Abstract
The differentiation and specificity of human CD4+ T follicular helper cells (TFH cells) after influenza vaccination have been poorly defined. Here we profiled blood and draining lymph node (LN) samples from human volunteers for over 2 years after two influenza vaccines were administered 1 year apart to define the evolution of the CD4+ TFH cell response. The first vaccination induced an increase in the frequency of circulating TFH (cTFH) and LN TFH cells at week 1 postvaccination. This increase was transient for cTFH cells, whereas the LN TFH cells further expanded during week 2 and remained elevated in frequency for at least 3 months. We observed several distinct subsets of TFH cells in the LN, including pre-TFH cells, memory TFH cells, germinal center (GC) TFH cells and interleukin-10+ TFH cell subsets beginning at baseline and at all time points postvaccination. The shift toward a GC TFH cell phenotype occurred with faster kinetics after the second vaccine compared to the first vaccine. We identified several influenza-specific TFH cell clonal lineages, including multiple responses targeting internal influenza virus proteins, and found that each TFH cell state was attainable within a clonal lineage. Thus, human TFH cells form a durable and dynamic multitissue network.
Subject terms: Adaptive immunity, Follicular T-helper cells, Vaccines
Schattgen et al. profiled the subsets and clonality of CD4+ TFH cells in the blood and lymph nodes of human volunteers who received two influenza vaccines 1 year apart to characterize their dynamics and clonal evolution over 2 years.
Main
Neutralizing antibodies against influenza viruses represent a key correlate of protection and the goal of vaccination. High-affinity antibodies are generated by activated B cells in germinal centers (GCs) located in secondary lymphoid organs. Mature CD4+ T follicular helper cells (hereafter TFH cells) home to GCs and support GC B cells through the production of cytokines and direct costimulatory molecule signaling through T–B cell contact1. While baseline frequencies of circulating TFH cells (cTFH cells) in peripheral blood are typically low2,3, the expansion of cTFH cells in response to vaccination is correlated with increased B cell activation and, consequently, increased antibody titers4–6. A diverse polyfunctional T cell response, including cTFH cells, is correlated with protection against symptomatic infection independent of serology5. Considering their influence on humoral immune response quality, a better understanding of the mechanisms that regulate TFH cell responses to vaccination is necessary for designing improved vaccine platforms.
TFH cells display substantial phenotypic heterogeneity and plasticity across location and time. CD4+ TH1 cell-like CXCR3+CXCR5loPD-1lo cTFH cells have impaired capacity to help B cells compared to CXCR3− cTFH cells, which more closely resemble GC TFH cells6–8. As cTFH cells are replenished by GC TFH cells that egress the secondary lymphoid organs, cells from both compartments display substantial overlap in core gene expression programs and T cell receptor (TCR) repertoires3,9–11. The trajectory of simultaneous TFH cell differentiation and maturation in secondary lymphoid organs and peripheral blood in humans following influenza vaccination has not been tracked over time. Previous characterization of GC B cell responses following influenza vaccination, performed by serial sampling of lymph nodes (LNs) and blood from human volunteers, indicated vaccine-specific B cells enter and persist in GCs for months while undergoing somatic hypermutation (SHM)12.
Here we characterized the phenotype and kinetics of TFH cells in the LN (hereafter LN TFH cells) and cTFH cells in five donors over the course of 2 years following two seasonal influenza vaccinations. In year 1, we observed a transient increase in cTFH cell frequencies in the blood during the first 2 weeks postvaccination, while LN TFH cells clonally expanded and slowly matured into IL21+CXCL13+ GC TFH cells. However, redifferentiation and cytokine production by GC TFH cells in year 2 postvaccination were more rapid. Furthermore, we observed the emergence of interleukin (IL)-10+ TFH cells13,14, characterized by a unique transcriptional profile, by 2 months postvaccination in year 1. Together, our data present a spatiotemporal view of TFH cell phenotype and repertoire dynamics following influenza vaccination.
Results
Influenza vaccination mobilizes human TFH cell responses
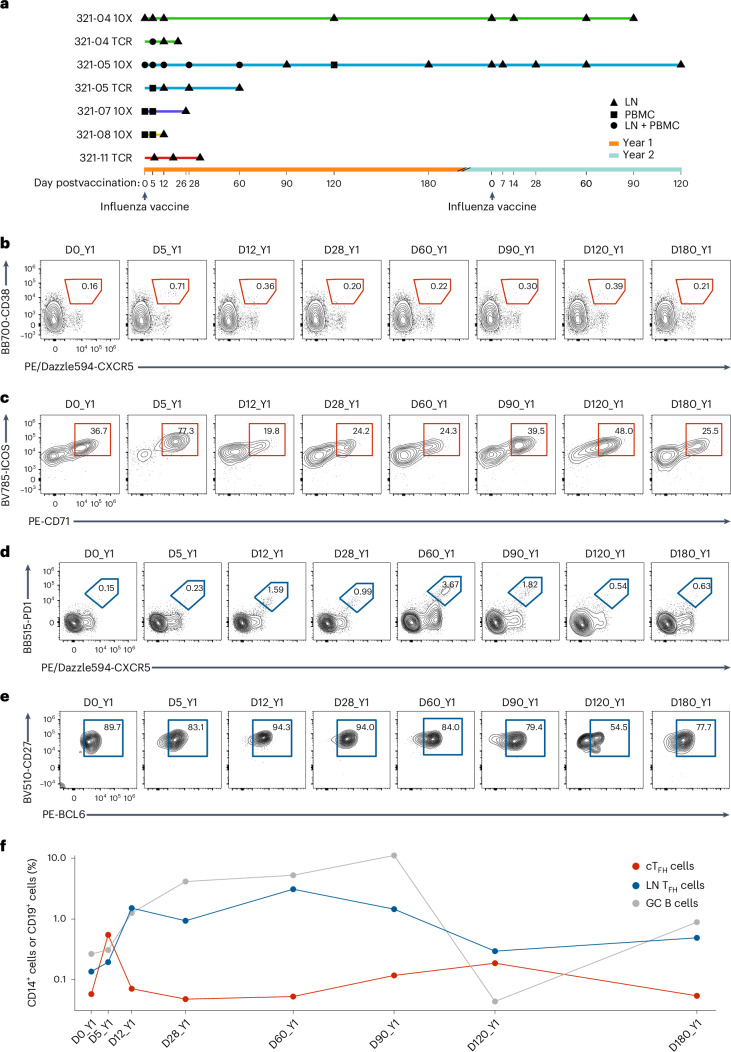
Cohort 1 contained five healthy adults (five males, 26–40 years old, referred to as 321-04, 321-05, 321-07, 321-08 and 321-11) enrolled in WU321 (ref. 12), a multiyear seasonal influenza vaccination study at Washington University, who had not received a seasonal influenza vaccine for at least 3 years before enrollment. Donors were first administered the 2018–2019 Northern Hemisphere seasonal quadrivalent influenza vaccine (QIV) in year 1 (hereafter year 1), then the 2019–2020 seasonal QIV in year 2 (hereafter year 2). LN samples were collected before year 1 vaccination (D0_Y1), on day 5 after year 1 vaccination (D5_Y1), D12_Y1, D120_Y1, D0_Y2, D14_Y2, D60_Y2, D90_Y2 (321-04), D26_Y1 (321-07), D12_Y1 (321-08), D7_Y2, D28_Y2, D60_Y2, D120_Y2 (321-05) and D6_Y1, D18_Y1, D21_Y1, D35_Y1 (321-11). Blood samples were taken at D5_Y1 (321-07 and 321-08) and D120_Y1 (321-05). Paired blood and LN samples were collected at D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D180_Y1 and D0_Y2 (321-05; Fig. 1a). Flow cytometry analysis of peripheral blood mononuclear cell (PBMC) and LN samples from year 1 for 321-05 showed that the frequency of CXCR5+CD38+ cTFH cells among CD4+ T cells in the blood was low (0.16%) before vaccination, expanded fourfold at D5_Y1, then contracted to baseline at D28_Y1 (Fig. 1b,d and Extended Data Fig. 1a,b). A similar transient increase in cTFH cell frequency was observed at D5_Y1 in 321-04 (Extended Data Fig. 1c). Expression of the activation markers ICOS and CD71 on cTFH cells peaked at D5_Y1 for 321-05 (Fig. 1c). The frequency of LN CXCR5+PD-1hi TFH cells in 321-05 began to increase at D5_Y1 and D60_Y1 before contracting at D90_Y1 and D180_Y1 (Fig. 1d). CXCR5+PD-1hi LN TFH cells expressed high levels of the TFH master transcription factor BCL6 across year 1 (Fig. 1e). The frequency of CD19+IgDloCD20hiCD38int GC B cells in the LN peaked and remained elevated from D28_Y1 to D90_Y1, coinciding with the peak in LN TFH cell frequency (Fig. 1f). Similarly, 321-04 showed an accumulation of LN TFH cells coinciding with increased GC B cells in the LN beginning at D5_Y1, which increased at D60_Y1, and peaked at D120_Y1 (Extended Data Fig. 1d).
