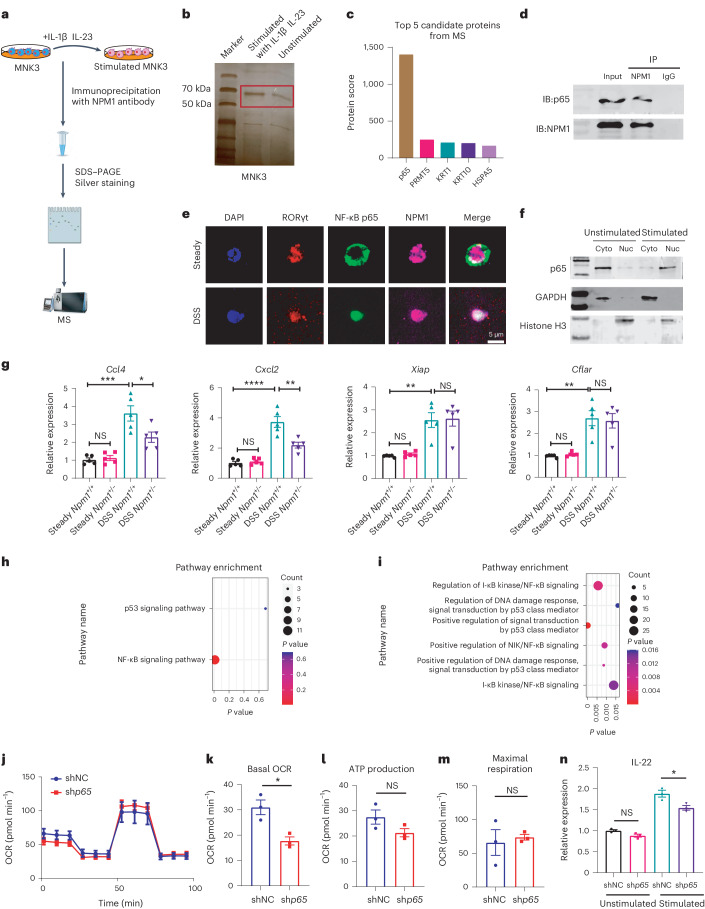
Fig. 1. NPM1 deficiency increases susceptibility to colitis and colonic adenocarcinoma.
a, Immunohistochemistry of NPM1 in colon tissue from patients with IBD (UC, n = 22 individual patients and CD, n = 20 individual patients) and non-IBD (n = 29 individual patients) controls. Scale bars = 10 μm. Immunohistochemistry score of NPM1. Statistical differences were determined by the Mann–Whitney test (**P < 0.01). b–f, Npm1+/− and control Npm1+/+ mice were administered 2.5% DSS for 7 days, followed by 3 days of recovery (H2O). Body weight (b), DAI (a score of inflammation) in the colon (c), colon length on day 10 (d,e) and colon histopathology on day 10 (f) were analyzed (n = 5 individual mice). Scale bars = 500 μm (left) and 100 μm (right). (g,h) RT–PCR analysis of mRNA abundance of Reg3b and Reg3g (g) and S100a8 and S100a9 (h) in the whole colon of mice at day 5 of administration of 2.5% DSS (n = 4 individual mice). i, Diagram of AOM/DSS CAC model. j, Total number of tumors and number of tumors larger than 2 mm in Npm1+/+ and Npm1+/− mice (n = 5 individual mice). k, Representative images of colons with tumors from Npm1+/+ and Npm1+/− mice on day 65 of the AOM/DSS CAC model. l, Histopathology of representative colon tumors from Npm1+/+ and Npm1+/− mice on day 65 of the AOM/DSS CAC model. Scale bars = 100 μm. Data in e, g, h and j are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t test (**P < 0.01, ***P < 0.001 and ****P < 0.0001), unless otherwise indicated. Ctrl, control; NS, not significant; CAC, colitis-associated colorectal cancer.
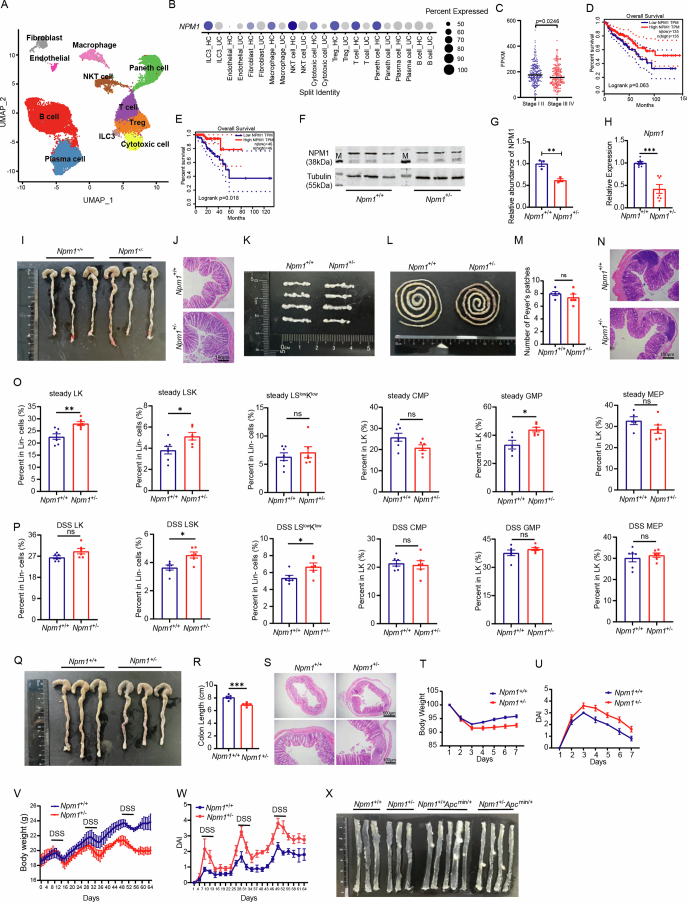
Extended Data Fig. 1. NPM1 deficiency increases susceptibility to enteritis and colonic adenocarcinoma.
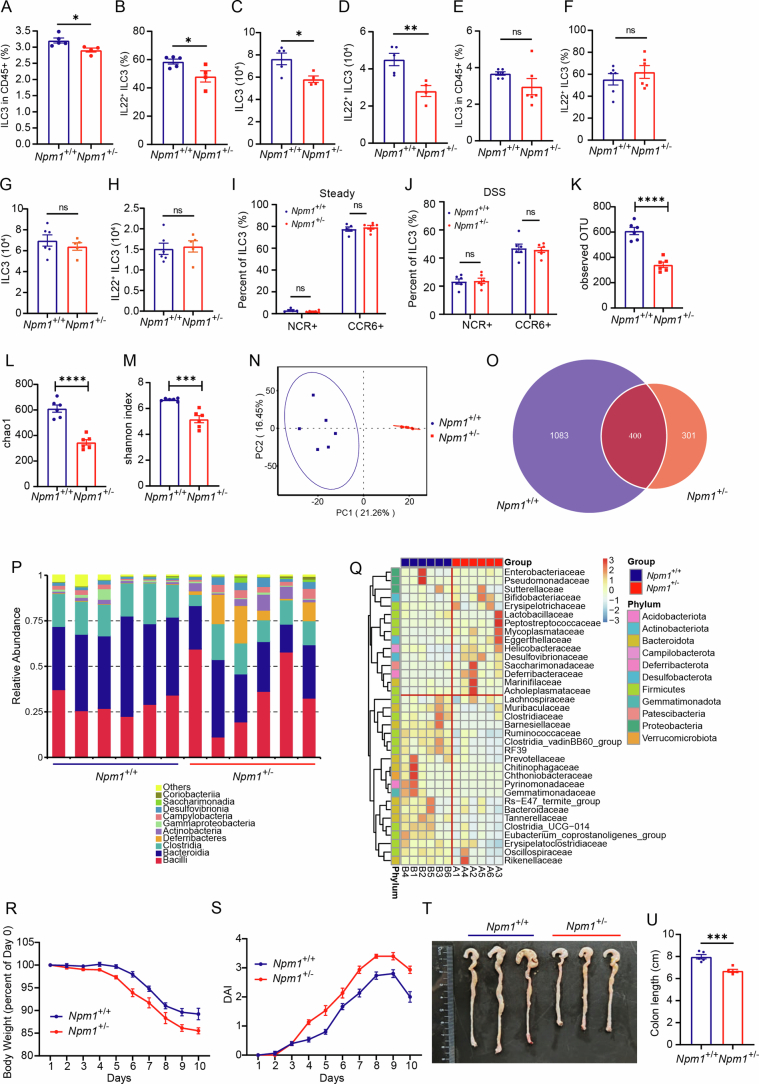
(a) UMAP analysis of GSE182270 shows different clusters of cells in human colonic biopsies. (b) Expression of NPM1 in different clusters (y axis). Dot size represents the fraction of cells within the cluster that express each NPM1. Colors indicate the z-scaled expression of genes in cells within each cluster. (c) Expression of NPM1 in different stages of 425 COAD patients in TCGA. (Stages I and II: n = 238 individual patients; stages III and IV: n = 187 individual patients). (d) Survival analysis of NPM1 in 270 COAD patients in TCGA, cutoff by the median expression of NPM1 in groups. (e) Survival analysis of NPM1 in 92 READ patients in TCGA, cutoff by the median expression of NPM1 in groups. (f,g) Protein expression analysis of NPM1 in the colon of Npm1+/+ and Npm1+/− mice. Quantitative analysis of protein levels of NPM1, relative to tubulin (e) (n = 3 individual mice). (h) RT-PCR analysis of mRNA expression of Npm1 in whole colon of Npm1+/+ and Npm1+/− mice (n = 7 individual mice). (i) Representative images of colons from Npm1+/+ and Npm1+/− mice in steady state. (j) H&E staining of colon from Npm1+/+ and Npm1+/− mice in steady state. Scale bars: 100 μm. (k) Comparison of mesenteric lymph nodes from Npm1+/+ and Npm1+/− mice in steady state (n = 4). (l,m) Representative images of Peyer’s patches from Npm1+/+ and Npm1+/− mice in steady state (l). Analysis of the number of Peyer’s patches (m) was performed (n = 5 individual mice). (n) H&E staining of solitary intestinal lymphoid tissue from Npm1+/+ and Npm1+/− mice in steady state. Scale bars: 100 μm. (o) Ratio of LK, LSK, Lin−Sca1low CD117low cells (Npm1+/+: n = 7 individual mice; Npm1+/−: n = 6 individual mice), CMP, GMP and MEP (Npm1+/+: n = 5 individual mice; Npm1+/−: n = 6 individual mice) in bone marrow from Npm1+/+ and Npm1+/− mice under steady-state. (p) Ratio of LK, LSK, Lin−Sca1low CD117low cells, CMP, GMP and MEP in bone marrow from Npm1+/+ and Npm1+/− mice under steady-state (n = 6 individual mice). (q–u) Npm1+/− and control Npm1+/+ mice were administered trinitrobenzene sulfonic acid (TNBS). Colon length (q,r), colon histopathology on day 7 (s), body weight (t) and DAI (u) were analyzed (n = 5 individual mice). Scale bars: 500 μm (up), 100 μm (down). (v,w) Npm1+/− and control Npm1+/+ mice were treated with AOM-DSS for 65 days, and body weight (v) and DAI (w) were analyzed (n = 5 individual mice). (x) Representative images of colons from CRC mouse model. Data in c, g, h, m, o, p and r are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined two-tailed unpaired Student’s t-test (*p < 0.05, **p < 0.01 and ***p < 0.001).
To explore the putative contribution of NPM1 in gastrointestinal homeostasis and inflammation, we generated Npm1-haploinsufficient (Npm1+/−) mice (Supplementary Fig. 1a–c) and confirmed reduced abundance of NPM1 in the colon (Extended Data Fig. 1f–h). Note that homozygous knockout was lethal. In the absence of injury, colon length and histology were similar between WT mice and Npm1+/− mice (Extended Data Fig. 1i,j). Concurrently, the organogenesis of secondary lymphoid structures, including Peyer’s patches (PP) and mesenteric lymph nodes (MLN), as well as solitary intestinal lymphoid tissue, was unaffected by Npm1 haploinsufficiency (Extended Data Fig. 1k–n). Given the critical role of NPM1 in MDS and AML, we also examined the change of bone marrow (BM) cells in Npm1+/− mice (Supplementary Fig. 2a). Results indicated that the ratio of Lin−c-Kit+ (LK) cells, Lin−Sca1+c-Kit+ (LSK) cells and LSlowKlow cells in the BM of Npm1+/− mice was elevated compared with that of Npm1+/+ mice both in steady state and DSS-induced colitis conditions (Extended Data Fig. 1o,p), which is a characteristic phenotype of MDS21,31,32. Meanwhile, within the LK cell population, the proportion of granulocyte–macrophage progenitors (GMP) increased in Npm1+/− mice, especially in a steady state (Extended Data Fig. 1o,p). Using a DSS colonic injury model (2.5% wt/vol for 7 days), we found that Npm1+/− mice had greater body weight loss and a greater increase in disease activity index (DAI), a marker of inflammation, compared to littermate controls (Fig. 1b,c). On day 10 (3 days into the recovery period), NPM1 deficiency exacerbated inflammation as indicated by reduced colon length and increased epithelial injury, submucosal edema and leukocyte infiltration in the colon (Fig. 1d–f). Additionally, at day 5 of DSS exposure, expression of genes encoding antimicrobial peptides (Reg3b and Reg3g) was reduced and calprotectin (S100a8 and S100a9), a marker of inflammation, was altered in colons of Npm1+/− mice (Fig. 1g,h). We also established a trinitrobenzene sulfonic acid (TNBS)-induced colitis model and evaluated the progress of colitis in WT and Npm1+/− mice. As anticipated, Npm1+/− mice also exhibited reduced colon length and enhanced inflammation, together with greater body weight loss and increased DAI (Extended Data Fig. 1q–u). Collectively, these data indicated that NPM1 has a protective role in the mouse colitis model.