Fig. 1. Influenza vaccination stimulates a CD4+ TFH cell response in blood and LN of humans.
a, Schematic representation indicating the time points of PBMC (D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1 and D120_Y1) and LN (D0_Y1, D5_Y1, D12_Y1, D26_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2) samples in five donors (321-04, 321-05, 321-07, 321-08 and 321-11) postvaccination with Flucelvax QIV on D0_Y1 (2018–2019 season) and D0_Y2 (2019–2020 season) 1 year apart. b, Representative flow cytometry plots of circulating CXCR5+CD38+ TFH cells (cTFH cells) in PBMCs from donor 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1 and D180_Y1, gated on CD45+CD14−CD19−CD3+CD4+ cells. c, Frequency of ICOS+CD71+ cTFH cells in PBMCs from donor 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1 and D180_Y1, gated on CXCR5+CD38+ TFH cells as in b. d, Representative flow cytometry plots of CXCR5+PD-1+ LN TFH cells in donor 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1 and D180_Y1, gated on CD14−CD19−CD4+ T cells. e, Frequency of CD27+BCL6+ LN TFH cells in 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1 and D180_Y1, gated on CXCR5+PD-1+ TFH cells as in d. f, Frequency of cTFH, LN TFH and CD19+IgDloCD20hiCD38int LN GC B cells in 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1 and D180_Y1. Shown as a percent of total CD4+ T cells or CD19+ B cells on log10 scale.
Extended Data Fig. 1. TFH flow gating strategy and donor 321-04 LN TFH and cTFH frequencies.
a,b, Gating strategies for defining activated cTFH cells (CXCR5+CD38+ICOS+CD71+; a) and LN GC TFH cells (CXCR5+PD−1+BCL-6+; b). c, Frequency of CXCR5+CD38+ cTFH in PBMCs for donor 321-04 in year 1 determined using flow cytometry. Shown as percent of total CD4+ T cells. d, Frequency of CXCR5+PD-1+BCL-6+ GC TFH and IgDloCD20hiCD38int GC B cell in LN biopsies taken from donor 321-04 in year 1 determined using flow cytometry. Shown as percent of total CD4+ T cells or total CD19+ B cells.
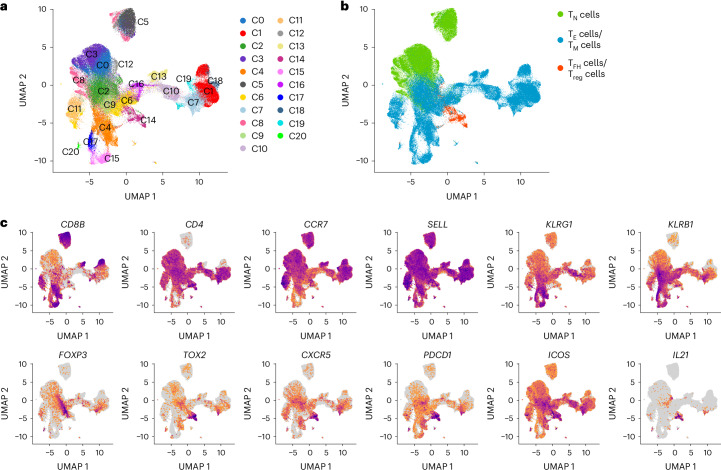
To characterize changes in T cell phenotypes elicited by repeated influenza vaccination, we performed single-cell gene expression (scGEX) and TCR sequencing on immune cells in all PBMC and LN samples from 321-04, 321-05 (except for the LN sample D120_Y1), 321-07 and 321-08 (Supplementary Table 1). The total T cell dataset comprised 154,109 cells and covered 127,329 unique TCR clonotypes grouped into 21 clusters (C0–C20) based on transcriptional similarities (Fig. 2a). Each cluster was categorized broadly into CCR7hiSELLhi CD4+ (C0, C3, C18 and C12) and CD8+ (C5) naive T cells (TN cells), CCR7loSELLloKLRG1+ effector (TE) or memory (TM) CD4+ (C1, C2, C6, C7, C9, C10, C14, C16 and C18) and CD8+ (C4, C11, C13, C15, C17 and C20) T cells (Fig. 2b,c and Extended Data Fig. 2a). Another CCR7loSELLloKLRG1+ cluster of CD4+ T cells (C14) containing cells from all donors expressed markers consistent with TFH (PDCD1, CXCR5, ICOS, TOX2 and IL21) and CD4+ regulatory T cells (FOXP3; herein called Treg; Fig. 2b,c and Extended Data Fig. 2a,b). Comparing clone size distributions across GEX clusters showed TN cells had few clones with more than one cell, whereas the clusters containing CD8+ or CD4+ TE, TM, TFH and Treg cells showed a high degree of clonal expansion (Extended Data Fig. 2c,d). Overall, the total T cell dataset deeply profiled the LN and blood T cell phenotypes of four donors following influenza vaccination.
Fig. 2. scGEX and TCR profiling capture diverse T cell phenotypes in blood and LN after influenza vaccination.
a, UMAP projection of the total T cell dataset generated from the PBMC (at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1 and D120_Y1) and LN (at D0_Y1, D5_Y1, D12_Y1, D26_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2) samples from donors 321-04, 321-05, 321-07 and 321-08 annotated by GEX cluster (n = 154,109 cells with 127,329 unique TCR clonotypes). b, UMAP projection of the total T cell dataset annotated by T cell phenotype as CCR7hiSELLhi TN, CCR7loSELLloKLRG1+ TE cells or TM cells or PDCD1+CXCR5+ TFH or FOXP3+ Treg cells. c, Feature plots displaying distinguishing T cell markers in the total T cell dataset.
Extended Data Fig. 2. Single-cell gene expression and TCR profiling capture diverse T cell phenotypes in blood and LN after influenza vaccination.
a, Dot plot showing the expressions of marker genes for each gene expression cluster of the total T cell dataset generated from the PBMC (at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1 and D120_Y1) and LN (at D0_Y1, D5_Y1, D12_Y1, D26_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2) samples from donors 321-04, 321-05, 321-07 and 321-08 annotated by GEX cluster. n = 154,109 cells with 127,329 unique TCR clonotypes. b, Frequency of cells by donor within each gene expression cluster. c, 2D UMAP projection of the total T cell dataset colored by log10(1 + n) transformed clone sizes. d, Distribution of log10(1 + n) transformed clone sizes across GEX clusters. e, Frequency of cells by donor within each gene expression cluster.
Influenza vaccination induces diverse phenotypes in TFH cells
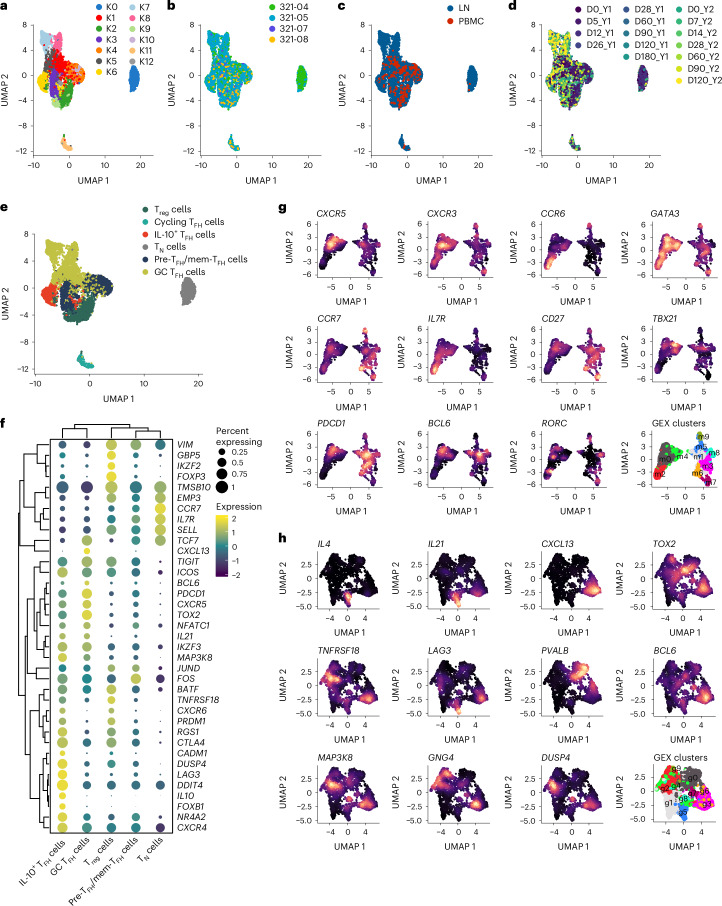
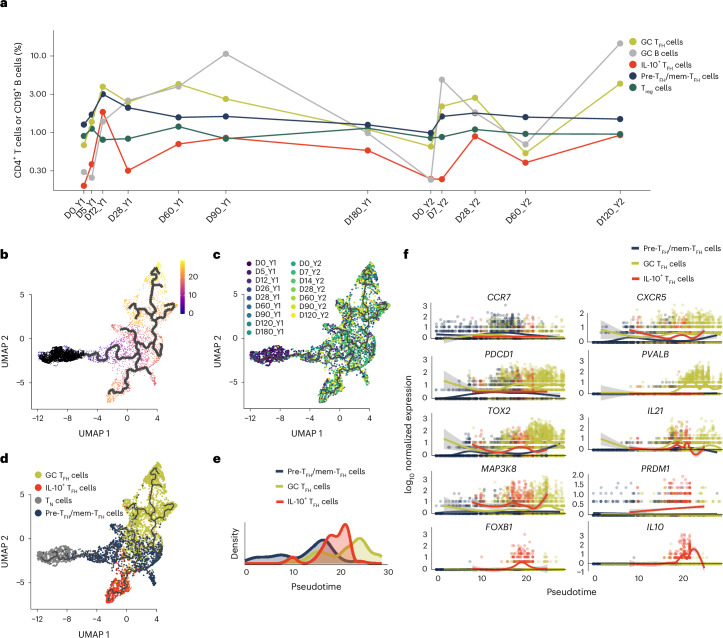
We next separated TFH cells from the total T cell dataset to more closely examine their phenotypes. Because CD4+ TH cells can differentiate into multiple subsets within clonal lineages that stem from a single founder15, we made a subset of all cells in the dataset with TCR sequences matching CD4+ T cells in the TFH/Treg cell cluster (C14; Fig. 2a,b). We also included 250 naive CD4+ T cells from clusters C0 and C3 (Fig. 2a) with 250 cells from LN and PBMCs (500 total; hereafter TN cells) as controls. This strategy yielded 6,109 CD4+ T cells with 4,418 unique TCR clonotypes (Supplementary Table 2), henceforth referred to as the TFH/Treg subset. Reclustering this subset, we discovered 13 scGEX clusters (K0–K12), with ‘donor’ accounting for much of the scGEX variation (Fig. 3a,b). Most cells in the TFH/Treg subset (excluding CD4+ TN cells) were from the LNs (4,737), while 539 cells were from the blood (Fig. 3c), indicating that our approach captured both the cTFH and LN TFH cell populations and their clonal relations. In addition to ‘donor’, scGEX varied substantially across time points and was roughly partitioned into early (D0_Y1–D28_Y1), middle (D60_Y1–D0_Y2) and late (D5_Y2–D120_Y2) time points in the projection (Fig. 3d). Because the single-cell dataset also contained LN B cells12, we looked for correlations between the frequency of LN TFH cells and LN B cell subsets. While we found no relationship between the frequency of TFH and plasmablasts, naive, resting memory or activated B cell subsets (markers defined in Methods) in the LN at matched time points, there was a significant positive correlation between the frequencies of TFH and the GC B cell subset (Extended Data Fig. 3a,b).