NPM1 inhibits colitis-associated colon tumorigenesis
Patients with IBD have a high risk of developing CAC33,34. To investigate the role of NPM1 in CAC development, we subjected WT mice and Npm1+/− mice to an azoxymethane (AOM)/DSS colon tumor model (Fig. 1i). By the end of the third cycle of DSS treatment, Npm1+/− mice failed to recover body weight and exhibited increased DAI (Extended Data Fig. 1v,w). Compared to WT mice, Npm1+/− mice developed more tumors and a greater number of larger tumors, indicative of a higher tumor burden (Fig. 1j–l). In addition to CAC, which is preceded by chronic inflammation, sporadic colorectal cancer (CRC) is a form of CRC that is often caused by mutations in the gene APC35. To examine the role of NPM1 in sporadic CRC, we crossed Npm1+/− mice with Apcmin/+ mice and fed them a Western diet to accelerate tumorigenesis. Results showed that there were no significant differences in colonic tumor load between Apcmin/+ mice and Npm1+/−Apcmin/+ mice (Extended Data Fig. 1x), suggesting that sporadic CRC arising from APC mutations does not involve NPM1. Collectively, these findings indicated that NPM1 has a pivotal role in impeding colitis-associated colon tumorigenesis by restricting tumor development and growth.
Protection against colitis requires NPM1 in hematopoietic cells
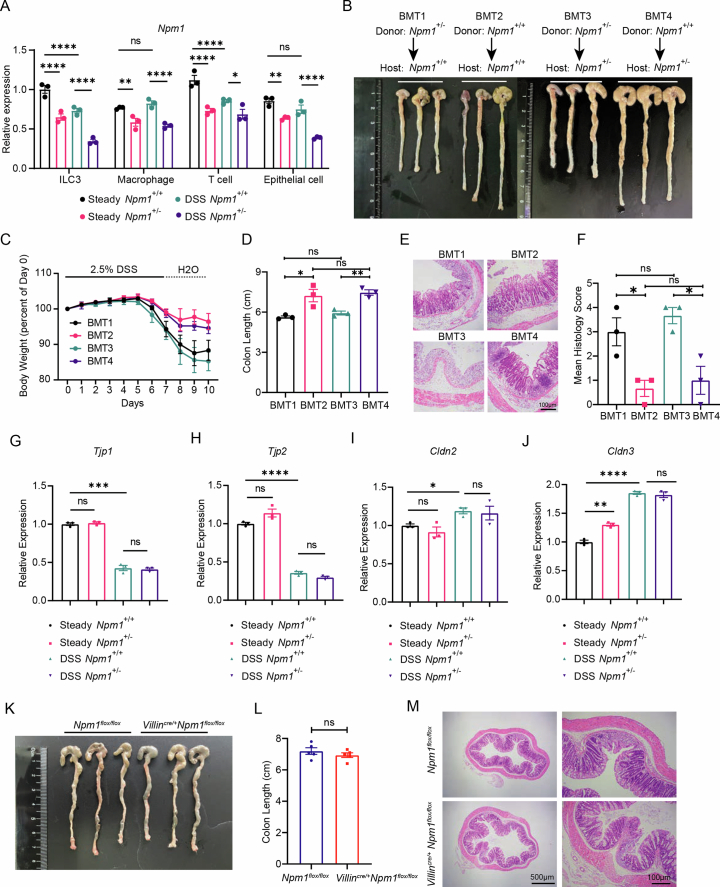
Given that Npm1 is expressed by many types of cells and decreased under pathological conditions (Extended Data Figs. 1b and 2a), it is unclear whether the exacerbated colitis in Npm1+/− mice is due to defects in hematopoietic or nonhematopoietic cells, particularly colonic epithelial cells. Thus, we established BM chimeras with Npm1 deficiency in these distinct cellular populations (Extended Data Fig. 2b). After a 7-day DSS treatment, mice receiving Npm1-haploinsufficient BM exhibited more severe colitis compared to mice receiving Npm1 WT BM cells. However, when the same donor BM was used regardless of the genotype of the host mice, there were no significant differences in body weight, colon length or histological features (Extended Data Fig. 2c–f), suggesting that the hematopoietic compartment is the main functional compartment for NPM1. We also detected the expression of tight junction genes (including Tjp1, Tjp2, Cldn2 and Cldn3) in epithelial cells, which are pivotal for the maintenance of intestinal barrier function36. With the exception of Cldn3, which is diminished in Npm1-haploinsufficient mice under physiological conditions, the expression of other tight junction genes remains relatively unchanged between two groups of mice in both physiological and pathological conditions (Extended Data Fig. 2g–j). We also generated Npm1flox/flox mice (Supplementary Fig. 1d–f) and crossed them with Villincre/+ mice to directly assess a role in protection against colitis for NPM1 in colonic epithelial cells. However, there was no obvious alteration in colon length and histological features between Villincre/+Npm1flox/flox mice and control mice (Extended Data Fig. 2k–m). Taken together, these data showed that impaired gut homeostasis and exacerbated inflammation in Npm1+/− mice are mainly caused by the heterozygous deletion of Npm1 in the hematopoietic compartment.
Extended Data Fig. 2. NPM1 in hematopoietic system is essential to colon against colitis.
(a) RT-PCR analysis of mRNA abundance of Npm1 in ILC3s, macrophages, T cells and epithelial cells from Npm1+/+ and Npm1+/− mice exposed to 2.5% DSS or water (steady state) (n = 3 individual mice). (b–f) Bone marrow chimeric mice of indicated genotypes were treated with 2.5% DSS water for 7 days, and representative images of the mouse colons on day 10 of the DSS model (b), body weight (c), colon length (d) and histopathology (e,f) were analyzed (n = 3 individual mice). Scale bars represent 100 μm. (g–j) RT-PCR analysis of mRNA expression of the indicated genes in epithelial cells from Npm1+/+ and Npm1+/− mice exposed to 2.5% DSS or water (steady state) (n = 3 individual mice). (k–m) Villincre/+Npm1+/− and control Npm1flox/flox mice were treated with 2.5% DSS for 7 days, and representative images (k), colon length (l) and histopathology (m) were analyzed (n = 5 individual mice). Scale bars: 500 μm (left), 100 μm (right). Data are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-way ANOVA (a) and two-tailed unpaired Student’s t-test (d,f–j,l) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
NPM1 is critical for maintaining IL-22-producing ILC3s
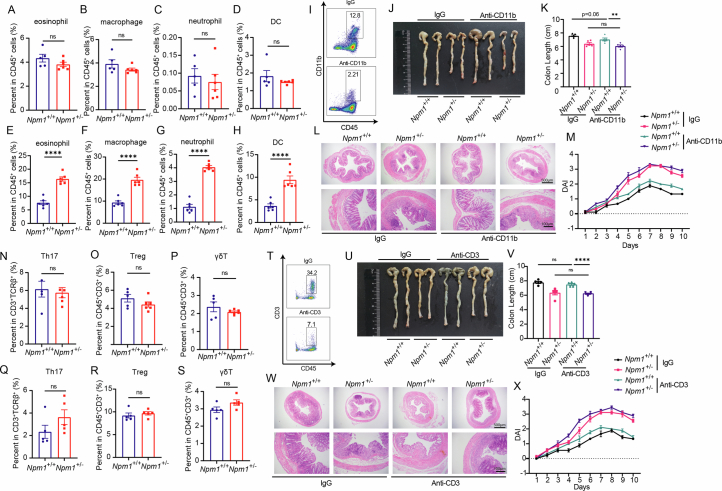
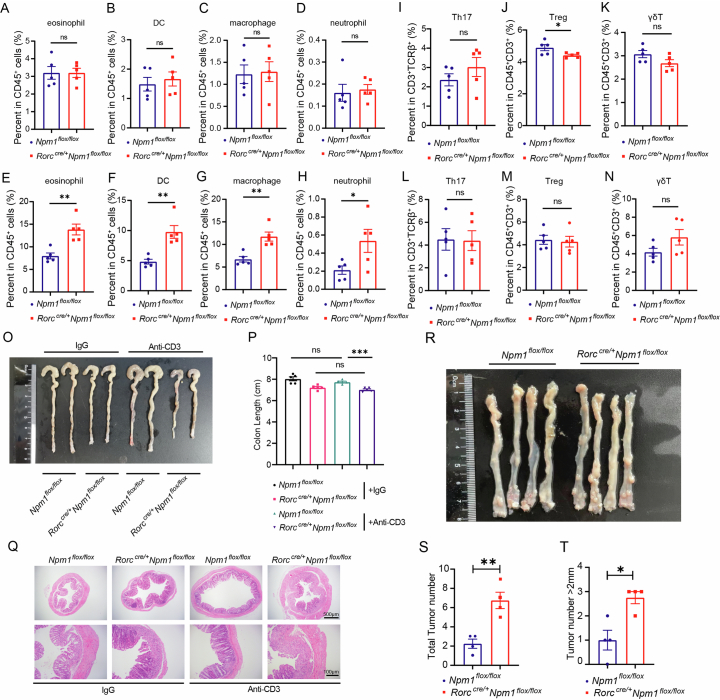
Subsequently, we investigated the type of gut immune cells involved in limiting gut inflammation by NPM1. The ratio of macrophages, neutrophils, eosinophils and dendritic cells (DCs) infiltrated in intestinal lamina propria leukocytes (LPLs) exhibited few changes between WT and Npm1+/− mice in steady state (Supplementary Fig. 2b and Extended Data Fig. 3a–d). However, in DSS-induced colitis, an elevation of these cells was observed in Npm1+/− mice compared to WT mice (Extended Data Fig. 3e–h). It’s known that infiltration of myeloid cells into the intestinal lamina propria is considered a common cause of progressive colitis37. Furthermore, clearance of CD11b+ myeloid cells failed to rescue the exacerbated enteritis in Npm1+/− mice, suggesting that NPM1 in myeloid cells was insufficient to regulate intestinal inflammation (Extended Data Fig. 3i–m). Likewise, evaluation of T cells (TH17, Treg and γδT cells) coupled with comparable colitis in two genotype mice after deletion of CD3+ T cells indicated that exacerbated colitis in Npm1-haploinsufficient mice was not attributed to T cells (Supplementary Fig. 2c and Extended Data Fig. 3n–x).
Extended Data Fig. 3. Regulation of colitis by NPM1 is independent of myeloid cells and T cells.
(a–h) Proportion of eosinophil, macrophage, neutrophil and dendritic cells (DCs) in lamina propria lymphocytes (LPLs) of Npm1+/+ and Npm1+/− mice under steady-state conditions (a–d) (Npm1+/+: n = 5 individual mice; Npm1+/−: n = 6 individual mice) and during DSS-induced colitis (e–h) (n = 6 individual mice) state are shown. (i–m) Colitis in Npm1+/+ and Npm1+/− mice was induced by DSS following administration with IgG or anti-CD11b blocking antibody. Deletion of CD11b+ cells by antibody (i). Representative images of colons (j), colon length (k) (n = 5 individual mice), colon histopathology on day 10 (l) and DAI (m) (n = 3 individual mice) are presented. Mice were injected with CD11b antibody (100 μg per mouse) every 2 days (from day −2 to day 6). Scale bars: 500 μm (up), 100 μm (down). (n–s) Proportion of TH17, Treg and γδT in LPLs of Npm1+/+ and Npm1+/− mice under steady-state conditions (n–p) (Npm1+/+: n = 5 individual mice; Npm1+/−: n = 6 individual mice) and during DSS-induced colitis (q–s) (n = 5 individual mice) state are shown. (t–x) DSS-induced colitis in Npm1+/+ and Npm1+/− mice was established following administration with IgG or anti-CD3 blocking antibody. Deletion of CD3+ cells by antibody (t). Representative images of colons (u), colon length (v) (n = 5 individual mice), colon histopathology (w) and DAI (x) (n = 3 individual mice) on day 10 are presented. Mice were injected with CD3 antibody (50 μg per mouse) once a day (from day −2 to day 6). Scale bars: 500 μm (up), 100 μm (down). Data in a–h, k, m, n–s, v and x are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined two-tailed unpaired Student’s t-test (**p < 0.01 and ****p < 0.0001).
We then investigated the effect of Npm1 haploinsufficiency on colonic ILC3s (Supplementary Fig. 2d). The population of colonic ILC3s and IL-22+ ILC3s decreased in Npm1+/− mice compared to Npm1+/+ mice after DSS administration, suggesting that haploinsufficient of Npm1 affects ILC3 expansion and function (Fig. 2a–e). Additionally, Npm1+/− ILC3 exhibited similar alterations in TNBS-induced colitis (Extended Data Fig. 4a–d). However, these changes were not observed under physiological conditions (Extended Data Fig. 4e–h). Further analysis revealed that there were no evident alterations in proportions of NCR+ ILC3 and CCR6+ ILC3 between WT and Npm1+/− mice under physiological or pathological conditions (Extended Data Fig. 4i,j). Moreover, isolated ILC3s from Npm1+/− mice produced less IL-22 compared with ILC3s from WT mice after DSS administration (Fig. 2f). In addition, the expression of Il22 was also decreased in isolated ILC3s from Npm1+/− mice exposed to DSS, but the expression of Il22 was similar in both genotypes under steady state (Fig. 2g). The decreased production of IL-22 in ILC3s may contribute to the observed dysregulation of Reg3b and Reg3g in Npm1+/− mice in DSS-induced colitis (Fig. 1g), and thus impaired intestinal microbiota homeostasis. There was a rapid decrease in observed operational taxonomic unit, Chao1 index and Shannon index in Npm1+/− mice (Extended Data Fig. 4k–m), indicating that microbiota diversity was repressed by Npm1 heterozygote deletion. Moreover, feces from Npm1+/− mice and WT mice showed a remarkable change in bacterial composition (Extended Data Fig. 4n–q). However, cohousing littermate Npm1+/− mice still exhibited more pronounced exacerbation of enteritis compared to WT mice (Extended Data Fig. 4r–u), indicating that changes in the gut microbiota are not the priori drivers of the exacerbated inflammation in Npm1+/− mice but may instead contribute to a certain extent to the exacerbation of enteritis. Collectively, our results indicated that NPM1 is important for the protective function of ILC3s in the gut immune microenvironment.
Fig. 2. NPM1 is required for maintaining the frequency and function of colonic ILC3s.
a, Colon LPLs were isolated from Npm1+/+ and Npm1+/− mice at day 5 of administration of 2.5% DSS. Analysis of ILC3s (live CD45+Lin−RORγt+ cells) and IL-22-producing ILC3s (live CD45+Lin−RORγt+IL-22+ cells) by flow cytometry. Numbers indicate percentages of cells in each outlined region. b,c, The proportion of CD45+ cells that are ILC3s (b; n = 6 individual mice) and the proportion of IL-22+ ILC3s in the total ILC3 population (c; n = 5 individual mice) in LPLs of Npm1+/+ and Npm1+/− mice after DSS administration are shown. d,e, Number of ILC3s (d) and IL-22+ ILC3s (e) in LPLs of Npm1+/+ and Npm1+/− mice after DSS administration are depicted (n = 5 individual mice). f, ILC3s, isolated by cell sorting from LPLs of Npm1+/+ and Npm1+/− mice after DSS administration, were analyzed by ELISA for IL-22 (n = 3 individual mice). g, Relative mRNA abundance of Il22 in ILC3s, isolated by cell sorting from the LPL of Npm1+/+ and Npm1+/− mice exposed to 2.5% DSS or water (steady state), was analyzed. The results are shown relative to the amount in cells from Npm1+/+ mice exposed to water (steady state; n = 6 individual mice). h–l, Npm1flox/flox and Rorccre/+Npm1flox/flox mice were administered 2.5% DSS for 7 d followed by 3 d of recovery. Body weight (h), DAI (i), colon length (j,k) and colon histopathology on day 10 (l) were analyzed (n = 5 individual mice). Scale bars = 500 μm (left) and 100 μm (right). m,n, LPLs were isolated from Npm1flox/flox and Rorccre/+Npm1flox/flox mice after 5 days of administration of 2.5% DSS (n = 5 individual mice). The proportion of CD45+ cells that are ILC3s (m) and the proportion of IL-22+ ILC3s in the total ILC3 population (n) are shown. o,p, The number of ILC3s (o) and IL-22+ ILC3s (p) in LPLs of Npm1flox/flox and Rorccre/+Npm1flox/flox mice after DSS administration are depicted (n = 5 individual mice). q, Relative mRNA abundance of Il22 in ILC3s, isolated by cell sorting from the LPL of Npm1flox/flox and Rorccre/+Npm1flox/flox mice at day 5 of administration of 2.5% DSS was analyzed (n = 4 individual mice). r, ILC3s, isolated by cell sorting from LPLs of Npm1flox/flox and Rorccre/+Npm1flox/flox, were analyzed by ELISA for IL-22 (n = 3 individual mice). s,t, IL-22 production by MNK3 cells after stimulation with IL-1β and IL-23 in vitro by flow cytometry (s; n = 5 individual mice) and by ELISA (t; n = 3 individual mice). Data in b–g, k and m–t are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001). LPLs, lamina propria leukocytes.
Extended Data Fig. 4. Changes of ILC3 may contribute to dysbiosis of fecal microflora under pathological conditions.
(a–d) LPLs were isolated from Npm1+/+ and Npm1+/− mice on day 5 after administration of TNBS. (a) Proportion of ILC3s in Lin− cells. (b) Proportion of IL-22+ ILC3s in the total ILC3 population. Number of ILC3s (c) and IL-22+ ILC3s (d) are shown (Npm1+/+: n = 5 individual mice; Npm1+/−: n = 4 individual mice). (e–h) ILC3s in LPLs of Npm1+/+ and Npm1+/− mice under steady-state. (e) Proportion of ILC3s in Lin− cells. (f) Proportion of IL-22+ ILC3s in the total ILC3 population (n = 6 individual mice). Number of ILC3s (g) and IL-22+ ILC3s (h) are depicted (Npm1+/+: n = 6 individual mice; Npm1+/−: n = 5 individual mice). (i,j) Proportion of NCR+ ILC3s and CCR6+ ILC3s in total ILC3s from Npm1+/+ and Npm1+/− mice under steady-state (i) (n = 5 individual mice) and during DSS-induced colitis (j) (n = 6 individual mice). (k–q) Feces from Npm1+/+ mice and Npm1+/− mice under colitis were collected to analyze intestinal microbiota by 16S rRNA sequencing. (k) Observed operational taxonomic unit (OTU), (l) Chao1 index, (m) Shannon–Wiener diversity index (Shannon index) and (n) principal coordinates analysis (PCoA). (o) Venn diagram of two groups of fecal microbiota. (p) The relative abundance of microbiota at phylum level in the fecal samples. (q) Heatmap analysis of the relative abundance of microbiota at family level in the fecal samples. (n = 6 individual mice). (r–u) Co-housed Npm1+/− and control Npm1+/+ mice were administered 2.5% DSS for 7 days, followed by 3 days of recovery (H2O). Body weight (r), DAI (s) and colon length on day 10 (t,u) were analyzed (n = 5 individual mice). Scale bars: 500 μm (left), 100 μm (right). Data are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t-test (a–h,k–m,u) and two-way ANOVA (i,j) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
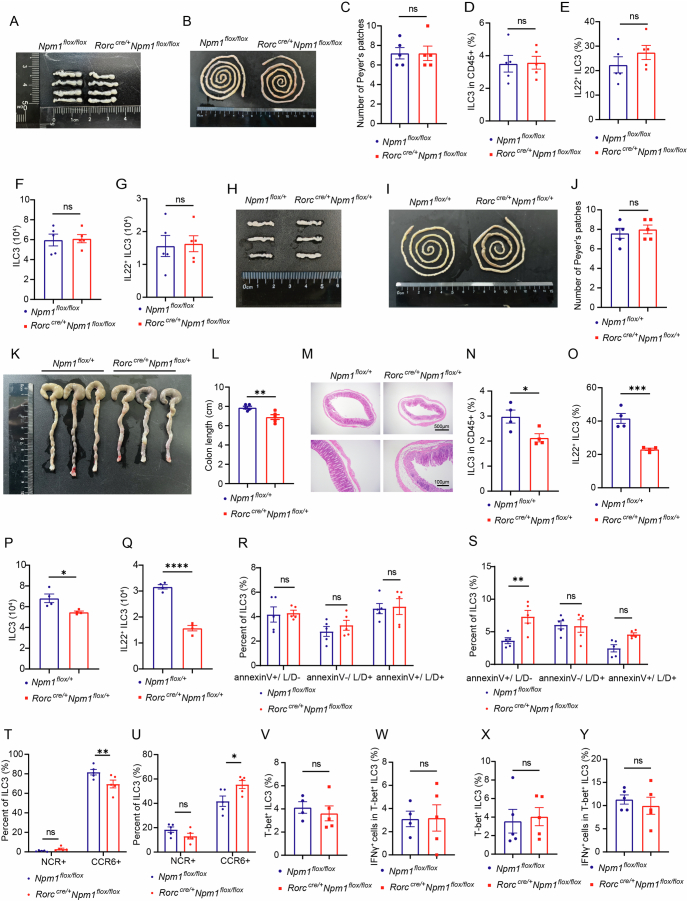
To specifically decipher the cell-intrinsic role of Npm1 in colonic ILC3s, we generated Rorccre/+ Npm1flox/flox mice that lack Npm1 on ILC3s and subjected the mice to DSS-induced colitis. The development of PP and MLN was unimpaired in Rorccre/+Npm1flox/flox mice (Extended Data Fig. 5a–c). Frequencies of intestinal ILC3 and IL-22+ ILC3 in Rorccre/+Npm1flox/flox mice were also comparable with those of the control group (Extended Data Fig. 5d–g). However, compared to Npm1flox/flox mice, Rorccre/+Npm1flox/flox mice exhibited greater loss of body weight and increased DAI (Fig. 2h–i), indicating exacerbated inflammation following DSS administration. When killed on day 10 (3 days after recovery), Rorccre/+Npm1flox/flox mice exhibited decreased colon length and greater features of colon injury (Fig. 2j–l). The frequencies of colonic ILC3s and IL-22+ ILC3s were also decreased in Rorccre/+Npm1flox/flox mice after DSS administration (Fig. 2m–p). Without development defects, heterozygous deletion of Npm1 in ILC3 also contributed to exacerbated enteritis and reduction of ILC3, which appears to be in a dose-dependent manner (Extended Data Fig. 5h–q). Furthermore, the percentage of apoptotic ILC3s was increased in Rorccre/+Npm1flox/flox mice in DSS-induced colitis (Supplementary Fig. 2e and Extended Data Fig. 5r,s). The proportion of CCR6+ ILC3 in total ILC3s was higher in Rorccre/+Npm1flox/flox mice than that in Npm1flox/flox mice under pathological conditions, which was opposite in steady state (Extended Data Fig. 5t,u). The proportion of interferon-γ (IFNγ)-producing ex-ILC3 was also unchanged between these two groups of mice with or without DSS administration (Supplementary Fig. 2f and Extended Data Fig. 5v–y). Additionally, consistent with changes observed in Npm1+/− mice, the increased infiltration of myeloid cells also existed in Rorccre/+Npm1flox/flox mice under pathological conditions (Extended Data Fig. 6a–h). Because RORc-Cre will also delete Npm1 in conventional T cells and γδT cells, we examined the function of various T cell subsets and excluded their contributions to exacerbated colitis in Rorccre/+Npm1flox/flox mice by depleting T cells using a CD3 antibody (Extended Data Fig. 6i–q). Moreover, isolated ILC3s from Rorccre/+Npm1flox/flox mice showed less Il22 expression and IL-22 production compared with Npm1flox/flox ILC3s (Fig. 2q,r). We also confirmed our findings in vitro using the ILC3 cell line, MNK3. MNK3 cells retain phenotypic and functional features characteristic of mouse primary ILC3s, including the production of IL-17A and IL-22 when stimulated with IL-23 and IL-1β38,39. Knockdown of Npm1 in MNK3 significantly suppressed the secretion of IL-22 upon stimulation (Fig. 2s,t). Furthermore, Rorccre/+Npm1flox/flox mice developed more tumors compared with Npm1flox/flox mice when subjected to AOM/DSS (Extended Data Fig. 6r–t). Collectively, these data supported that NPM1 in ILC3s is critical for gut homeostasis under injury conditions and limiting inflammation.
Extended Data Fig. 5. NPM1 is required for maintaining the frequency and function of colonic ILC3s.
(a) Images of mesenteric lymph nodes from Npm1flox/flox and Rorccre/+Npm1flox/flox mice (n = 4 individual mice). (b,c) Representative images of Peyer’s patches from Npm1flox/flox and Rorccre/+Npm1flox/flox mice in steady state. The number of Peyer’s patches (c) was analyzed (n = 5 individual mice). (d–g) ILC3s in LPLs of Npm1flox/flox and Rorccre/+Npm1flox/flox mice under steady-state. (d) Proportion of ILC3s in Lin− cells. (e) Proportion of IL-22+ ILC3s in the total ILC3 population. Number of ILC3s (f) and IL-22+ ILC3s (g) are depicted (n = 5 individual mice). (h) Images of mesenteric lymph nodes from Npm1flox/flox and Rorccre/+Npm1flox/flox mice (n = 3 individual mice). (i,j) Representative images of Peyer’s patches from Npm1flox/+ and Rorccre/+Npm1flox/+ mice in steady state (i). The number of Peyer’s patches (j) was analyzed (n = 5 individual mice). (k–m) Npm1flox/+and Rorccre/+Npm1flox/+mice were administered 2.5% DSS for 7 days followed by 3 days of recovery. Representative images of colons (k), colon length (l) and colon histopathology on day 10 (m) are presented. (n = 4 individual mice). Scale bars: 500 μm (up), 100 μm (down). (n–q) ILC3s in LPLs of Npm1flox/+ and Rorccre/+Npm1flox/+mice with colitis. (n) Proportion of ILC3s in Lin− cells. (o) Proportion of IL-22+ ILC3s in the total ILC3 population. Number of ILC3s (p) and IL-22+ ILC3s (q) are provided (n = 4 individual mice). (r,s) Apoptotic percentage of ILC3s in LPLs from Npm1flox/flox and Rorccre/+Npm1flox/flox mice was detected by Annexin V staining under steady-state (r) and during DSS-induced colitis (s) (n = 5 individual mice). (t,u) Proportion of NCR+ ILC3s and CCR6+ ILC3s in total ILC3s from colon of Npm1flox/flox and Rorccre/+Npm1flox/flox mice under steady-state (t) and during DSS-induced colitis (u) (n = 5 individual mice). (v–y) Proportion of T-bet+ ILC3s in LPLs and IFNγ+ cells in T-bet+ ILC3s from Npm1flox/flox and Rorccre/+Npm1flox/flox mice under steady-state (v,w) (n = 4 individual mice) and during DSS-induced colitis (x,y) (n = 5 individual mice). Data are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t-test (c–g, j, l, n–q and v–y) and two-way ANOVA (r–u) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Extended Data Fig. 6. Exacerbate enteritis in Rorccre/+Npm1flox/flox mice under DSS is independent of T cells.
(a–n) Proportions of eosinophil, dendritic cells (DCs), macrophages, neutrophils, TH17, Treg and γδT in LPLs from Npm1flox/flox and Rorccre/+Npm1flox/flox mice under steady-state (a–d,i–k) and during DSS-induced colitis (e–h,l–n) (n = 5 individual mice). (o–q) Colitis in Npm1flox/flox and Rorccre/+Npm1flox/flox mice was induced by DSS following administration with IgG or anti-CD3 blocking antibody. Representative images of colons (o), colon length (p) and colon histopathology (q) on day 10 are presented (n = 5 individual mice). Mice were injected with CD3 antibody (50 μg per mouse) once a day (from day −2 to day 6). Scale bars: 500 μm (up), 100 μm (down). (r) Representative images of colons with tumors from Npm1flox/flox and Rorccre/+Npm1flox/flox on day 65 of the AOM/DSS CAC model. (s,t) Total number of tumors (s) and number of tumors larger than 2 mm (t) in Npm1flox/flox and Rorccre/+Npm1flox/flox mice (n = 4 individual mice). Data in a–n, p, s and t are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined two-tailed unpaired Student’s t-test (*p < 0.05, **p < 0.01 and ***p < 0.001).