Fig. 3. Influenza vaccination stimulates the differentiation of distinct TFH cell subsets.
a–d, UMAP projections of the TFH/Treg subset annotated by scGEX cluster (a), donor (b), tissue (c) and time postimmunization (d) in a subset of the total T cell dataset in Fig. 2 that contained all cells clonally related to those in the TFH/Treg cell cluster C14 from the PBMC and LN of all donors (n = 6,109 cells with 4,418 unique TCR clonotypes). e, UMAP projection of the TFH/Treg subset annotated as CCR7loCXCR5loPDCD1loICOS+ pre-TFH/mem-TFH cells, CCR7−CXCR5hiPDCD1hiICOS+ GC TFH cells, CCR7−CXCR5hiPDCD1hiICOS+LAG3+ IL-10+ TFH cells, FOXP3+ Treg cells, MKI67+ cycling cells or CCR7+CD4+ TN cells. f, Dot plot of select TFH cell and Treg cell subset-specific markers in TFH/Treg subset grouped by each subset (excluding cycling) defined in e. g,h, Reclustering and feature plots of CCR7loCXCR5loPDCD1loICOS+ pre-TFH/mem-TFH cells (g) and CCR7−CXCR5hiPDCD1hiICOS+ GC TFH cells (h) displaying marker genes associated with scGEX clusters within the pre-TFH/mem-TFH and GC TFH cells.
Extended Data Fig. 3. B cell subset frequency correlation and marker genes for TFH/Treg subset.
a, Heatmap of Pearson correlation between frequency of LN TFH (all TFH subsets) and naive, resting memory, activated, GC and plasmablast (PB) B cell subsets in the lymph node. b, Scatter plot comparing the relative frequencies of germinal center B cells versus TFH cells in LN samples. Correlation analyses were performed using the Pearson correlation metric and P values were computed using two-sided t-tests. c, Heatmap of the top marker genes for each GEX cluster for the TFH clonal lineages subset. d, Volcano plot of DEGs between indicated TFH subset, Treg cells and TN cells. e, Violin plots of mouse full TFH module score as defined in ref. 19 in human TFH cells from this study grouped by subset.
Differentially expressed gene (DEG) analysis across TFH cell clusters indicated that clusters K1, K5, K7 and K8 had scGEX profiles characteristic of mature GC TFH cells (CXCR5, PDCD1 and IL21), while clusters K3, K10 and K12 expressed genes consistent with the early and memory stages of TFH cell differentiation (KLF2 and CCR7; Extended Data Fig. 3c). Cluster K6 expressed IL10 and was consistent with IL-10+ TFH cells (Extended Data Fig. 3c)13,14. Clusters K2 and K9 corresponded to FOXP3+ Treg cells, while clusters K0 and K11 corresponded to CD4+ TN cells and cycling cells, respectively (Extended Data Fig. 3c). Therefore, we classified each of the TFH cell clusters as either CCR7loCXCR5loPDCD1loICOS+ pre-TFH/mem-TFH cells, CCR7−CXCR5hiPDCD1hiICOS+ GC TFH cells, CCR7−CXCR5hiPDCD1hiICOS+LAG3+ IL-10+ TFH cells, FOXP3+ Treg cells, MKI67+ cycling cells or CD4+ TN cells (Fig. 3e). Compared to TN, GC TFH cells had increased expression of TOX2 (ref. 16) and CXCL13 (Fig. 3f and Extended Data Fig. 3d). Top DEGs in pre-TFH/mem-TFH cells were associated with adhesion and cytoskeletal rearrangement, including VIM, EMP3 and TMSB10, as well as KLF2, a negative regulator of TFH differentiation17 (Fig. 2f and Extended Data Fig. 3d). Pre-TFH/mem-TFH and GC TFH cells had high expression of TCF7, which promotes TFH cell differentiation via suppression of TH1 cell-promoting transcription factor PRDM1 (ref. 18), and low expression of PRDM1 (Fig. 3f and Extended Data Fig. 3d). In contrast, IL-10+ TFH cells expressed low levels of TCF7 and high levels of PRDM1 (Fig. 3f and Extended Data Fig. 3d). We then compared the human TFH cell subsets with mouse transitional and mature GC TFH cell subsets by scoring DEGs characteristic of these subsets across human TFH subsets19,20. We found that the mouse mature GC TFH module scores were highest in the human GC TFH and IL-10+ TFH cells and lowest in the CD4+ TN cells, while pre-TFH/mem-TFH cells were in the middle (Extended Data Fig. 3e), indicating they aligned with the mouse transitional GC TFH cells.
Reclustering of the pre-TFH/mem-TFH cell subset identified ten subclusters (m0–m9) that improved the resolution between the pre-TFH and mem-TFH cells (Fig. 3g). Clusters m6 and m7 had high expression of CCR7 and CD27 and moderate expression of PDCD1 and BCL6 (Fig. 3g), corresponding to the mem-TFH cells. CXCR3+CCR6−TBX21+ TH1-like in pre-TFH cell clusters m0 and m4 and CXCR3−CCR6+RORC+ TH17-like in pre-TFH cluster m2 aligned well with previously defined CXCR3+ cTFH and CCR6+ pre-TFH cell subsets (Fig. 3g). Similarly, ten subclusters of GC TFH cells (g0–g9) that expressed BCL6 and TOX2 were identified after reclustering (Fig. 3h). Clusters g3 and g6 coexpressed CXCL13 and IL21, while cluster g5 coexpressed IL4 and IL21 (Fig. 3h). The inhibitory coreceptors LAG3 and TNFRSF18 were expressed in clusters g1–g6, g8 and g9 (Fig. 3h). Cluster g0 expressed PVALB but lacked expression of CXCL13, IL21 and signaling molecules associated with mature GC TFH cells (MAP3K8, DUSP4 and GNG4; Fig. 3h).
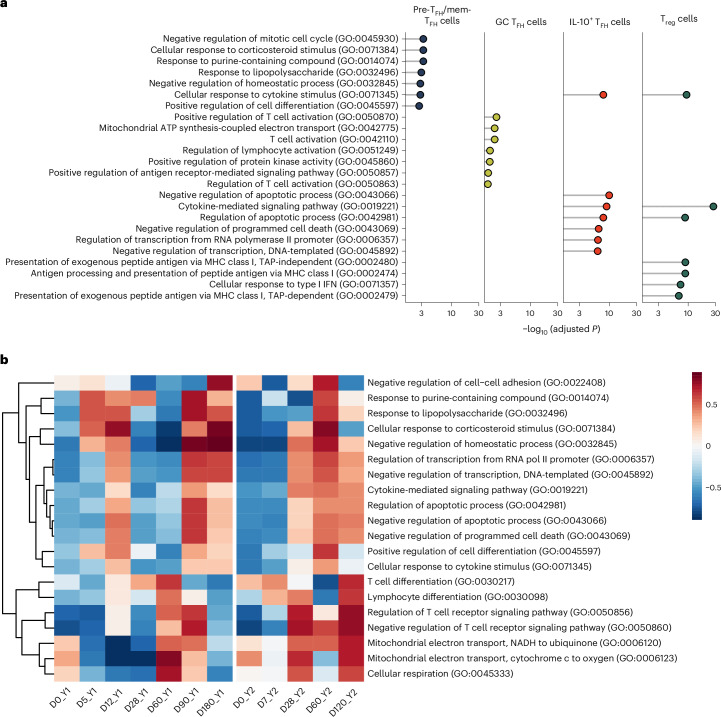
Next, we conducted pathway enrichment analysis to identify Gene Ontology (GO) biological processes enriched in Treg and TFH cell subsets (Fig. 4a). GC TFH cells showed upregulation of genes related to the electron transport chain (ETC; Fig. 4a). IL-10+ TFH and Treg cells shared enrichment in cytokine signaling and regulation of apoptosis pathways (Fig. 4a). Gene set variation analysis (GSVA) was performed to look at the variation of these pathways over time in LN TFH cells for 321-05 and 321-04. In 321-05, pathways associated with T cell activation and cytokine signaling increased between D0_Y1 and D60_Y1 before decreasing to baseline by D0_Y2 (Fig. 4b). ETC pathways were sharply upregulated at D60_Y1 and D90_Y1 before decreasing at D180_Y1 (Fig. 4b). Compared to year 1, the ETC module was upregulated at D28_Y2 and remained elevated at D60_Y2 and D120_Y2 (Fig. 4b). Similar kinetics in these pathways were detected in 321-04, although the ETC was elevated on D0_Y1 and displayed kinetics during year 2 like those of 321-05 during year 1 (Extended Data Fig. 4a). Overall, influenza vaccination stimulated subset-specific changes in the transcriptional profiles related to cell migration, activation and metabolism of LN TFH cells.