NPM1 promotes mitochondrial gene expression in ILC3s
To uncover mechanisms by which NPM1 regulates ILC3 expansion and function, we performed RNA-seq (smart-seq2) of Live+Lin−CD45lowCD90high LPLs27,40 from colon of WT and Npm1+/− mice with colitis induced by DSS treatment (Fig. 3a). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that the OXPHOS pathway is a top differentially modulated pathway in Npm1-haploinsufficient mice compared to WT mice (Fig. 3b). We also observed decreased expression of several genes encoding mitochondrial complex subunits of OXPHOS in Npm1+/− mice, specifically those for electron transport chain (ETC) complex I (mt-Nd3, mt-Nd4, mt-Nd4l and Ndufa12-ps), complex IV (mt-Co2 and mt-Co3) and complex V (mt-Atp6 and mt-Atp8; Fig. 3c) and confirmed these findings by RT–PCR (Fig. 3d). However, the universal decrease of mtDNAs was not observed in epithelial cells, macrophages or T cells of Npm1+/− mice (Extended Data Fig. 7a–c). The abundance of NDUFB8 (complex I), mt-CO1 (complex IV) and mt-ATP6 (complex V) was also notably reduced due to Npm1-knockdown in MNK3 cells (Fig. 3e). These results indicated that NPM1 has a role in regulating the OXPHOS pathway in ILC3s.
Fig. 3. NPM1 regulates the OXPHOS pathway in ILC3s.
a, RNA-seq analysis of colonic ILC3 isolated from Npm1+/+ and Npm1+/− mice at day 5 of administration of 2.5% DSS (n = 3). b, KEGG pathway enrichment analysis of downregulated genes in Npm1+/− mice (n = 3 individual mice). c, Heatmap of selected DEGs encoding proteins involved in OXPHOS in ILC3s between Npm1+/+ and Npm1+/− mice (n = 3 individual mice). d, RT–PCR analysis of mRNA abundance of the indicated genes in ILC3s, isolated by cell sorting from the LPL of Npm1+/+ and Npm1+/− mice at day 5 of administration of 2.5% DSS (n = 3 individual mice). e, Western blot showing the abundance of selected mitochondrial complex components in MNK3 cells. The samples were derived from the same experiment, and the blots were processed in parallel. f, Single-cell analysis of colonic samples of patients with UC from the GEO database (GSE182270). Representative DEGs (x axis) by cluster (y axis) with dot size representing the fraction of cells within the cluster that express each gene and colors indicating the z-scaled expression of genes in cells within each cluster. g, KEGG pathway enrichment analysis of upregulated genes in NPM1high ILC3s compared to NPM1low ILC3s from the data of patients with UC. Data in d is representative of two independent experiments, shown as the mean ± s.e.m., and statistical significance was determined by two-way ANOVA (*P < 0.05 and **P < 0.01).
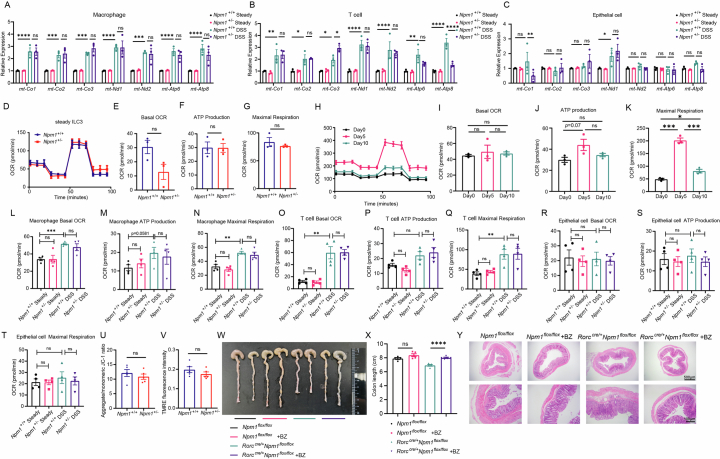
Extended Data Fig. 7. NPM1 is essential for mitochondrial function in ILC3s.
(a–c) RT-PCR analysis of mRNA abundance of the indicated genes in macrophage (a), T cells (b) and epithelial (c), sorted from the LPLs of Npm1+/+ and Npm1+/− mice in steady state or at day 5 of administration of 2.5% DSS (n = 3 individual mice). (d–g) Cell mito stress test was performed with sorted colonic ILC3s from Npm1+/+ and Npm1+/− mice under steady-state. Representative oxygen consumption rate profile (OCR) (d), basal OCR (e), ATP production (f) and maximal respiration (g) are shown (n = 3 individual mice). (h–k) Cell mito stress test was conducted with sorted colonic ILC3s from wild-type (WT) mice on days 0, 5 and 10 of DSS-induced colitis. Representative OCR (h), basal OCR (i), ATP production (j) and maximal respiration (k) are presented (n = 3 individual mice). (l–t) Cell mito stress test was performed with sorted colonic macrophages (l–n), T cells (o–q), epithelial cells (r–t) from LPLs of Npm1+/+ and Npm1+/− mice at day 5 of administration of 2.5% DSS (n = 4 individual mice). (u,v) Mitochondrial membrane potential was assessed with the indicator JC-1 (u) and TMRE (v) in isolated colonic ILC3s from Npm1+/+ and Npm1+/− mice in steady state (n = 5 individual mice). (w–y) Npm1flox/flox and Rorccre/+Npm1flox/flox mice were treated with bezafibrate (i.g., 10 mg/kg) and administered 2.5% DSS for 7 days followed by 3 days of recovery. Representative colon images (w), colon length (x) and colon histopathology (y) are shown (n = 5 individual mice). Scale bars: 500 μm (up), 100 μm (down). Data are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-way ANOVA (a–c) and two-tailed unpaired Student’s t-test (e–g,i–v,x) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
To validate that NPM1 regulates OXPHOS in ILC3s in humans as well, scRNA-seq data of human colonic biopsies (GSE182270)30 was analyzed, and the ILC3 cluster was identified based on higher expression of KIT, RORC but lower expression of CTLA4, CD3D and CD3G (markers of T cells; Fig. 3f). Although NPM1 is broadly expressed across all clusters, ILC3s were among those with comparatively high expression (Fig. 3f). Similar to our mouse data, KEGG pathway enrichment analysis of differentially expressed genes (DEGs) between NPM1high and NPM1low ILC3s in patients with UC identified the OXPHOS pathway among the top five pathways regulated by NPM1 (Fig. 3g). These findings suggested that altered cellular metabolism through the OXPHOS pathway in ILC3s represents a potential mechanism by which NPM1 activity influences UC.
Lack of NPM1 impairs mito-OXPHOS and biogenesis in ILC3s
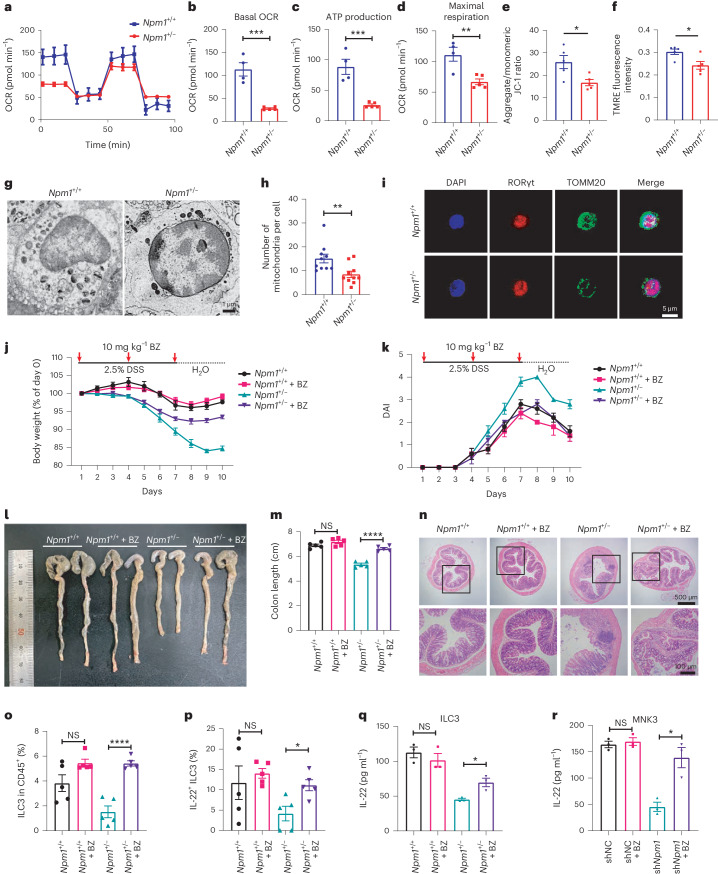
According to the abovementioned results, mitochondrial OXPHOS is probably impaired in Npm1-haploinsufficient ILC3s (Fig. 3c–e). Therefore, we evaluated OXPHOS in isolated ILC3s from DSS-induced Npm1+/− mice and WT mice. Heterozygous deletion of Npm1 in ILC3s reduced oxygen consumption rate (OCR) in response to DSS (Fig. 4a). Compared to WT ILC3s, Npm1+/− ILC3s exhibited a marked reduction in basal OCR, ATP production and maximal respiration (Fig. 4b–d), indicating that mitochondrial OXPHOS in ILC3s was impaired by insufficient NPM1. However, such impaired mitochondrial function in Npm1+/− ILC3s was not observed under physiological conditions (Extended Data Fig. 7d–g). In the DSS model, mouse intestinal ILC3s exhibited a dramatic mitochondrial activation in the acute tissue damage phase (day 5) and then partially restored to a normal state in the repair phase (day 10; Extended Data Fig. 7h–k). The inadequate mitochondrial activation of ILC3 in the acute phase caused by heterozygous deletion of Npm1 could lead to exacerbated colitis (Fig. 4b–d). Besides, epithelial cells, macrophages and T cells in Npm1+/− mice exhibited few differences in OXPHOS compared to those in WT mice in both steady state and DSS-induced colitis conditions (Extended Data Fig. 7l–t). Moreover, the mitochondrial membrane potential of Npm1+/− ILC3s was also reduced significantly compared with that of WT ILC3s only under pathological conditions (Fig. 4e,f and Extended Data Fig. 7u,v). These results showed the importance of NPM1 in maintaining mitochondrial OXPHOS in ILC3s.
Fig. 4. Npm1 is essential for maintaining mitochondrial OXPHOS and biogenesis in ILC3s.
a–d, Cell mito stress test was performed with isolated colonic ILC3s from Npm1+/+ (n = 4 individual mice) and Npm1+/− (n = 5 individual mice) mice. Representative OCR profile (a), basal OCR (b), ATP production (c) and maximal respiration (d) are shown. e,f, Mitochondrial membrane potential was assessed with the indicator 5,5ʹ,6,6ʹ-tetrachloro-1,1ʹ,3,3ʹ-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (e) and TMRE (f) in isolated colonic ILC3s from Npm1+/+ and Npm1+/− mice under DSS (n = 5 individual mice). g, Ultrastructural analysis of mitochondria by SEM of isolated colonic ILC3s from Npm1+/+ and Npm1+/− mice. Scale bar = 1 μm. h, The number of mitochondria per cell was counted in SEM images (n = 10 fields per group). i, TOMM20 in ILC3s from Npm1+/+ and Npm1+/− mice was detected by immunofluorescence staining. Scale bar = 5 μm. j–n, Npm1+/+ and littermate control Npm1+/− mice were treated with bezafibrate (i.g., oral gavage) and administered 2.5% DSS for 7 days followed by 3 days of recovery. Body weight (j), DAI (k), representative colon images (l), colon length (m) and colon histopathology (n) are shown (n = 5 individual mice). Scale bars = 500 μm (up) and 100 μm (down). o,p, The proportion of CD45+ cells that are ILC3s (o) and the proportion of IL-22+ ILC3s in the total ILC3 population (p) in LPLs from mice of the indicated genotypes with or without bezafibrate treatment (n = 5 individual mice). q,r, Analysis of IL-22 production by isolated colonic ILC3s from mice treated with or without bezafibrate (10 mg kg−1, i.g.; q) and MNK3 (r) cells with or without bezafibrate (200 μM) addition by ELISA (n = 3 individual mice). Data in b–f, h, m, o–r are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001). BZ, bezafibrate.
To determine whether reduced transcription of OXPHOS genes in Npm1-haploinsufficient ILC3s (Fig. 3c–e) is associated with decreased mitochondrial biogenesis, we quantified the number of mitochondria using scanning electron microscopy (SEM; Fig. 4g). We found fewer mitochondria per cell in Npm1+/− ILC3s (Fig. 4h). ILC3s from Npm1+/− mice also had reduced staining of TOMM20, a mitochondrial protein (Fig. 4i). Collectively, these data indicated that NPM1 has a critical function in maintaining mitochondria numbers and mitochondrial metabolism in ILC3s and that impairment of such metabolism represents a mechanism by which heterozygous deletion of Npm1 exacerbates DSS-induced colitis.
To confirm that mitochondrial biogenesis and function were impaired by Npm1 heterozygous deletion and that such impairment contributed to colitis severity, we used bezafibrate, an agonist of the transcription factors peroxisome proliferator-activated receptor (PPAR) γ coactivator 1α (PGC1α) that stimulates mitochondrial OXPHOS and biogenesis41,42. Compared with Npm1+/− mice without bezafibrate treatment during the course of DSS administration, Npm1+/− mice receiving bezabrifate exhibited greater recovery of body weight, greater reduction in DAI, longer colons and reduced inflammation (Fig. 4j–n), suggesting that maintaining mitochondrial function through bezafibrate limited colitis severity in Npm1+/− mice. However, bezafibrate had minimal impact on mice with sufficient NPM1 function, suggesting that both NPM1 and bezafibrate maintain mitochondrial function to limit colitis (Fig. 4j–n). Similarly, bezafibrate succeeded in reversing the colitis in Rorccre/+Npm1flox/flox mice (Extended Data Fig. 7w–y). Moreover, in Npm1+/− mice exposed to DSS, bezafibrate resulted in increased percentages of total colonic ILC3s, IL-22+ ILC3s and IL-22 production by ILC3s (Fig. 4o–q), suggesting that sufficient mitochondrial OXPHOS and biogenesis are required for ILC3 activity in DSS-induced colitis.
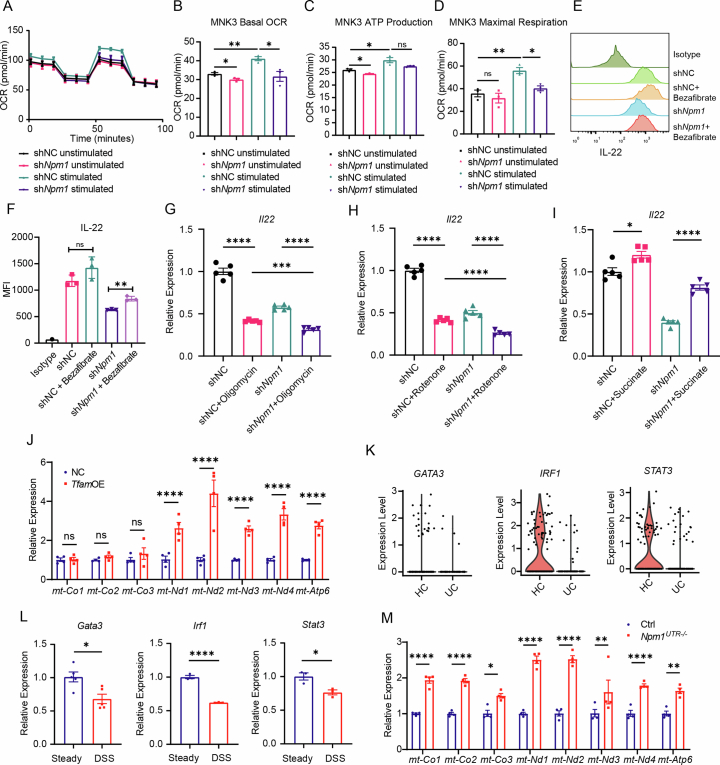
MNK3 also exhibited mitochondrial activation after IL-1β/IL-23 stimulation, which was regulated by NPM1 (Extended Data Fig. 8a–d). Knockdown of Npm1 in MNK3 suppressed the secretion of IL-22 in response to IL-23 and IL-1β (Fig. 4r). However, bezafibrate rescued ILC3 function in terms of IL-22 secretion in Npm1-knockdown cells (Fig. 4r and Extended Data Fig. 8e,f). In contrast, OXPHOS inhibitors oligomycin and rotenone suppressed the activation of MNK3 (Extended Data Fig. 8g,h). However, the difference in Il22 expression between shNC and shNpm1 MNK3 after OXPHOS inhibitor administration indicated that NPM1 may participate in other biological processes to sustain ILC3 activation (Extended Data Fig. 8g,h). The tricarboxylic acid (TCA) cycle, a crucial component of mitochondrial metabolism, is known to participate in the activation of immune cells43. Because succinate is a substrate for the TCA cycle, its addition partially rescued the impaired ILC3 activation resulting from Npm1 heterozygous deletion (Extended Data Fig. 8i). These results revealed that the defect in mitochondrial function resulting from Npm1 deficiency accounts for the impairment of ILC3 activation and function, leading to exacerbated colitis.
Extended Data Fig. 8. Adequate mitochondrial function is critical to ILC3 activation.
(a–d) Cell mito stress test was performed with unstimulated and stimulated MNK3 cell lines (shNC and shNpm1). Representative oxygen consumption rate profile (OCR) (a), basal OCR (b), ATP production (c) and maximal respiration (d) are shown (n = 3 biological samples). (e,f) MNK3 cells with or without Npm1-knockdown were treated with bezafibrate, and the MFI of IL-22 in MNK3 was analyzed by FC (n = 3 biological samples). (g–i) MNK3 cells with or without Npm1-knockdown were treated with oligomycin (g), rotenone (h) and succinate (i), and the expression of Il22 was analyzed by RT-PCR (n = 5 biological samples). (j) RT-PCR analysis of mRNA abundance of the indicated genes in control and TfamOE MNK3 cells (n = 4 biological samples). (k) Expression of indicated genes in ILC3s of UC and HC in GSE182270. (l) RT-PCR analysis of mRNA abundance of Gata3 (n = 5 indicated mice), Irf1 (n = 3 indicated mice), Stat3 (n = 3 indicated mice) in ILC3s of mice in steady state or in DSS-induced colitis. (m) RT-PCR analysis of mRNA abundance of the indicated genes in wild-type and Npm1UTR−/− ILC3s at day 5 of administration of 2.5% DSS (n = 4 indicated mice). Data are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t-test (b–d, f–i and l) and two-way ANOVA (j,m) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
NPM1 regulates TFAM transcription by binding to p65
To uncover the molecular mechanism by which NPM1 regulates mitochondrial homeostasis of ILC3s, we immunoprecipitated MNK3 cells with or without stimulation (Fig. 5a). Proteins associated with NPM1 were separated by SDS–PAGE and then silver stained. A band of ~70 kDa was enriched in stimulated MNK3 cells compared to unstimulated cells (Fig. 5b). Mass spectrometry (MS) revealed that p65, a component of the nuclear factor kappa B (NF-κB) transcription factor, is the top candidate for ~70 kDa protein that co-immunoprecipitated with NPM1 (Fig. 5c), which is consistent with a previous study reporting an interaction between NPM1 and p65 (also known as RelA), RelB and p50 (ref. 44). The two proteins, NPM1 and p65, were co-immunoprecipitated from stimulated MNK3 cells, confirming the MS findings and suggesting that the proteins interacted (Fig. 5d). Immunofluorescence analysis of ILC3s revealed that p65 was localized in the cytoplasm and NPM1 was localized in the nucleus in the noninflammatory steady state, whereas p65 accumulated in the nucleus after DSS-induced colitis and colocalized with NPM1 (Fig. 5e). Stimulation of MNK3 cells with IL-1β and IL-23 also promoted the accumulation of p65 in the nucleus (Fig. 5f). These results indicated that inflammatory stimulation induces subcellular translocation of p65 and promotes the interaction between p65 and NPM1 in the nucleus of ILC3s.
Fig. 5. The role of NPM1 in p65 signaling.
a, Schematic diagram of protein–protein interaction analysis with NPM1. b, Silver-stained gel showing proteins that were immunoprecipitated with NPM1 and exhibited higher intensity in stimulated than unstimulated cells. Red rectangle shows bands that were excised for MS analysis. c, Top five candidate NPM1-interacting proteins identified by MS. d, IP and IB of the interaction between NPM1 and p65 in MNK3 cells. The samples were derived from the same experiment, and the blots were processed in parallel. e, Immunofluorescence staining of the subcellular location of p65 in ILC3s isolated from mice at day 5 of administration of 2.5% DSS or under the steady state (water). Scale bar = 5 μm. f, Unstimulated or stimulated MNK3 cells were subjected to cellular fractionation into cyto and nuc fractions followed by western blotting for p65. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and histone H3 were used as markers for cytosolic and nuclear proteins, respectively. g, RT–PCR analysis of mRNA abundance of p65 target genes in isolated colonic ILC3s from Npm1+/+ and Npm1+/− mice at day 5 of administration of 2.5% DSS (n = 5 individual mice). h, KEGG pathway enrichment analysis of upregulated transcription factor-related pathways in ILC3s from patients with UC compared with ILC3s from healthy participants. i, Gene Ontology (GO) analysis of downregulated pathways in NPM1low ILC3s compared with NPM1high ILC3s from patients with UC, which was identified using a median expression cutoff for NPM1 in ILC3 of patients with UC. j–m, Cell mito stress test was performed with stimulated MNK3 cell line with or without p65 knockdown. Representative OCR (j), basal OCR (k), ATP production (l) and maximal respiration (m) are shown (n = 3 biological samples). n, Expression of Il22 in unstimulated and stimulated MNK3 cell line (shNC and shp65; n = 3 biological samples). Data in g and k–n are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001). IB, immunoblot; cyto, cytosol; nuc, nuclear.
To investigate whether NPM1 functions as a transcriptional cofactor that binds to p65 and influences the transcription of p65 target genes in activated ILC3s, we thus monitored the expression of four p65-regulated genes (Cxcl2, Ccl4, Xiap and Cflar) and found that they were dramatically induced in the colitis condition compared with the steady-state condition. However, only the expression of Cxcl2 and Ccl4 was markedly decreased in Npm1+/− ILC3s compared to WT ILC3s from mice with DSS-induced colitis (Fig. 5g). These transcriptional results indicated that the NF-κB pathway in ILC3s was activated by DSS-induced colitis and that NPM1 contributes to the regulation of a subset of NF-κB target genes. We also observed that the NF-κB signaling pathway in ILC3s from patients with UC was significantly upregulated compared to HCs in GSE182270 (Fig. 5h). Additionally, several NF-κB-related signaling pathways were also enriched when comparing NPM1high ILC3s and NPM1low ILC3s from patients with UC (Fig. 5i). In vitro tests of MNK3 cells with p65 knockdown exhibited a decrease in OCR, especially basal OCR (Fig. 5j–m). More notably, the knockdown of p65 resulted in the downregulation of Il22 expression after stimulation (Fig. 5n). Hence, our findings showed that p65 signaling was critical for the activation of ILC3. Meanwhile, NPM1 bound to p65 and participated in downstream transcriptional regulation in ILC3s in colitis.
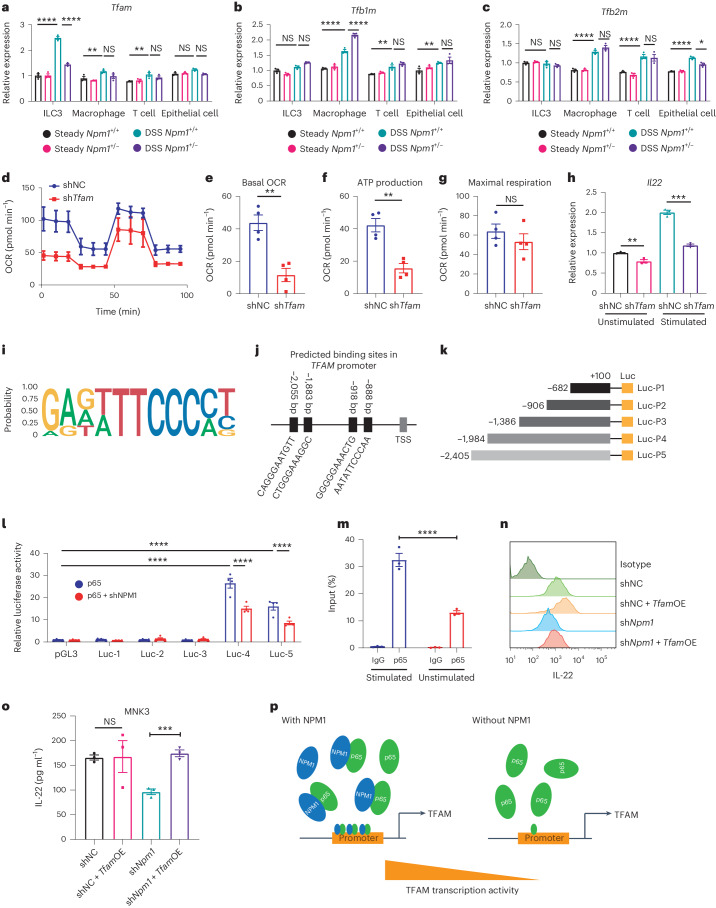
To investigate a transcriptional regulatory role for NPM1 in mitochondrial OXPHOS and biogenesis, we examined the expression of the following three mitochondrial transcription factors in ILC3s: Tfam, mitochondrial transcription factor B1 (Tfb1m) and mitochondrial transcription factor B2 (Tfb2m). These transcription factors participate in mtDNA transcription and are stimulated by PGC1ɑ45. The expression levels of the three mitochondrial transcription factors in ILC3s showed no differences between Npm1+/+ and Npm1+/− mice in steady state (Fig. 6a–c). However, under pathological conditions, a remarkable decrease in Tfam expression was only observed in ILC3s, not macrophages, T cells and epithelial cells, of Npm1+/− mice when compared to Npm1+/+ mice (Fig. 6a–c), suggesting that NPM1 has an indispensable role in upregulation of Tfam in ILC3s upon DSS treatment. However, Tfb1m and Tfb2m were significantly increased in macrophages, T cells and epithelial cells, but not in ILC3s after DSS treatment (Fig. 6a–c). These data suggested that mitochondrial activation in ILC3s is primarily dependent on TFAM rather than on TFB1M or TFB2M. Overexpression of Tfam in MNK3 markedly enhanced the expression of mtDNAs, including mt-Nd1, mt-Nd2, mt-Nd3, mt-Nd4 and mt-Atp6 (Extended Data Fig. 8j). Knockdown of Tfam in MNK3 notably impaired its mitochondrial function and attenuated the production of IL-22 (Fig. 6d–h). Accordingly, NPM1 is crucial for the heightened demand of TFAM to subsequently increase mitochondrial function in ILC3s, not other cell types, during DSS-induced colitis.
Fig. 6. NPM1 regulates TFAM transcription by binding to p65.
a–c, RT–PCR analysis of mRNA expression of Tfam (a), Tfb1m (b) and Tfb2m (c) in isolated ILC3s, macrophages, T cells and epithelial cells from Npm1+/+ and Npm1+/− mice exposed to 2.5% DSS or water (steady state; n = 3 individual mice). d–g, Cell mito stress test was performed with stimulated MNK3 cell line with or without Tfam knockdown. Representative OCR (d), basal OCR (e), ATP production (f) and maximal respiration (g) are shown (n = 4 biological samples). h, Expression of Il22 in unstimulated and stimulated MNK3 cell line (shNC and shTfam; n = 3 biological samples). i, Logo plot of the consensus binding motif of the transcription factor p65. j, The positions and sequences of the four predicted binding sites of p65 in the TFAM promoter. k, Diagram of the pGL3-TFAM promoter luciferase reporter plasmids. l, TFAM reporter activity measured in HEK293T cells (n = 4 biological samples). m, ChIP–qPCR assays of the binding efficiency of p65 to the Tfam promoter in MNK3 cells with or without stimulation by IL-23 and IL-1β. IgG served as the negative control (n = 3 biological samples). n,o, Analysis of the effect of Tfam overexpression (TfamOE) on IL-22 production in MNK3 cells by flow cytometry (n) and ELISA (o), (n = 3 biological samples). p, Model depicting transcription activity change of Tfam in ILC3 cells with or without NPM1. Data are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-way ANOVA (a–c,l,m) and two-tailed unpaired Student’s t test (e–h,o; *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001). TSS, transcription start site; shNC, nontargeted short hairpin RNA; shNpm1, short hairpin RNA targeting Npm1.