Fig. 4. Influenza vaccination alters TFH cell metabolism and signaling with time.
a, Enrichr analysis showing enriched GO biological processes in using LN pre-TFH/mem-TFH, IL-10+ TFH, GC TFH and Treg cells from the TFH/Treg subset from donors 321-04 and 321-05 at time points D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2. Marker DEGs for the four subsets were redefined by comparing them against the global background of subsetted LN pre-TFH/mem-TFH, GC TFH, IL-10+ TFH and Treg cells included in the analysis. b, GSVA across D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D180_Y1, D0_Y2, D7_Y2, D28_Y2, D60_Y2 and D120_Y2 in LN TFH cells from donor 321-05 (excluding Treg cells) for top TFH cell-specific GO terms for a.
Extended Data Fig. 4. Influenza-vaccination stimulated alterations in TFH metabolism and signaling in donor 321-04.
GSVA of donor 321-05 LN TFH cells with respect to time for TFH-specific upregulated GO terms.
Influenza vaccination stimulates a dynamic TFH cell response
Next, we assessed changes in the subset composition for LN TFH cells over the entire time course in 321-04 and 321-05. In 321-05, pre-TFH/mem-TFH cells represented the largest fraction (1.2% of CD4+ T cells in LN), followed by Treg cells (0.86%), GC TFH cells (0.65%) and a few IL-10+ TFH cells (0.19%) at D0_Y1 (Fig. 5a). The GC TFH cell frequency increased sixfold (3.86%) at D12_Y1, peaked at D60_Y1 (4.18%) and declined to a similar frequency as D0_Y1 by D180_Y1 (1.05%; Fig. 5a). The IL-10+ TFH cell frequency increased at D12_Y1 (1.79%), declined at D28_Y1 (0.30%) and D60_Y1 (0.67%) and again increased to 1.3% at D90_Y1 (Fig. 5a). Treg frequency increased at D5_Y1 (1.07%) and D60_Y1 (1.15%), before returning to baseline by D180_Y1 (1.09%), and varied little during year 2 (1.1–2.5%; Fig. 5a). Pre-TFH/mem-TFH cells were also the predominant subset (0.94%) at the time of revaccination on D0_Y2 (Fig. 5a). GC TFH cells expanded threefold between D0_Y2 and D7_Y2 (0.63–2.12%) and remained elevated out to D120_Y2 (4.24%; Fig. 5a), while IL-10+ TFH cell frequency increased threefold between D7_Y2 and D28_Y2 (0.23–0.85%) and remained elevated out to D120_Y2 (0.88%; Fig. 5a). In 321-04, the frequency of GC TFH cells (1.75%) was over twofold higher than pre-TFH/mem-TFH cells (0.75%) on D0_Y1 (Extended Data Fig. 5a). IL-10+ TFH cell frequency increased fourfold at D5_Y1 (0.95%) and 15-fold at the peak time D120_Y1 (3.13%; Extended Data Fig. 5a). Treg cell frequency increased from D0_Y1 to D5_Y1 (0.53% and 1.23%) and remained elevated through D120_Y1 (1.73%; Extended Data Fig. 5a). The frequency of GC TFH cells (14.47%), IL-10+ TFH cells (3.13%) and GC B cells (21.96%) each peaked at D120_Y1 (Extended Data Fig. 5a). During year 2, the frequencies of pre-TFH/mem-TFH, GC TFH and GC B cells increased between D0_Y2 (1.12%, 0.81% and 0.47%) and D14_Y2 (2.15%, 2.67% and 5.90%; Extended Data Fig. 5a). Frequencies of GC TFH cells, IL-10+ TFH cells and GC B cells further increased between D14_Y2 and D60_Y2 (7.35%, 1.94% and 20.9%) and decreased at D90_Y2 (4.50%, 0.81% and 5.53%; Extended Data Fig. 5a).
Fig. 5. Influenza vaccination elicits dynamic alterations in TFH cell composition.
a, Relative frequency of LN GC TFH cell, GC B cells, IL-10+ TFH cells, pre-TFH/mem-TFH cell and Treg cell subsets in donor 321-05 at time points D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D180_Y1, D0_Y2, D7_Y2, D28_Y2, D60_Y2 and D120_Y2. b–d, UMAP projections of LN pre-TFH/mem-TFH, GC TFH, IL-10+ TFH and CD4+ TN cells from all donors at D0_Y1, D5_Y1, D12_Y1, D26_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2 subsetted from the TFH/Treg subset, reclustered and subjected to pseudotime analysis showing calculated pseudotime values with selected root nodes where pseudotime scale was set to zero are indicated by black points on the left side of the projection (b), time points (c) or TFH cell subset (d). n = 4,418 cells. e, Density plots of pseudotime value distributions by TFH cell subset in the division of the TFH/Treg subset defined in b. f, Scatter plot of log10 normalized expression of indicated TFH cell subset marker genes versus pseudotime values per cell in the division of the TFH/Treg subset defined in b. Each dot represents a cell, and the color indicates its assigned TFH cell subset.
Extended Data Fig. 5. Influenza-vaccination induced changes to TFH composition and phenotypes over time in donor 321-04.
a, Relative frequencies of pre/memory, GC and IL-10+ TFH subsets in LN samples from donor 321-04 over time. b, Volcano plots showing DEGs within LN GC TFH cells (all subsets) from donor 321-05 across matched time points (or all time points) after vaccination for years 1 and 2. D5_Y1 vs D7_Y2 (top), D28_Y1 vs D28_Y2 (middle) and year 1 vs year 2 (bottom). c, Heatmap of scaled GEX in LN GC TFH cells from donor 321-05 at indicated time points. Genes shown are the top 10 marker genes for each time point from b. d, Violin plots showing expressions of IL21, CXCL13 and CXCR5 in LN GC TFH cells for donors 321-04 (top) and 321-05 (bottom) at time points D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2. e, Comparison of somatic hypermutation frequency of QIV-specific antibody lineages to LN GC TFH frequency after year 1 vaccination. The box-and-whisker plots show the distribution of SHM frequency for the QIV-specific antibody lineage with the number indicating the median value for the time point. The red point indicates the frequency of GC TFH within CD4+ T cells of the LN for the time point. Donors 321-04 and 321-05 are shown in the top and bottom panels, respectively.
Recall of TM cell responses on re-exposure to antigen enables rapid responses through clonal expansion and/or accelerated redifferentiation into TE cell types. While the sum frequency of all TFH cell subsets in the LN was comparable at D5_Y1 (3.59% of CD4+ T cells) and D7_Y2 (8.70%) for 321-05 (Fig. 5a), we looked for transcriptional differences in LN GC TFH cells between D5_Y1 and D7_Y2, D28_Y1 and D28_Y2 and combined year 1 and combined year 2 time points for 321-05 (Extended Data Fig. 5b). MT-ATP6 and MT-ND3, critical ETC components, were increased in year 2 time points for the combined year 1 and year 2, D28_Y2 versus D28_Y1 and D28_Y1 versus D28_Y2 comparisons (Extended Data Fig. 5b,c). In 321-04, IL21 and CXCL13 were upregulated at D5_Y1 and D12_Y1 and at D14_Y2 compared to D0_Y1 or D0_Y2, respectively (Extended Data Fig. 5d). In 321-05, induction of IL21 and CXCL13 occurred at D12_Y1 and continued to rise until the peak at D90_Y1 before decreasing to similar levels as D0_Y1 at D180_Y1 (Extended Data Fig. 5d). Modest levels of IL21 and little CXCL13 were observed at D0_Y2; however, their expression at D7_Y2 had risen to levels comparable to those between D28_Y1 and D90_Y1 (Extended Data Fig. 5d). Overall, GC TFH cells, despite similar overall frequencies, produced higher levels of IL21 and CXCL13 for supporting GC B cell recruitment and proliferation when re-exposed to influenza vaccine in year 2 compared to initial postvaccination responses in year 1.
GC TFH cells support the activation and maturation of B cells through SHM. The rate of SHM in IGH chains of QIV-specific B cell clonal lineages in 321-04 and 321-05 at time points postinfluenza vaccination year 1 has been reported12, so we compared the frequency of LN GC TFH cells during the same time points. Here we found that the SHM peak in the QIV-specific B cell lineages aligned with the peak frequency of GC TFH (D60_Y1 and D120_Y1 for 321-05 and 321-04, respectively; Extended Data Fig. 5e).