To determine if NPM1 and p65 regulate TFAM expression, we examined whether they directly bind to the TFAM promoter and affect its transcription. We identified four putative binding sites for p65 in the TFAM promoter and constructed luciferase reporter plasmids (Fig. 6i–k). Using luciferase reporter assays in HEK293T cells, we found that p65 significantly enhanced TFAM promoter-dependent reporter expression in plasmids with either third or fourth binding sites (Fig. 6l). However, knockdown of NPM1 inhibited promoter activity (Fig. 6l), indicating that NPM1 contributes to TFAM transcription.
To validate a direct interaction between p65 and the Tfam promoter in ILC3s, we performed chromatin immunoprecipitation (ChIP) using MNK3 cells and a p65 antibody and tested for the presence of Tfam promoter sequences. To determine the effect of inflammatory signals on the interaction, we evaluated MNK3 cells with and without stimulation by IL-1β and IL-23. We found that p65 is bound to the Tfam promoter in ILC3s under both conditions, with stimulation enhancing this interaction (Fig. 6m). To confirm the importance of Tfam in ILC3 activation, we overexpressed Tfam in MNK3 cells and found that expression of Tfam mostly restored secretion of IL-22 in MNK3 cells in which Npm1 was knocked down (Fig. 6n,o). Collectively, our findings indicated that NPM1 acts as a partner of p65 to promote Tfam transcription, thereby supporting ILC3 mitochondrial function and activation (Fig. 6p).
Npm1 overexpression (Npm1OE) protects against DSS-induced colitis
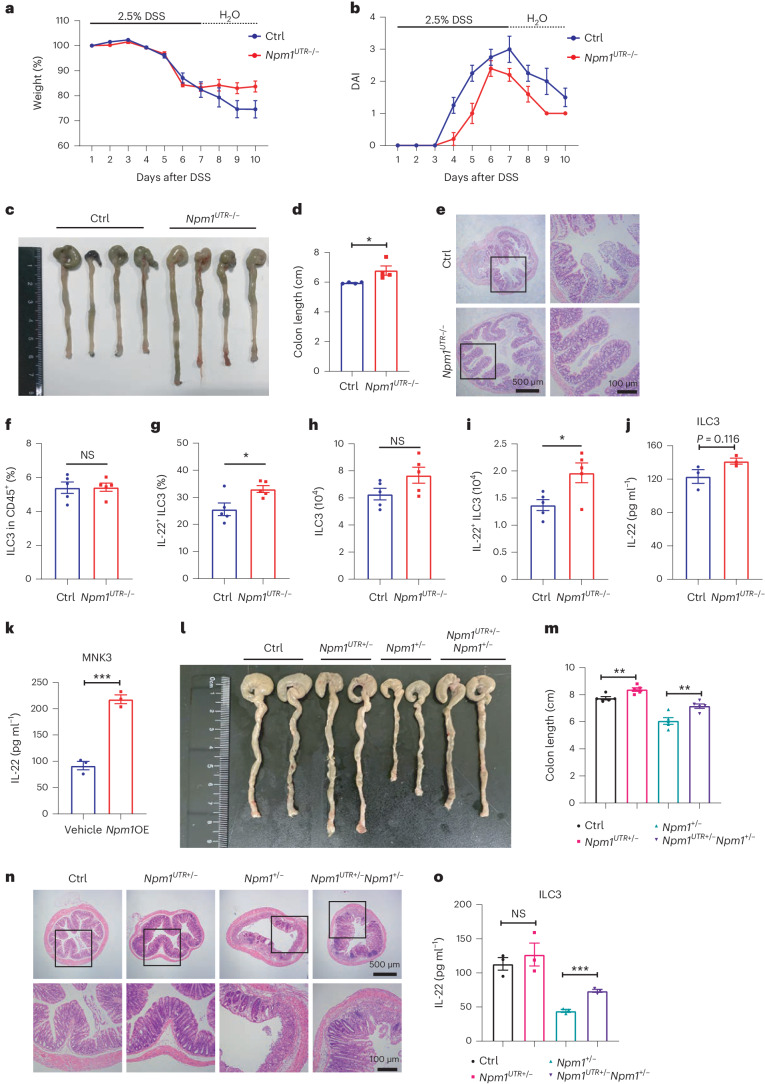
Our subsequent investigation aimed to explain why NPM1 is downregulated in UC and whether overexpression of NPM1 could ameliorate colitis. GATA binding protein 3 (GATA3), interferon regulatory factor 1 (IRF1) and signal transducer and activator of transcription 3 (STAT3), which are predicted transcriptional factors associated with NPM1, demonstrated reduced expression in ILC3s of patients with UC and enteritic mice in comparison to the control groups (Extended Data Fig. 8k,l). These findings may provide insights into the mechanisms underlying the downregulation of NPM1 in IBD. To confirm the protective function of NPM1 in colitis, we generated Npm1UTR−/− mice that have a genetic knockout of the 3′-UTR region of Npm1 and overexpress Npm1 (Supplementary Fig. 1g–j). Compared to control (Ctrl) mice, Npm1UTR−/− mice had less severe DSS-induced colitis, as evidenced by the increased recovery of body weight, decreased DAI, increased colon length and reduced inflammation (Fig. 7a–e). Although overexpression of Npm1 did not enhance the frequency of colonic ILC3s, a higher proportion were producing IL-22, indicating that Npm1OE enhanced the defense function of ILC3s against colitis (Fig. 7f–j). Expression of various mtDNA was upregulated in Npm1UTR−/− ILC3 compared to control group (Extended Data Fig. 8m). Overexpression of Npm1 in MNK3 cells also increased IL-22 secretion (Fig.7k). Eventually, we crossed Npm1UTR−/− with Npm1+/− mice and generated Npm1UTR+/−Npm1+/− mice. As expected, heterozygous overexpression of Npm1 prevented the exacerbated DSS-induced colitis caused by the Npm1 haploinsufficiency (Fig. 7l–n). ILC3s isolated from Npm1UTR+/−Npm1+/− mice also showed increased IL-22 secretion compared with ILC3s from Npm1+/− mice (Fig. 7o). Taken together, these results demonstrated that Npm1OE has a protective function against colitis.
Fig. 7. Npm1OE protects against DSS-induced colitis.
a–e, Npm1UTR−/− and littermate control mice were fed with 2.5% DSS for 7 days and allowed to recover for 3 days. Body weight (a), DAI (b), representative colon images (c), colon length (d) and colon histopathology (e) are shown (n = 4 individual mice). Scale bars = 500 μm (left) and 100 μm (right). f,g, LPLs were isolated from Npm1UTR−/− and control mice on day 5 of administration of 2.5% DSS (n = 5 individual mice). The proportion of ILC3s (live CD45+Lin−RORγt+ cells) within the CD45+ population (f) and of IL-22-producing ILC3s (live CD45+Lin−RORγt+IL-22+ cells) with the ILC3 population (g) was determined by flow cytometry. h,i, Number of ILC3s (h) and IL-22+ ILC3s (i) in LPLs of Npm1UTR−/− and control mice after DSS administration are depicted (n = 5 individual mice). j,k, IL-22 production by isolated colonic ILC3s from Npm1UTR−/− and control mice on day 5 of administration of 2.5% DSS (j) and MNK3 cells in response to Npm1OE (k) was determined by ELISA (n = 3 biological samples). l–n, Control, Npm1UTR+/−, Npm1+/− and Npm1UTR+/−Npm1+/− mice were administered 2.5% DSS for 7 days and allowed to recover for 3 days. Representative images of the mouse colons (l), colon length (m) and colon histopathology (n) are shown (n = 5 individual mice). Scale bars = 500 μm (top) and 100 μm (bottom). o, IL-22 production by isolated colonic ILC3s from the indicated groups of mice was determined by ELISA (n = 3 individual mice). Data in d, f–k, m and o are representative of two independent experiments, shown as the means ± s.e.m., and statistical significance was determined by two-tailed unpaired Student’s t test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Discussion
In this study, we demonstrated that NPM1, a protein that is abundant in colonic ILC3s, is critical for the activation of IL-22 production in response to colitis. We found that NPM1 binds to p65 and regulates transcription of the mitochondrial transcription factor TFAM, thereby having a role in maintaining mitochondrial biogenesis and OXPHOS. Our findings revealed the protective role of NPM1 in gut homeostasis and suggested that a deficiency in the activity of NPM1 is a key factor linking IBD and MDS/AML.
The NF-κB family of transcription factors has a crucial role in responding to various stimuli by regulating the expression of genes involved in diverse biological processes such as inflammation, metabolism, cancer and development44. Here we identified p65 as the top interacting protein with NPM1 in ILC3s in DSS-induced colitis and observed the subcellular translocation of p65 and colocalization with NPM1 in the nucleus of ILC3s to activate downstream gene transcription after inflammatory stimulation. A previous study revealed that NPM1 interacts with the N-terminal DNA-binding domain of p65 and enhances binding to target gene promoters44. We also found that TFAM is a potential target of p65 in ILC3s and that p65 regulates TFAM transcription in a manner enhanced by NPM1. TFAM is a mitochondrial transcription factor that controls mtDNA replication and transcription46. Tfam−/− mice are embryonically lethal, and tissue-specific deficiency of Tfam leads to severe OXPHOS defects, which is the main cause of human mitochondrial diseases47. Furthermore, Tfam∆ILC3 mice exhibit a substantial reduction of ILC3s by 6 weeks of age48. Here we demonstrated that TFAM is highly expressed in ILC3s and acts as a key downstream effector of NPM1 in DSS-induced colitis. Moreover, mitochondrial activation in ILC3s is primarily dependent on TFAM, rather than on TFB1M or TFB2M. In contrast to ILC3s, macrophages, T cells and epithelial cells are primarily depend on TFB1M and/or TFB2M in DSS-treated mice. This indicates the indispensable role of NPM1 for ILC3s, not T cells, macrophages or epithelial cells. By maintaining mtDNA replication, mitochondrial number and OXPHOS levels in activated ILC3s, NPM1-stimulated TFAM expression supports the cells’ high energy requirements. Although there is no direct evidence that TFAM regulates IL-22, the lack of mitochondrial-derived energy by insufficient TFAM could limit the activation of ILC3s and effector cytokine secretion in colitis. Meanwhile, although it cannot be ruled out that NPM1 affects ILC3 mitochondrial function and cell activation through interactions with other molecules that participate in mitochondria functions, such as NPM1’s known partners c-Myc49,50, SP1 (refs. 51,52), p53 (refs. 53,54) and IRF1 (refs. 55,56), the effects of p65 knockdown and Tfam knockdown on ILC3 function in MNK3 cells are similar to those of knocking down Npm1. Therefore, it is believed that the p65-TFAM axis is an important effector for NPM1 to increase the function and metabolism of ILC3.
Mitochondria have an important role in the activation of immune cells. Activation of ETC in mitochondria is essential for T cell activation, expansion and cytokine production57. The proliferation and cytokine secretion of ILC3s depend on glycolysis and also mitochondrial ROS following in vitro activation by IL-1β and IL-23 or in vivo during bacterial infection39. By analyzing mitochondria in primary ILC3s from Npm1+/− mice under DSS administration, we observed diminished mitochondrial numbers, consistent with impaired biogenesis, and reduced OXPHOS. To confirm that the deficiency in IL-22 secretion was due to mitochondrial dysfunction, bezafibrate was used to activate mitochondrial function and successfully rescued the production of IL-22 in Npm1-deficient ILC3s. Overexpression of Tfam also increased IL-22 production, providing additional support for mitochondrial biogenesis and OXPHOS were crucial for ILC3 activation.
MDS is a hematopoietic disorder involving clonal abnormalities of cells caused by mutations in oncogenes and tumor suppressor genes, as well as chromosomal abnormalities9. The subsequent alterations in the function and properties of BM-derived immune cells can lead to the development of immune-mediated disorders including IBD. Genetic mutations provide insight into the relationship between MDS and IBD. For example, mutations in PTPN11, a driver gene in MDS/AML, result in exacerbation of intestinal inflammation by disrupting BM-derived macrophage responsiveness to IL-10 (ref. 58). NPM1 acts as a top driver mutation in high-risk MDS and AML6,17. In our study, we demonstrated that NPM1 functions in BM-derived ILC3s to control the gut microenvironment, particularly through a protective IL-22-related immune response. Our data provide insight with potential relevance for the diagnosis and treatment of patients with concurrent IBD and MDS.
In summary, our study highlights the role of NPM1 in maintaining mitochondrial function and IL-22 production in ILC3s in the progression of colitis. Our findings suggest that NPM1 might be a therapeutic target for IBD and provide insights into a connection between MDS/AML and IBD.
Methods
Generation of Npm1+/−, Npm1UTR+/− and Npm1flox/+ mice
Npm1+/− and Npm1UTR+/− mice were generated by knocking out the DNA-binding domain (including partial exon 8, exon 9, 10 and partial exon 11) and 3′-UTR domain with the binding sites of microRNAs using CRISPR–Cas9 technology from the CRO company Shanghai Model Organisms Center. In brief, Cas9 mRNA and gRNA were synthesized in vitro and then injected into fertilized eggs of C57BL/6J mice. The resulting F0 mice were screened for Npm1+/− genotype using specific PCR primers (PI, 5′-GAAAAGGTCCCAGTGAAGAAAGTGA-3′; PII, 5′-TGGCAAGTGAACCTGGACAACAT-3′; PIII, 5′-GGCTGACCCACAGGCTGAGGAG-3′ and PIV, 5′-CCAACAGATTGGCTATCAATAGAGGA-3′) or Npm1UTR+/− (PI, 5′-CCACAGGCTGAGGAGGCAACAC-3′; PII, 5′-AAAAGGTTCAGGCACGAAGCAG-3′; PIII, 5′-GTCAGATGTGGAAATGGTAGGGAGA-3′ and PIV, 5′-AAAAGGTTCAGGCACGAAGCAG-3′) and crossed with WT C57BL/6J mice to get F1 heterozygous mice which were identified by genotyping PCR. F1 heterozygous mice were crossed with WT C57BL/6J mice to get F2 heterozygous mice. The third and further generations of Npm1+/− and Npm1UTR+/− mice were used in the experiments.