To infer the developmental trajectories of LN TFH cells over the course of the GC response, we performed pseudotime analysis using the LN TFH cells (excluding the Treg cells; Fig. 5b,d). Comparing pseudotime values across TFH cell states showed that the inferred trajectories of the pre-TFH/mem-TFH cells were distributed across early and middle pseudotime (~0 to 20), IL-10+ TFH cells were in middle pseudotime (~12 to 22) and GC TFH cells had the latest pseudotime values (~11 to 30; Fig. 5e). Comparing the expression of marker genes representative of TFH cell subsets relative to pseudotime values indicated that CCR7 expression was highest in the early and middle pseudotime pre-TFH/mem-TFH cells, the GC TFH cell markers PDCD1, PVALB, TOX2 and CXCR5 increased in expression during mid to late pseudotime and IL-10+ TFH cell markers FOXB1 and IL10 were specifically expressed in middle pseudotime (Fig. 5f). These results showed dynamic alterations in the magnitude and phenotypic composition of LN TFH cells in response to influenza vaccination.
Influenza vaccine stimulates clonal TFH cell responses
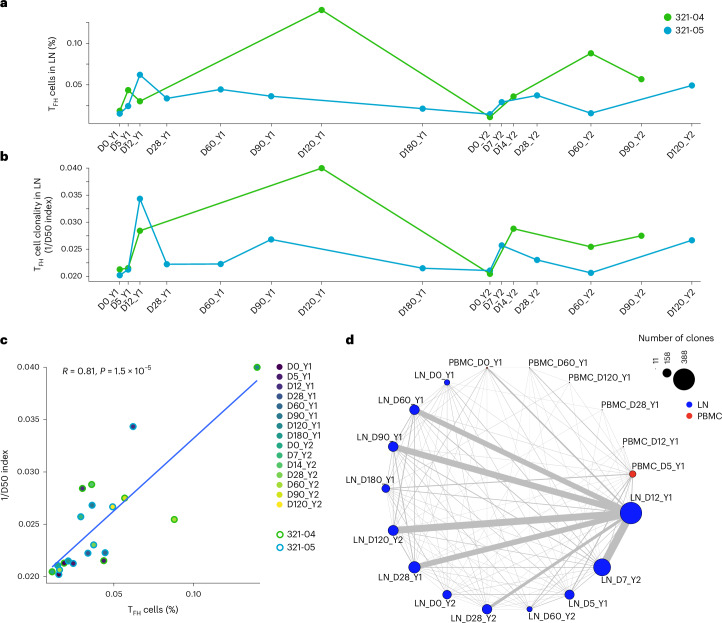
Next, we characterized the TCR repertoires and clonal dynamics of TFH cell responses. In 321-05, the frequency of TFH cells among all T cells in the LN increased between D0_Y1 and D5_Y1 and peaked at D28_Y1 before decreasing to baseline at D90_Y1 (Fig. 6a). In 321-04, TFH cell frequency increased starting at D5_Y1 and peaked at D120_Y1 during year 1 (Fig. 6a). LN TFH cell frequency increased between D0_Y2 and D7_Y2 (321-05) or D14_Y2 (321-04) and remained elevated through D28_Y2 and D120_Y2 (321-05) or D60_Y2 and D90_Y2 (321-04) for year 2 (Fig. 6). TFH cell clonality followed a similar trend as frequency. For 321-05, the peak in TFH cell frequency coincided with their maximum clonality (Methods) at D12_Y1 (Fig. 6b). For 321-04, the clonality of TFH cells increased at D12_Y1 and remained elevated for the remainder of year 1 (Fig. 6b). TFH cell frequency and clonality in both donors contracted at baseline (D0_Y2) but quickly expanded in a clonal manner at D7_Y2 (321-05) and D14_Y2 (321-04), contracted at D28_Y2 and D60_Y2 (321-05) or D60_Y2 (321-04), expanded again to a second peak at D90_Y2 and D120_Y2 for 321-04 and 321-05, respectively (Fig. 5b). TFH cell frequency and clonality were positively correlated across all time points (Fig. 5c).
Fig. 6. Influenza vaccination leads to TFH cell clonal expansion.
a, Relative frequency of all subsets of TFH cells in LN (TFH/Treg subset excluding Treg and TN cells) from donors 321-04 and 321-05 over D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2 time points. b, TCR clonality of all subsets of TFH cells in LN (TFH/Treg subset excluding Treg and TN cells) from donors 321-04 and 321-05 over D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2 time points by inverse D50 index. c, Scatter plot comparing the relative frequency and clonality from a and b (n = 20). Correlation analyses were performed using the Pearson correlation metric, and P values were computed using two-sided t test. d, Network graph depicting the connection between TFH cell clonal lineages in PBMCs and LN in donor 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D180_Y1, D0_Y2, D7_Y2, D28_Y2, D60_Y2 and D120_Y2 time points. Node sizes correspond to the number of unique clones, and edge widths correspond to the number of clones connecting each node.
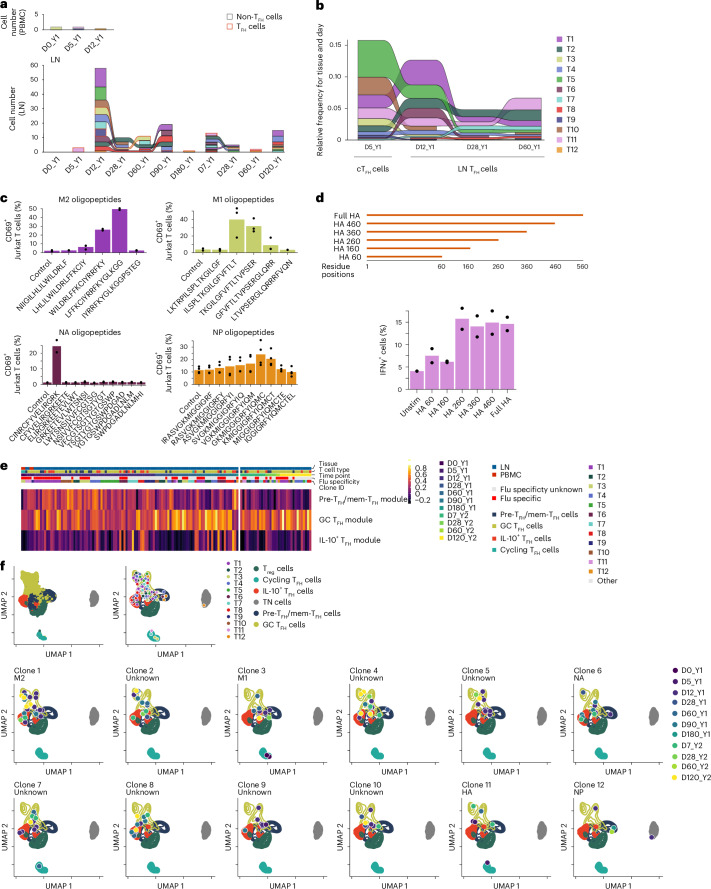
We tracked TFH clonotypes across all time points for 321-04 and 321-05, and across LN and blood for 321-05. In 321-05, the number of unique LN TFH cell clonotypes remained low at D0_Y1 (101) and D5_Y1 (170) and expanded on D12_Y1, when the largest number of unique TFH clonotypes was observed (388; Fig. 6d). In 321-05, several of the TFH clones detected in the LN at D12_Y1 were detected in the blood at D0_Y1 (Fig. 6d), indicating that some of the clonotypes engaging in GC reactions were derived from cTFH cells. Many of the TFH clonotypes from D12_Y1 were detected at subsequent time points, particularly at D28_Y1, D60_Y1 and D90_Y1 (Fig. 6d), with fewer detectable by D180_Y1 (Fig. 6d). Very few TFH clonotypes detected in year 1 were detectable on D0_Y2 but were 1-week postsecondary vaccination (D7_Y2) and persisted until D120_Y2 (Fig. 6d). In 321-04, several of the TFH clones detected at D12_Y1 persisted up to D120_Y1, contracted by D0_Y2, were detected again at D14_Y2 and persisted through D90_Y2 (Extended Data Fig. 6a). Shared clonotypes in the PBMCs of 321-07 and 321-08 were detected across D0_Y1 and D5_Y1; however, no clones were shared between the blood and LN TFH compartments in these donors (Extended Data Fig. 6b,c).
Extended Data Fig. 6. Bulk and single-cell TCR repertoire profiling of cTFH and LN TFH cells after influenza vaccination.
a–c, Network graph depicting the connections between TFH clonal lineages in PBMCs and LN for donors: 321-04 (a), 321-08 (b) and 321-07 (c) at D0_Y1, D5_Y1, D12_Y1, D120_Y1, D0_Y2, D14_Y2, D60_Y2 and D90_Y2 time points. Node sizes correspond to the number of unique clones, and edge widths correspond to the number of clones connecting each node. d, 2D UMAP projection of total T cell dataset highlighting cells with matching paired TCR sequences to TCR data generated by scPCR of individually sorted CXCR5+CD38+ cTFH and CXCR5+PD-1+ LN TFH cells for donors 321-04 and 321-05. e, Frequency cells within each GEX cluster matching to TCRα or TCRβ sequences in the bulk TCR repertoire sequencing from bulk-sorted CXCR5+CD38+ cTFH and CXCR5+PD-1+ LN TFH cells for donors 321-04 and 321-05. TCRs were sequenced from the following combinations of donor, time point and tissue: donor 321-05: D12_Y1, D28_Y1, D60_Y1 in LN and D5_Y1 PBMCs; donor 321-04: D5_Y1 and D12_Y1 in LN and D5_Y1 PBMCs; donor 321-11: D6_Y1, D18_Y1, D21_Y1, D35_Y1 in LN. f, Morisita–Horn index of overlapping clonotypes for TCRα (left) and TCRβ (right) in bulk TCR sequencing from e.
We performed additional single-cell and bulk TCR-seq on sorted cTFH cells and LN TFH cells from 321-04, 321-05 and 321-11 postvaccination year 1 (Fig. 1a). TCR sequences from 321-04 and 321-05 PBMC and LN TFH cells mostly matched to cells within the TFH/Treg cell cluster (C14) in the total T cell dataset (Extended Data Fig. 6d,e). Measuring the overlap between cTFH cells and LN TFH cells for year 1 time points showed that the overlap of TCRα and TCRβ chains was highest among samples taken from the same donor, regardless of tissue (Extended Data Fig. 6f). These results indicated that the TCR repertoire of TFH cells was shared among tissues and remained durable over the course of multiple years.
Vaccination stimulates the maturation of influenza-specific TFH cells
Next, to determine whether the expanded and persistent TFH cell lineages were specific for influenza vaccine-derived antigens, we chose 12 paired (α and β chains) TCRs (herein called lineages T1–T12) from 321-05, prioritizing clonally expanded lineages detected in both PBMC and LN samples and/or at multiple time points in postvaccination year 1 (Fig. 7a and Supplementary Data 1). T3, T9, T11 and T12 were detected in both blood and LN (Fig. 7a). T3 was detected in cTFH cells at D0_Y1 (Fig. 7a), while T1, T2, T4, T5, T6, T7 and T10 were detected in bulk cTFH cells sequenced on D5_Y1 (Fig. 7b). To determine whether each TFH TCR picked was influenza-specific, we transduced TCRs T1–T12 into TCR-deficient Jurkat cells to create stable T1–T12 cell lines. Artificial antigen-presenting cells (aAPCs) expressing individual major histocompatibility (MHC) class II alleles from 321-05 in K562 cells (Supplementary Data 2), or purified B cells from PBMCs from HLA-matched individuals not in the study, were used as APCs. We cocultured T1–T12 cell lines with aAPCs infected with influenza virus or transfected with plasmids expressing individual virus segments and measured upregulation of CD69 or interferon-γ (IFNγ) as the readout of activation. T1, T3 and T12 showed a positive response to infected aAPCs expressing HLA-DR3*03:01, HLA-DPA1*01:03/DPB1*13:01 and HLA-DR5*01:01, respectively (Extended Data Fig. 7a,b). Overexpression of influenza proteins showed that T1 and T3 responded to the matrix (M) protein and T12 responded to nucleoprotein (NP; Extended Data Fig. 7c). T6 showed a positive response to neuraminidase (NA) peptides presented by B cells expressing HLA-DPA1*01:03/DPB1*04:02, which could be blocked with an anti-HLA-DP antibody (Extended Data Fig. 7d,e). Using partially overlapping peptides tiled across the M, M2, NP and NA proteins, we found that the peak response for T1 was to M246–62, T3 to M151–67, T6 to NA417–431 and T12 to NP30–43 (Fig. 7c). Coculturing T11 with aAPCs pulsed with full-length recombinant hemagglutinin (HA) showed a positive response to HA (H1N1) presented by HLA-DR5*01:01 aAPCs (Extended Data Fig. 7f). More precise HA epitope mapping determined that T11 responded to an epitope within the HA160–260 region (Fig. 7d).
Fig. 7. Vaccination evokes maturation of influenza-specific TFH cell clonal lineages.
a, Number of cells detected in PBMCs (top) and LN (bottom) for TFH cell clonal lineages T1–T12 in donor 321-05 at D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1, D90_Y1, D180_Y1, D0_Y2, D7_Y2, D28_Y2, D60_Y2 and D120_Y2. b, Relative abundance of TFH cell clonal lineages T1–T12 in bulk TCRβ sequencing of sorted cTFH cells and LN TFH cells from donor 321-05 at D5_Y1, D12_Y1, D28_Y1 and D60_Y1. c, Frequency of CD69+ cells in T1, T3 and T12 cell lines after 24 h of coculture with aAPCs expressing HLA-DR3*03:01, HLA-DPA1*01:03/DPB1*13:01 or HLA-DR5*01:01 pulsed with peptides derived from influenza M2, M1, NA or NP, and in the T6 cell line after 24 h of coculture with B cells expressing HLA-DPA1*01:03/DPB1*04:02 pulsed with NA peptides. DMSO alone was used as a control (n = 2–4 technical replicates per condition). Representative of two experiments. d, Frequency of IFNγ+ T11 Jurkat T cell line after 24 h coculture with aAPCs transfected with plasmids containing full HA protein, residues 1–460 (HA 460), 1–360 (HA 360), 1–260 (HA 260), 1–160 (HA 160) or the empty vector control (unstim; n = 2 technical replicates per condition). Representative of n = 2 experiments. e, Heatmap of pre-TFH/mem-TFH, GC TFH and IL-10+ TFH cells gene set module scores for TFH cell clonal lineages T1–T12 from donor 321-05 across time points in a. f, UMAP projection showing the localization of all cells encompassed by TFH cell clonal lineages T1–T12 assessed in donor 321-05 across time points from within the embedding from Fig. 2e.
Extended Data Fig. 7. Identification and profiling of influenza-specific TFH clonotypes in donor 321-05.
a, Gating strategy for assessing Jurkat TFH TCR cell line activation in co-culture experiments. b, Frequency of CD69+ Jurkat T cells expressing TCRs T1, T3 and T12 after co-culture with aAPCs infected with influenza PR8 for 24 hours. c, Frequency of CD69+ T1, T3 and T12 Jurkat T cell lines after co-culture with aAPCs transfected with plasmids expressing individual segments of the IAV genome compared to empty vector control for 24 hours. d, Frequency of CD69+ Jurkat T cells expressing TCRs T4, T6, T7, T9 and T10 after co-culture for 24 hours with partially HLA-matched B cells pulsed with influenza protein peptide pools. e, Frequency of CD69+ in T6 Jurkat cells stimulated as in d in the presence of antibodies blocking specific MHC class II molecules. f, Frequency of CD69+ T11 cell line co-culture with aAPCs pulsed with recombinant HA protein, PMA/ionomycin or DMSO control for 24 hours. g, PC1 scores of individual cells from the picked TFH lineages with respect to time. h, Heatmap showing the expressions of genes corresponding to the head and tail PC1 loadings in the twelve TFH clonal lineages.
Principal-component analysis (PCA) analysis of the highly variable features in cells belonging to the T1–T12 lineages indicated that the first principal component (PC1) scores varied with respect to time (Extended Data Fig. 7g). The highest positive PC1 scores were observed on D0_Y1, D5_Y1 and D12_Y1, decreased to values around 0 at D28_Y1, continued to decrease until D60_Y1 and remained stable at D180_Y1, D7_Y2, D28_Y2, D60_Y2 and D120_Y2 (Extended Data Fig. 7g). Examining the top contributing genes in PC1 (positive and negative) showed that genes in the positive loadings contained several pre-TFH/mem-TFH cell markers (TMSB10 and KLF2), while those in the negative loadings were enriched with GC TFH cell markers (TOX2; Extended Data Fig. 7h). Scoring marker gene sets for pre-TFH/mem-TFH, GC TFH and IL-10+ TFH cell subsets as modules showed that pre-TFH/mem-TFH cell module scores were highest between D0_Y1 and D12_Y1 and decreased at D28_Y1, while GC TFH cell and IL-10+ TFH cell scores increased and continued to rise at D60_Y1 and D90_Y1 (Fig. 7e and Supplementary Data 3; Methods). Pre-TFH/mem-TFH cell module scores increased little in response to vaccination in year 2, while GC TFH cell module values were consistent between D0_Y2 and D120_Y2, comparable to those observed at D90_Y1 (Fig. 7e). Finally, we plotted the daughter cells, or cells derived from a shared clonal ancestor as defined by TCR sequence, for T1–T12 lineages across all time points in which they were detected (Fig. 7a) in the context of the TFH/Treg cell embedding. This analysis showed that while the daughter cells localized to clusters corresponding to the pre-TFH/mem-TFH, GC TFH and IL-10+ TFH cell subsets, their distribution among the TFH subsets varied with time postvaccination both years (Fig. 7f). These observations indicated that TFH subset composition among clonally expanded influenza-specific TFH clonotypes could change rapidly within the first week postvaccination and durably over the course of years.
Discussion
Here we found that seasonal influenza vaccination mobilized and matured the TFH response in the blood and LN. Pre-TFH/mem-TFH, GC TFH and IL-10+ TFH cell subsets followed distinct kinetics and could be populated by cells from a single clonal lineage. IL-10+ TFH cells may represent a further matured GC TFH cell subset, but our pseudotime analysis suggested this was not the case. Instead, we observed a bifurcation in the developmental trajectories toward either GC TFH cells or IL-10+ TFH cell subsets within a clonal lineage. This suggested that differentiation into either state was stochastic rather than due to the qualities of TCR-antigen recognition. Plasticity between these subsets on a per-cell basis could not be ruled out. Further work in this area will be necessary to elucidate the mechanisms regulating this fate decision.
The quality of the TFH cell response to the seasonal influenza vaccination varied between the first and second vaccinations. We observed substantial alterations in LN TFH cell transcriptional profiles at matched time points postvaccination year 2 versus year 1, corresponding to changes in signaling, costimulation and metabolic pathways. The magnitude and kinetics of IL21 and CXCL13 expression following vaccination also differed between year 1 and year 2. Previous work in mice has shown that CD4+ TM cells previously engaged in GC reactions can dedifferentiate, persist long-term (at least 200 days) in the LN and redifferentiate more quickly into GC TFH cells upon antigen re-exposure21. Our findings support the idea that a similar accumulation of memory TFH cells occurs in human lymphoid organs. Long-lived TFH cells in mice undergo epigenetic alterations that enforce their reprogramming and support their survival and plasticity22. Whether a similar mechanism exists for human TFH cells should be further investigated.