Npm1flox/+ mice were generated by introducing two loxp sequences into Npm1 using CRISPR–Cas9 technology from the CRO company Cyagen Biosciences. Briefly, Cas9 mRNA and gRNA were synthesized in vitro with a homologous arms-encompassed targeting vector and injected into fertilized eggs of C57BL/6J mice. The resulting F0 mice were screened using specific PCR primers (FI, 5′-AACAGCTAGATGGGAAGTATGGA-3′; RI, 5′-AGTTCCCAAGTTTGCTTTGAACAG-3′ and FII, 5′-ACGTTGCAGATAGCTGTACTGATG-3′; RII, 5′-GCTAAAGCGAATCTTGTCTGTTCA-3′) and crossed with WT C57BL/6J mice to get F1 heterozygous mice, which were identified by genotyping PCR with primer pairs (F2 and R2). Positive F1 Npm1flox/+ mice were crossed with WT C57BL/6J mice to get F2 heterozygous mice. The third and further generations of Npm1flox/+ mice were used in the experiments.
Mice
All mice used in this study were bred in the animal facility of Suzhou Institute of Biomedical Engineering and Technology and Shandong University and were approved in accordance with the Institutional Animal Care and Use Committee guidelines at Suzhou Institute of Biomedical Engineering and Technology and Shandong University. Mice were housed in individually ventilated cages under a 12-h light/12-h dark cycle with normal food and water. All experiments were performed using C57BL/6J mice, which also served as controls for Npm1+/−, Npm1UTR−/− and Apcmin/+ mice. Npm1flox/flox mice served as controls for Rorccre/+Npm1flox/flox and Villincre/+ Npm1flox/flox mice. Male mice aged 6–8 weeks were used for the experiments. For the DSS model, drinking water containing 2.5% DSS was given to age-matched male mice for 7 days, followed by regular water for 3 days, with DSS water being replaced each day. For the rescue experiment, mice were treated with 10 mg kg−1 bezafibrate (i.g.) every other day. Throughout the experiment, body weight was monitored. To induce colon cancer model, WT and Npm1+/− mice were injected intraperitoneally with AOM (10 mg kg−1). After 5 days, 2.5% DSS was added to the drinking water for seven consecutive days, followed by 14 days of regular water. This cycle was repeated three times. Mice were killed for analysis on day 65 of the experiments. In the TNBS model, mice were anesthetized and then treated with 2 mg of TNBS dissolved in 50% ethanol via rectal administration using a polyethylene catheter (2 mm in outer diameter). Following administration, the mice were maintained in an inverted position for a minimum of 1 min. Control mice were treated rectally with 50% ethanol alone. The progression of colitis was monitored daily, assessing parameters such as diarrhea, presence of blood in stools, body weight and survival rates. Note that littermate mice are generally genotyped at 3–4 weeks of age and then placed in separate cages when grouping, according to their genotype. The DAI is calculated by combining the following three parameters: the percentage weight loss of the mice, the consistency of stool and the presence of stool blood. The scoring for each parameter is as follows: (1) weight loss—0 points if weight remains stable, 1 point for a 1–5% weight loss, 2 points for a 5–10% weight loss, 3 points for a 10–15% weight loss and 4 points for a weight loss greater than 15%; (2) stool consistency—0 points for normal stool, 2 points for loose stool and 4 points for diarrhea and (3) stool blood—0 points for no blood, 2 points for occult blood positivity and 4 points for overt bleeding. The DAI is calculated as follows: DAI = (weight loss index + stool consistency + blood in stool)/3. Note that mice are generally genotyped and caged at 3–4 weeks of age and then placed in separate cages when grouping, according to their genotype.
Generation of BM chimera
The generation of BM chimeras was achieved by collecting BM cells from both WT and Npm1+/− mice and subsequently flushing them with 1× PBS. The cell suspension, comprising 1 × 07 BM cells, was then intravenously injected into lethally irradiated recipient mice of both WT and Npm1+/− genotypes, with a dose of 102.2 cGy min−1 for 9 min. Experiments were conducted 4 weeks following reconstitution.
In vivo T cell and myeloid cell blocking
To deplete T cells, anti-CD3ɛ (Bio X Cell, BE0001-1; clone 145-2C11) was administered intravenously daily (50 µg per mouse, from day −2 to day 6), and control mice were administered an equivalent amount of IgG (Bio X Cell, BE0091). To deplete myeloid cells, antimouse/antihuman CD11b (Bio X Cell, BE0007; clone M1/70) were administered intravenously every 2 days (100 µg per mouse, from day −2 to day 6), and control mice were administered an equivalent amount of IgG (Bio X Cell, BE0091; clone LTF-2). The DSS-induced colitis model was initiated on day 0.
Histology
We dissected the colons from the indicated mice, fixed them in 10% formalin and stained them with hematoxylin and eosin (H&E) using paraffin-embedded sections. We used the following scoring system to evaluate colon tissue histologically: 0 = no evidence of inflammation, 1 = low level of inflammation with scattered infiltrating mononuclear cells (1–2 foci), 2 = moderate inflammation with multiple foci, 3 = high level of inflammation with increased vascular density and marked wall thickening and 4 = maximal inflammation with transmural infiltration and loss of goblet cells.
Flow cytometry and isolation of lamina propria leukocytes
To isolate leukocytes from the lamina propria, we incubated intestinal segments of approximately 0.5 cm at 37 °C for 1.5 h in complete Roswell Park Memorial Institute (RPMI) medium (Suzhou Haixing Biosciences), supplemented with DNase I (150 µg ml−1; Sigma) and collagenase VIII (300 U ml−1; Sigma). The digested fragments were triturated and filtered through a 100 µm cell strainer. The cells were collected from the interface of the 80% and 40% Percoll gradients after centrifugation at 660g for 15 min at room temperature. Before surface staining, Fc receptors were blocked using CD16/32 antibody (eBioscience; dilution 1:100). Leukocytes isolated from the intestinal lamina propria were then stained with antibodies against the following markers: CD45 eFlour 506 (dilution 1:100), RORγt PE (dilution 1:50), Ly-6G PE (dilution 1:100), CD127 Super Bright 645 (dilution 1:100), F4/80 FITC (dilution 1:100), CD3 Alexa-488 (dilution 1:100), CD34 FITC (dilution 1:100), CD117 APC (dilution 1:100), CD19 eFlour (450 dilution 1:100), IL-22 PE (dilution 1:50), CD4 APC (dilution 1:100), IL-17A BV421 (dilution 1:50), Lineage Percp-cy5.5 Cocktail (dilution 1:50), T-bet PE (dilution 1:100), IFNγ-APC (dilution 1:50), NKp46-PerCPcy5.5 (dilution 1:50), FOXP3-eFlour 450 (dilution 1:100), CCR6-BV421 (dilution 1:50), TCR γ/δ-APC (dilution 1:50) and CD127-FITC (dilution 1:100). For cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA) (50 ng ml−1) and ionomycin (500 ng ml−1) for 2 h, along with the addition of brefeldin A (2 µg ml−1). Live and dead cells were distinguished using the Live and Dead Violet Viability Kit (BioLegend).
Smart-seq
Live+Lin−CD45lowCD90.2high ILC3s were sorted from colon LPLs of the indicated mice. The SMARTer cDNA synthesis protocol was used to synthesize cDNA, which was then fragmented using dsDNA Fragmentase (New England Biolabs (NEB), M0348S) and incubated at 37 °C for 30 min. Library construction commenced with fragmented cDNA, where blunt-end DNA fragments were generated through a combination of fill-in reactions and exonuclease activity. Size selection was carried out using the provided sample purification beads. An A-base was added to the blunt ends of each strand, indexed Y adapters were ligated to the fragments and the ligated products were amplified using PCR. Subsequently, paired-end sequencing was conducted on NovaSeq 6000 (Illumina), following the protocol recommended by the vendor.
scRNA-seq data processing
scRNA-seq dataset (GSE182270) was downloaded from the Gene Expression Omnibus (GEO) database and was performed on cells extracted from colonic biopsies of inflamed mucosa (patients with UC, n = 5) and normal colonic mucosa (HCs, n = 4). Count tables were analyzed using the Seurat 4.0 package following the standard workflow with default settings. The number of principal components (PCs) was determined based on Elbow plots, PCs = 13. Next, FindNeighbors and FindClusters functions were used for cell clustering, and the UMAP method was used for visualization. Cell-type-specific markers were found by the FindMarkers function; cell-type identities were manually annotated by matching cluster-specific upregulated marker genes with cell-type markers in the CellMarker 2.0 database. NPM1low ILC3s and NPM1high ILC3s were identified using a median expression cutoff for NPM1 in ILC3s. Note that the cell dropout of NPM1 was not included in the analysis. FindMarkers function was used to identify significantly regulated genes in NPM1high ILC3. The ClusterProfiler package was applied for functional annotation.
Immunoprecipitation (IP) and western blot analysis
To perform IP, cells were lysed in an IP lysis buffer containing 20 mmol l−1 Tris (pH 7.5), 150 mmol l−1 NaCl and 1% Triton X-100, supplemented with a cocktail of protease and phosphatase inhibitors. Following lysis, the supernatants were collected after centrifugation and incubated overnight at 4 °C with constant rotation with the indicated antibodies. The antibody–antigen complexes were then precipitated using protein A/G magnetic beads (Millipore) and washed with PBS. For western blot analysis, cell lysates were prepared using radio immunoprecipitation assay (RIPA) lysis buffer (CoWin Biosciences) containing protease inhibitors and phosphatase inhibitors (CoWin Biosciences). Equal amounts of protein were loaded onto SDS–PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dried milk for 1 h at room temperature before being incubated with primary antibodies overnight at 4 °C, including SDHB (Proteintech; 1:2,000), NDUFB8 (Proteintech; 1:2,000), MT-ATP6 (Abclonal; 1:1,000), MT-CO1 (Abclonal; 1:1,000), UQCRC2 (Proteintech; 1:1,000), NPM1 (Abclonal; 1:1,000) and p65 (Cell Signaling Technology (CST); 1:1,000). After washing, the membranes were incubated with IRDye 800cw or 680cw conjugated secondary antibodies (LICORbio; 1:10,000) for 1 h. The membranes were then imaged using an Odyssey CLx Infrared Imaging System.
ChIP
The ChIP assay was conducted using the ChIP-IT Kit (Beyotime). In brief, the cells were initially fixed with formaldehyde and subsequently lysed. To precipitate the DNA fragment, either 2 μg of anti-p65 or normal IgG were used. The DNA–protein complexes were then pulled down with magnetic beads and subjected to decross-linking. The extracted DNA samples were finally amplified using specific Tfam promoter primers for the sequences containing the binding site 5′-GGGAAAGGC-3′.
Luciferase reporter assay
HEK293T cells that overexpressed p65 were transfected with the specified pGL3-luciferase reporter plasmid that contained the TFAM promoter, along with the Renilla pRL-TK plasmid as the internal control. After incubation for 24–48 h, the cell lysates were subjected to luciferase activity analysis using the Dual-Luciferase Reporter Assay kit (Promega).
Immunofluorescence staining
Glass slides were pre-inserted into 12-well plates, and cells were seeded onto these plates. After 24 h, when the cells had reached 40–50% confluence, they were washed with PBS and subsequently fixed with 4% paraformaldehyde for 30 min. The cells were then permeabilized with a 0.5% Triton X-100 solution for an additional 20 min. A blocking buffer containing 5% bovine serum albumin was added next. Primary antibodies comprising anti-NPM1 (Proteintech; 1:200), anti-RORγt (Thermo Fisher Scientific; 1:200), anti-TOMM20 (Proteintech; 1:200) and anti-p65 (CST; 1:200) were used in this experiment. The corresponding secondary antibodies conjugated with Alexa Fluor 488, 555 and 647 were also used at a concentration of 1:2,000 (Invitrogen).
MS
The Q Exactive Mass Spectrometer (Thermo Fisher Scientific) and Dionex Ultimate 3000 RSLCnano (Thermo Fisher Scientific) were used to analyze the affinity-purified samples according to the manufacturer’s instructions. The proteins were first reduced with 0.05 M Tris (2-carboxyethyl) phosphine (TCEP) and then alkylated with 55 mM methyl methanethiosulfonate (MMTS). The sample was then centrifuged and subjected to centrifugation steps before being digested with trypsin. After digestion, the resulting peptides were loaded onto a reversed-phase analytical column and underwent high-performance liquid chromatography (HPLC)–MS analysis. The peptide detection was conducted using an Orbitrap at a resolution of 70,000, with tandem mass spectrometry (MS/MS) using normalized collision energy (NCE) setting as 27, and MASCOT software was used to identify proteins. The peptide mass tolerance was 20 ppm, while the fragment mass tolerance was 0.6 Da, and the significance threshold was 0.05.
RNA extraction and quantitative real-time PCR analysis
The procedures were conducted as described previously59. Supplementary Table 2 lists all PCR primer sequences used for the detection of mt-Co1, mt-Co2, mt-Co3, mt-Nd1, mt-Nd2, mt-Nd3, mt-Nd4, mt-Atp6, Cxcl2, Ccl4, Xiap, cFlip, Tfam, Tfb1m, Tfb2m, Il22, Npm1, Gata3, IRF1, Stat3, Tjp1, Tjp2, Cldn2, Cldn3 and Gapdh. Moreover, Tfam ChIP–qPCR primers are also listed in Supplementary Table 2.
SEM
The cells were centrifuged to precipitate, and the medium was removed. Then 500 μl of 2.5% glutaraldehyde (Ted Pella) was slowly added, avoiding suspending the precipitated cells, and left at room temperature for 1 h, followed by keeping at 4 °C for 3 h. The glutaraldehyde solution was replaced with PBS, and cells were left at 4 °C overnight. The cells were stained following the reported protocol60. The cells were then embedded in resin (Eponate 12 Kit, Ted Pella). Ultrathin sections of 50 nm thickness were cut (UC7, Leica) and collected on carbon-coated Kapton tapes. EM images were acquired with an SEM (GeminiSEM 300, Zeiss).