TCR sequencing permitted us to track individual TFH cell clonotypes over time and across tissues. In other studies, a comparison of the TFH cells from the tonsils to cTFH in the blood of a single donor showed that these two compartments partially overlap in their TCR repertoires10,11,23. We identified influenza-specific TFH cell clonotypes that persisted in the LN 3–6 months after the first vaccination and reappeared in year 2 upon revaccination in the same LN, where they persisted for 4 months.
Among the five TFH cell TCRs with specificities identified in this study, three recognized epitopes from internal M1, M2 or NP proteins. These clones were detectable on day 5 of the first vaccination, indicating that they were likely derived from TM rather than TN cells. This implies that a substantial portion of the TFH cell response to seasonal influenza vaccination is memory-based and targets conserved epitopes present in split-inactivated vaccines24,25, rather than the desired new responses to HA or NA epitopes26. Along those lines, the investigation into the quality of adaptive immune responses after Flublok administration, which contains only recombinant HA, showed improved CD4+ T cell functionality and antibody responses compared to FluZone, a split-inactivated QIV vaccine27. We speculate that the presence of internal proteins in split-inactivated influenza vaccines stimulates memory TFH cell responses that blunt the ability of CD4+ TN cells and B cells to engage in a GC response and generate antibodies targeting new HA subtypes. Future studies on the quality and kinetics of the TFH cell response comparing split-inactivated virus versus subunit vaccines are needed to address this possibility.
A limitation of our study is that we examined only five individuals due to the cost and procedural intensity of the experimental plan. Future studies will build on these observed trajectories to characterize human TFH cell responses in more individuals and with matched time points postvaccination between years and diverse vaccination strategies.
Methods
Human donors
The WU321 study12 enrolled eight participants (one female and seven males, ages 26–40 years) who had not been vaccinated with the vaccine for at least 3 years before the initiation of the study. Cohort 1 studied here contains PBMC (D0_Y1, D5_Y1, D12_Y1, D28_Y1, D60_Y1 and D120_Y1) and LN (D0_Y1, D5_Y1, D12_Y1, D26_Y1, D28_Y1, D60_Y1, D90_Y1, D120_Y1, D180_Y1, D0_Y2, D7_Y2, D14_Y2, D28_Y2, D60_Y2, D90_Y2 and D120_Y2) samples from five donors (321-04, 321-05, 321-07, 321-08 and 321-11) of the WU321 study. Paired PBMC–LN samples from donor 321-05 were available for D0_Y1, D5_Y1, D12_Y1, D28_Y1 and D60_Y1. All studies were approved by the Institutional Review Board (IRB) of Washington University in St. Louis. Written consent was obtained from all participants.
Sample collection, preparation and storage
PBMCs were isolated using Vacutainer CPT tubes (BD Biosciences), the remaining red blood cells were lysed with ammonium chloride lysis buffer (Lonza) and cells were immediately used or cryopreserved in 10% dimethylsulfoxide in FBS. Ultrasound-guided fine-needle aspiration (FNA) of axillary LNs was performed by a qualified physician’s assistant under the supervision of a radiologist. LN dimensions and cortical thickness were measured before each FNA. For each FNA sample, six passes were made using 25-gauge needles, each of which was flushed with 3 ml of RPMI 1640 supplemented with 10% FBS and 100 U ml−1 penicillin/streptomycin, followed by three 1-ml rinses. Red blood cells were lysed with ammonium chloride buffer (Lonza), washed twice with PBS supplemented with 2% FBS and 2 mM EDTA and immediately used or cryopreserved in 10% DMSO in FBS. Participants reported no adverse effects of phlebotomy, serial FNA or vaccination. No statistical methods were used to predetermine the sample size. Investigators were not blinded to experiments and outcome assessment.
Vaccine
The Flucelvax QIV influenza vaccine for the North American 2018/2019 and 2019/2020 seasons was purchased from Seqirus.
Single-cell RNA-seq library preparation and sequencing
Activated and memory B cells were enriched from PBMCs by first staining with IgD-PE (IA6-2; 1:200 dilution) and MojoSort anti-PE Nanobeads (BioLegend) and then processing with the EasySep Human B Cell Isolation Kit using the EasyEights Magnet (STEMCELL Technologies) to negatively enrich IgDlo B cells. Enriched IgDlo B cells, whole PBMCs and whole FNA were processed using the following: 10x Genomics Chromium Single Cell 5′ Library and Gel Bead Kit v2, Chromium Single Cell V(D)J Enrichment Kit and Chromium i7 Multiplex Kit (PN-120262). The cDNAs were prepared after GEM generation and barcoding, followed by GEM reverse transcription reaction and bead cleanup steps. Purified cDNA was amplified for 10–14 cycles before cleaning with SPRIselect beads. Then, samples were evaluated on a bioanalyzer to determine the cDNA concentration. TCR/B cell receptor (BCR) target enrichments were performed on full-length cDNA. scGEX and enriched TCR/BCR libraries were prepared as recommended by the 10x Genomics Chromium Single Cell V(D)J Reagent Kit (v1 Chemistry) user guide, with appropriate modifications to the PCR cycles based on the calculated cDNA concentration. The cDNA libraries were sequenced on NovaSeq 6000 S4 (Illumina), targeting a median sequencing depth of 50,000 and 5,000 read pairs per cell for gene expression and TCR/BCR libraries, respectively.
Cell sorting and flow cytometry
Staining for analysis and sorting was performed using fresh or cryopreserved PBMCs or FNA single-cell suspensions in 2% FBS and 2 mM EDTA in PBS (P2). For sorting, cells were stained for 30 min on ice with IgD-PerCP-Cy5.5 (IA6-2; 1:200), CD4-Alexa 700 (SK3; 1:400), CD20-APC-Fire750 (2H7; 1:100) and Zombie Aqua, along with CD38-BV605 (HIT2; 1:100), CD71-FITC (CY1G4; 1:200) and CD19-PE (HIB19; 1:200) for PBs or CD19-BV421 (HIB19; 1:100), CD71-PE (CY1G4; 1:400), CXCR5-PE-Dazzle 594 (J252D4; 1:40) and CD38-PE-Cy7 (HIT2; 1:200) for GC B cells (all from BioLegend). For donors 321-07 and 321-08, the PBMC samples for days 0 and 5 were stained with TotalSeq-C antihuman hashtag oligos 9 and 10, respectively, for downstream demultiplexing. Cells were washed twice, and single PBs (live singlet CD19+ CD4− IgDlo CD38+ CD20− CD71+) and GC B cells (live singlet CD19+ CD4− IgDlo CD71+CD38int CD20+ CXCR5+) were sorted using a FACSAria II into 96-well plates containing 2 μl lysis buffer (Clontech) supplemented with 1 U μl−1 RNase inhibitor (New England Biolabs) or bulk sorted into buffer RLT Plus (QIAGEN) and immediately frozen on dry ice. For analysis, cells were stained for 30 min on ice with biotinylated recombinant HAs and PD-1-BB515 (EH12.1; BD Horizon; 1:100) diluted in P2, washed twice and then stained for 30 min on ice with IgA-FITC (M24A; Millipore; 1:500), CD45-PerCP (2D1; BD Bioscience; 1:25), IgG-BV480 (goat polyclonal; Jackson ImmunoResearch; 1:100), IgD-SB702 (IA6-2; Thermo Fisher Scientific; 1:50), CD38-BV421 (HIT2; 1:100), CD20-Pacific Blue (2H7; 1:400), CD27-BV510 (O323; 1:50), CD4-BV570 (OKT4; 1:50), CD24-BV605 (ML5; 1:100), streptavidin-BV650, CD19-BV750 (HIB19; 1:100), CXCR5-PE-Dazzle 594 (J252D4; 1:50), CD71-APC (CY1G4; 1:100), CD14-A700 (HCD14; 1:200) and IgM-APC-Cy7 (MHM-88; 1:400; all from BioLegend) diluted in Brilliant Staining Buffer (BD Horizon). Cells were washed twice, then fixed and permeabilized for intranuclear staining for 1 h at 25 °C with True Nuclear fixation buffer (BioLegend), washed twice with permeabilization/wash buffer and stained for 30 min at 25 °C with BCL6-PE (7D1; 1:50) and Ki-67-PE-Cy7 (Ki-67; 1:400; both from BioLegend). Cells were washed twice with permeabilization/wash buffer and resuspended in P2 for acquisition on an Aurora using SpectroFlo v.2.2 (Cytek Biosciences). Flow cytometry data were analyzed using FlowJo v.10 (Treestar).
Processing of 10x Genomics single-cell 5′ gene expression data
The demultiplexed FASTQ pair-end reads were preprocessed on a by-sample basis using the cellranger count command from 10x Genomics’ Cell Ranger (v.6.0.0) for alignment against the GRCh38 human reference (v.3.0.0; refdata-cellranger-GRCh38-3.0.0). Individual samples are assigned to one of three initial datasets—one containing samples from donors 321-05 and 321-04, one containing samples from donors 321-07 and 321-08 and the last containing additional CD4+ PBMCs from donor 321-05 at D0_Y1 time point.