Mitochondrial membrane potential assay
Primary ILC3s were loaded with the JC-1 primer (Beyotime, C2006) and potentiometric dye TMRE (Beyotime, C2001S) at 37 °C for 20 min and washed with buffer or cell medium three times. Δψm was measured using a microplate reader. When detecting JC-1 monomers, the excitation light can be set to 490 nm and the emission light can be set to 530 nm. When detecting JC-1 polymer, the excitation light can be set to 525 nm and the emission light can be set to 590 nm. The maximum excitation wavelength of TMRE is 550 nm, and the maximum emission wavelength is 575 nm.
Immunohistochemical staining
Immunohistochemical staining was performed as follows: after deparaffinization and hydration, paraffin slides were repaired by boiling in Tris–EDTA buffer (pH 8.0) for 10 min. Next, sections were treated with 3% H2O2 for 20 min to bleach endogenous peroxidase. After blocking with donkey serum, primary antibodies against NPM1 were diluted 1:100 and then incubated at 4 °C overnight. After three washes with PBS, the tissue slides were treated with horseradish peroxidase (HRP), conjugated donkey anti-rabbit or mouse secondary antibody (Dako) for 45 min and then stained by 3,3’-diaminobenzidine (DAB). Semi-quantitative immunohistochemistry is generally divided into the following three levels: low (+), medium (++) and high (+++). These levels are scored as follows: low (+) = 1, medium (++) = 2 and high (+++) = 3. Then calculate the value based on (+)% × 1 + (++)% × 2 + (+++)% × 3. The final score is (+) for a value less than 1.0, (++) for a value between 1.0 and 1.5 and (+++) for a value greater than 1.5.
Seahorse metabolic analysis
Cellular OCR was quantified using the Agilent Seahorse XFe24, following the manufacturer’s protocol. Primary ILC3s, macrophages, T cells, epithelial cells and MNK3 cells were plated on 24-well plates precoated with poly-d-lysine and incubated with the complete RPMI medium over night. Following the incubation period, the cells were washed and transferred into seahorse assay medium supplemented with 1 mM pyruvate, 2 mM glutamine and 10 mM glucose and cultured for an additional hour at 37 °C in a CO2-free environment. To measure OCR, indicated inhibitors such as oligomycin (1.5 μM), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), 1 μM), rotenone (0.5 μM) and antimycin A (0.5 μM) were introduced where specified, and the rates of OCR (pmol O2 per min) were monitored in real-time.
Ethics
Pathology sections were obtained from patients with UC, patients with CD and healthy individuals after approval was obtained from the Ethics Committee of Shandong University School of Basic Medicine (ECSBMSSDU2020-1-035). All animal experiments were approved and are in accordance with the Institutional Animal Care and Use Committee guidelines at Suzhou Institute of Biomedical Engineering and Technology (2021-C058) and Shandong University (ECSBMSSDU2020-2-057).
Randomization and blinding
For DSS/TNBS/AOM-DSS animal studies to assess the changes in Npm1 deficiency, no method of randomization was used. Mice were grouped according to genotype, and all experiments were performed with sex-matched littermates. For the bezafibrate experiment, mice were first grouped by genotype and then randomly assigned to two groups (bezafibrate-treated group and control group). For CD11b/CD3 antibody treatment experiments, mice were first grouped by genotype and then randomly assigned to two groups (CD11b/CD3 antibody-treated group and IgG antibody-treated group). Animal studies were not blinded (mice were named with mouse ID and genotyped within 6 weeks of birth). Group allocation was not applicable because mice were grouped based on and compared across different genotypes. Histological analyses were conducted by two independent investigators, who had limited knowledge of the group of mice and patients. DAI was analyzed by two independent investigators, who had limited knowledge of the group of mice and patients. Data from FACS, qPCR and enzyme-linked immunosorbent assay (ELISA) were collected by an investigator with only the knowledge of mouse ID (without grouping information).
Statistical analysis
Flow cytometry data were analyzed by FlowJo (v10), and immunofluorescence images were analyzed by Image J 64-bit Java 8. Statistical analyses were performed using GraphPad Prism (v8). Data were presented as mean ± s.e.m. Statistical significance was assessed by Student’s t test (unpaired) or two-way analysis of variance (ANOVA) analyses. All statistical tests were two-tailed, and a P value <0.05 was considered statistically significant. Data distribution was assumed to be normal, but this was not formally tested. The number of patients, mice and biological repeats are indicated in the figure legends, as well as the number of independent experiments. No animal or data point was excluded from the analyses. In all studies using at least three to five animals per group, all experiments were performed at least twice to ensure reproducibility.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-024-01921-x.
Supplementary information
Supplementary Figs. 1 and 2 and Supplementary Tables 1 and 2.
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Acknowledgements
We thank Z. Zhang’s laboratory, W. Feng and H. Zhu for their help and suggestions. Advanced Optical Microscopy Application Open Platform in Suzhou Institute of Biomedical Engineering and Technology (SIBET) is acknowledged for professional assistance in image acquisition. We thank N.R. Gough (BioSerendipity) for professional editing services. This work was funded by the Chinese Academy of Sciences (CAS) Project for Young Scientists in Basic Research (grant YSBR-067 to G.S.), the Basic Research Pilot Program of Suzhou (SJC2022007 to M.S.), National Natural Science Foundation of China (82273336 to M.S. and 82071854 to S.L), Pre-Research and Construction Project of Major Scientific Research Facilities in Jiangsu Province (BM2022010 to M.S.), National Key R&D Program of China (2020YFA0804400 to S.L.) and Innovative Research Group Project (82321002 to S.L.).
Extended data
Author contributions
R.C.Z., J.Y., S.Y.L. and M.X.S. conceptualized the project. R.C.Z. and J.Y. developed the methodology. R.C.Z., Y.J.Z., H.Z., J.L.P., L.H., D.S.L. and J.Y.Y. performed validation. Y.S.Z. and D.T.Y. carried out a formal analysis. R.C.Z., Y.J.Z., H.Z. and L.H. conducted the investigation. M.X.S., S.Y.L., Y.X.L., D.J.P., R.L.X. and C.G.P. arranged the resources. R.C.Z., Y.S.Z. and D.T.Y. curated the data. R.C.Z. wrote the original draft. J.Y., S.S., S.Y.L., D.T.Y., C.G.P. and M.X.S. did the writing, reviewing and editing of the manuscript. M.X.S., S.Y.L., C.G.P., R.B.Z. and G.H.S. provided supervision.
Peer review
Peer review information
Nature Immunology thanks Matthew Hepworth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Data availability
Smart-seq analysis of primary colonic ILC3 in Npm1+/+ and Npm1+/− mice have been deposited in the GEO under the accession code GSE271455. scRNA-seq data from patients with UC and healthy individuals was downloaded from GEO under the accession code GSE182270. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rongchuan Zhao, Jiao Yang.
Contributor Information
Changgeng Peng, Email: changgeng.peng@tongji.edu.cn.
Shiyang Li, Email: lishiyang@sdu.edu.cn.
Minxuan Sun, Email: minxuan.sun@sibet.ac.cn.
Extended data
is available for this paper at 10.1038/s41590-024-01921-x.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-024-01921-x.
References
- 1.Neurath, M. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol.14, 688 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Primers6, 74 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Baumgart, D. C. & Sandborn, W. J. Crohn’s disease. Lancet380, 1590–1605 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Danese, S. & Fiocchi, C. Ulcerative colitis. N. Engl. J. Med.365, 1713–1725 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Hibi, T. & Ogata, H. Novel pathophysiological concepts of inflammatory bowel disease. J. Gastroenterol.41, 10–16 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Ogawa, S. Genetics of MDS. Blood133, 1049–1059 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejar, R., Levine, R. & Ebert, B. L. Unraveling the molecular pathophysiology of myelodysplastic syndromes. J. Clin. Oncol.29, 504–515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tefferi, A. & Vardiman, J. W. Myelodysplastic syndromes. N. Engl. J. Med.361, 1872–1885 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Kono, M. et al. Induction of complete remission by azacitidine in a patient with myelodysplastic syndrome-associated inflammatory bowel disease. J. Crohns Colitis12, 499–502 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Hebbar, M. et al. Association between myelodysplastic syndromes and inflammatory bowel diseases. Report of seven new cases and review of the literature. Leukemia11, 2188–2191 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Harewood, G. C., Loftus, E. V. Jr., Tefferi, A., Tremaine, W. J. & Sandborn, W. J. Concurrent inflammatory bowel disease and myelodysplastic syndromes. Inflamm. Bowel Dis.5, 98–103 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Wang, Z., Zhou, Y. & Liu, Y. Concurrent inflammatory bowel disease and myelodysplastic syndrome: report of nine new cases and a review of the literature. Dig. Dis. Sci.53, 1929–1932 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Nakamura, F. et al. Simultaneous occurrence of inflammatory bowel disease and myelodysplastic syndrome due to chromosomal abnormalities in bone marrow cells. Digestion79, 215–219 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Kristinsson, S. Y. et al. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J. Clin. Oncol.29, 2897 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komrokji, R. S. et al. Autoimmune diseases and myelodysplastic syndromes. Am. J. Hematol.91, E280–E283 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Khan, N. et al. Incidence of acute myeloid leukemia and myelodysplastic syndrome in patients with inflammatory bowel disease and the impact of thiopurines on their risk. Am. J. Gastroenterol.116, 741–747 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Makishima, H. et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat. Genet.49, 204–212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hingorani, K., Szebeni, A. & Olson, M. O. Mapping the functional domains of nucleolar protein B23. J. Biol. Chem.275, 24451–24457 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Uckelmann, H. J. et al. Mutant NPM1 directly regulates oncogenic transcription in acute myeloid leukemia. Cancer Discov.13, 746–765 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, X. Q. D. et al. Mutant NPM1 hijacks transcriptional hub to maintain pathogenic gene programs in acute myeloid leukemia. Cancer Discov.13, 724–745 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morganti, C. et al. NPM1 ablation induces HSC aging and inflammation to develop myelodysplastic syndrome exacerbated by p53 loss. EMBO Rep.23, e54262 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raval, A. et al. Effect of nucleophosmin 1 haploinsufficiency on hematopoietic stem cells. Leukemia26, 853–855 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature517, 293–301 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol.5, 64–73 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science334, 1561–1565 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity36, 92–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity40, 25–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenberg, G. F. et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science336, 1321–1325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature491, 259–263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uzzan, M. et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat. Med.28, 766–779 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schinke, C. et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood125, 3144–3152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muto, T. et al. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J. Exp. Med.210, 2627–2639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gobert, A. P. et al. Protective role of spermidine in colitis and colon carcinogenesis. Gastroenterology162, 813–827 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah, S. C. & Itzkowitz, S. H. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology162, 715–730 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vu, T. et al. Serine-threonine kinase receptor-associated protein is a critical mediator of APC mutation-induced intestinal tumorigenesis through a feed-forward mechanism. Gastroenterology162, 193–208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo, W. T. et al. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology161, 1924–1939 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell182, 655–671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allan, D. S. et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol.8, 340–351 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Di Luccia, B., Gilfillan, S., Cella, M., Colonna, M. & Huang, S. C.-C. ILC3s integrate glycolysis and mitochondrial production of reactive oxygen species to fulfill activation demands. J. Exp. Med.216, 2231–2241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong, L. et al. Nutrition impact on ILC3 maintenance and function centers on a cell-intrinsic CD71–iron axis. Nat. Immunol.24, 1671–1684 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chamoto, K. et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl Acad. Sci. USA114, E761–E770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inak, G. et al. Defective metabolic programming impairs early neuronal morphogenesis in neural cultures and an organoid model of Leigh syndrome. Nat. Commun.12, 1929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg, S. E., Sena, L. A. & Chandel, N. S. Mitochondria in the regulation of innate and adaptive immunity. Immunity42, 406–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin, J., Kato, M., Nagata, K. & Okuwaki, M. Efficient DNA binding of NF-κB requires the chaperone-like function of NPM1. Nucleic Acids Res.45, 3707–3723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barshad, G., Marom, S., Cohen, T. & Mishmar, D. Mitochondrial DNA transcription and its regulation: an evolutionary perspective. Trends Genet.34, 682–692 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Wculek, S. K. et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity56, 516-+ (2023). [DOI] [PubMed] [Google Scholar]
- 47.Ekstrand, M. I. et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet.13, 935–944 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Fu, Z. et al. Mitochondrial transcription factor A in RORγt+ lymphocytes regulate small intestine homeostasis and metabolism. Nat. Commun.12, 4462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee, K. M. et al. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab.26, 633–647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, Z. & Hann, S. R. Nucleophosmin is essential for c-Myc nucleolar localization and c-Myc-mediated rDNA transcription. Oncogene32, 1988–1994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarpulla, R. C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev.88, 611–638 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Dhar, S. K., Xu, Y., Chen, Y. M. & Clair, D. K. S. Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. J. Biol. Chem.281, 21698–21709 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, H. et al. p53 contributes to cardiovascular diseases via mitochondria dysfunction: a new paradigm. Free Radic. Biol. Med.208, 846–858 (2023). [DOI] [PubMed] [Google Scholar]
- 54.Ånensen, N. et al. Correlation analysis of p53 protein isoforms with mutations and therapy response in acute myeloid leukemia. Oncogene31, 1533–1545 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Zhong, Z. Y. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature560, 198–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondo, T. et al. Identification and characterization of nucleophosmin/B23/numatrin which binds the anti-oncogenic transcription factor IRF-1 and manifests oncogenic activity. Oncogene15, 1275–1281 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Ron-Harel, N. et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab.24, 104–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao, P. et al. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. J. Exp. Med.216, 337–349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, Y. et al. USF1/CD90 signaling in maintaining glioblastoma stem cells and tumor-associated macrophages adhesion. Neuro Oncol.24, 1482–1493 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hua, Y., Laserstein, P. & Helmstaedter, M. Large-volume en-bloc staining for electron microscopy-based connectomics. Nat. Commun.6, 7923 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1 and 2 and Supplementary Tables 1 and 2.
Statistical source data.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Data Availability Statement
Smart-seq analysis of primary colonic ILC3 in Npm1+/+ and Npm1+/− mice have been deposited in the GEO under the accession code GSE271455. scRNA-seq data from patients with UC and healthy individuals was downloaded from GEO under the accession code GSE182270. Source data are provided with this paper.