Processing 10x Genomics single-cell TCR reads
Reads for each sample were parsed using cellranger vdj from 10x Genomics’ Cell Ranger (v.6.0.0) for alignment against the cellranger-vdj-GRCh38-alts-ensembl-5.0.0 reference. Additional quality control was performed using the make_10x_clones_file function from CoNGA software package using settings stringent = True to correct for spurious chain pairings based on other paired clonotypes in the dataset. Only cells with paired TCRαβ sequences were retained. The inverse D50 index in Fig. 6b was calculated as the reciprocal of the quotient from dividing the number of unique clones occupying the top 50% of the repertoire (after ranking by abundance) by the total number of unique clonotypes for the time point multiplied by 100.
Processing single-cell TCR sequencing from sorted TFH cells
TCRα and TCRβ chains were amplified from blood and LN TFH cells sorted into individual wells of a 384-well plate (described above) by RT–PCR using variable gene and constant region primers and sequenced by the Sanger method as previously described28. Sequencing reads were parsed using the TCRdist pipeline as previously described29.
Bulk TCR sequencing of sorted TFH cells
TCRα and TCRβ chains from bulk-sorted cTFH and LN TFH were amplified using 5′ rapid amplification of cDNA ends procedure with unique molecular identifiers (UMIs) for error correction essentially as described in ref. 30. RNA was extracted from bulk-sorted blood and LN TFH cells from donors 321-04, 321-05 and 321-11 using the RNeasy Micro Kit (QIAGEN). Reverse transcription was carried out using SmartScribe RT (Takara) and Q5 polymerase (NEB) was used during the first and second rounds of amplification. Barcoded TCRα and TCRβ amplicons generated by the second round of PCR were pooled by equal volume, prepped and indexed for sequencing on Illumina platforms using a KAPA HyperPrep Kit (Roche). We performed 150-bp paired-end sequencing on the NovaSeq 6000 (Illumina).
Processing bulk TCR sequencing
Paired-end FASTQ reads were processed using migec (v.1.2.9) software31. Reads corresponding to individual samples were demultiplexed before assembling nucleotide sequence reads within each UMI group. Variable–diversity–joining (VDJ) junction mapping of assembled contigs was performed using the NCBI-BLAST+ package within migec. Additional quality control was performed on the filtered clonotype tables from migec using the vdjtools (v.1.2.1)32 FilterNonFunctional, Correct and Decontaminate functions to remove potentially erroneous clonotypes within and between samples. The immunarch (v.0.6.5) package33 for R was used for the Morisita–Horn index calculations.
scGEX analysis
Analysis was performed using Seurat (v.4.1.1). The three datasets noted above were each processed in the following manner before aggregation. First, an object containing all cell types was generated before filtering down to only T cells. Quality control was performed by removing cells with greater than 10% mitochondrial content, expressing fewer than 300 or greater than 4,000 genes, and containing fewer than 900 or greater than 40,000 UMIs to filter out presumably lysed/dead cells and doublets. MULTIseqDemux was used for demultiplexing hashtag oligos for the dataset containing donors 321-07 and 321-08 (ref. 34). After preprocessing, the three datasets were merged, and SCTranform was used for data normalization before feature extraction, scaling and PCA35. The Harmony algorithm was applied for batch integration across donors and datasets36. The FindClusters and RunUMAP tools were applied to the harmonized embedding to generate a 2D projection of scGEX space. TCR clonotype information (described above) was then mapped back to cells in the object by matching their corresponding UMI barcodes.
The total T cell dataset was subset from all cell datasets by selecting clusters where the majority of cells expressed CD3E and then further subsetting those to only cells with paired TCR sequence information. Cluster identities for the total T cell dataset were assigned based on the expression of T cell markers distinguishing each of the major subtypes, including CD4, CD8B, KLRB1, ZBTB16, KLRG1, CCR7, SELL, FOXP3, PRF1 and TBX21. PDCD1, CXCR5 and ICOS were used to identify TFH clusters. TFH cells were further subset and reprocessed to identify heterogeneity in their scGEX phenotypes. Here we chose to include all cells clonally linked to those in the two TFH clusters that might otherwise be excluded due to non-TFH scGEX cluster assignments.
B cell subsets were defined using the following markers: BCL6, RGS13, MEF2B, STMN1, ELL3 and SERPINA9 for GC B cells; XBP1, IRF4, SEC11C, FKBP11, JCHAIN and PRDM1 for plasmablasts; TCL1A, IL4R, CCR7, IGHM and IGHD for naive B cells; TBX21, FCRL5, ITGAX, NKG7, ZEB2 and the lack of CR2 for activated B cells; TNFRSF13B, CD27 and CD24 for resting memory B cells.
Monocle3 (refs. 37–39) R package (v.1.1.0) was used for pseudotime analysis on our data. The getEnrichedPathways function in the Cerebro40 (v.1.3.1) R package was used to find enriched GO terms for the TFH populations. GO terms enriched in IL-10+ TFH cells with respect to all other TFH and Treg cells were used for GSVA41 analysis in Fig. 4b (GSVA R package, v.1.42.0). Genes defining the pre/memory, GC and IL-10+ TFH modules (Supplementary Data 3) 6F were taken from intersecting positive markers defining the subset relative to the two other subsets based on the results of the differential expression analysis results using Seurat’s FindMarkers function using default parameters (Extended Data Fig. 3). Module scores were calculated using the AddModuleScore function in Seurat. The scCustomize package was used to generate some visualizations.
HLA typing
HLA typing was conducted with the research-grade AllType NGS 11-loci Amplification Kit (One Lambda) as described in 10.1038/s41590-022-01184-4. Libraries were sequenced on the Illumina MiSeq platform at 150 × 150 bp, and data were parsed using TypeStream Visual software (One Lambda). HLA typing results are in Supplementary Data 2.
Construction of TFH TCR cell lines and aAPC lineages
Twelve TCR clonotypes were picked from donor 321-05 for cloning based on the following two criteria: the clone was detected at multiple time points after vaccination, and it had matching single-cell TCR and bulk sequencing results from the sorted TFH cells. Full-length TCR nucleotide sequences of the picked clones were reconstructed using stiTChR42 with the TCRα and TCRβ chains linked with a P2A auto-cleavage site. To generate APCs expressing specific HLA, the nucleotide sequences of α and β chains for donor-specific HLA class II haplotypes were linked via the T2A site to form the full HLA constructs (HLA-DR, HLA-DP and HLA-DQ). Constructs were synthesized and cloned (Genscript) into a custom pLVX-EF1a-P2A-GFP-IRES-Puro lentiviral vector. Lentivirus particles were subsequently generated by cotransfecting 293T cells (American Type Culture Collection; ATCC) with one of the generated constructs, psPAX2 (Addgene, 12260) and pMD2.G (Addgene, 12259), using GeneJuice transfection reagent (EMD Millipore) or Lipofectamine3000 transfection kit (Thermo Fisher Scientific). To generate the aAPC lineages, K562 cells (ATCC, CCL-243) expressing HLA-DM, CD80 and CD64 molecules were transduced with the donor’s HLA-II vectors to generate a set of aAPC lines. Similarly, Jurkat cells were transduced with the panel TCR lentiviruses. Both K562 and Jurkat cells were placed under 1 μg ml−1 puromycin selection for 2 weeks before sorting for GFP+ cells.
Generation of APCs
B cells were used as APCs and were generated from PBMCs. The cells were isolated using CD19+ microbeads (Miltenyi Biotec) and maintained in culture with irradiated NIH3T3 CD40L feeder cells, as previously described43,44. Cells were expanded for 5 days in B cell medium—Iscove’s modified Dulbecco’s medium (ATCC) containing 10% human serum, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin, 2 mM l-glutamine and 200 U ml−1 IL4 (Peprotech). Following the expansion, B cells were used in coculture assays with the Jurkat T cell lines.
Cell line antigen-stimulation assays
TCR-negative Jurkat cells were cloned with a curated set of donor-derived TCR sequences. The TCR-expressing clonotypes were subsequently screened for activation upon coculture with aAPC-encoding cognate HLA class II alleles. The HLA-II-expressing aAPCs were preincubated with β-propiolactone-inactivated influenza A virus (strain A/Puerto Rico/8/1934 H1N1) at multiplicity of infection 1 for 2 hours before adding the TCR-expressing lines in a ratio of 1:1. The cocultured cells were incubated at 37 °C and 5% CO2 for an additional 24 h. Cells were washed with cold PBS and then stained with a cocktail of fluorescently labeled antibodies, including antihuman CD3-PE-Cy7 (OKT3; 1:200), Ghost viability dye (Tonbo Biosciences; 1:1000), TCRαβ-BV421 (IP26; 1:100), CD69-PerCP-eFluor-710 (FN50; 1:100) and IFNγ-BV785 (4S.B3; 1:100). Similarly, HLA-II-expressing aAPCs were transfected with pHW2000 vectors encoding the genomic viral segments (PB2, PB1, PA, NP, M and NS) 48 h before coculturing with the TCR-expressing lineages. The transfection was conducted via a Neon Electroporation System (Thermo Fisher Scientific, MPK5000S) using 1 pulse at 1,000 V and 50 ms width, following the manufacturer’s protocol. To identify the driving peptide motifs that triggered activation in the responding clones, a pool of overlapping oligopeptide sequences spanning M1, M2 and NP proteins of the H1N1 PR8 strain (Mimotopes) was used for the peptide mapping experiments. Libraries covering M1 and M2 consisted of 17-mer peptides that were overlapping by 11 residues. The NP library consisted of 15-mer peptides overlapping by 11 residues. All samples were acquired on an Aurora (CyteK), and the data were analyzed using FlowJo software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-024-01926-6.
Supplementary information
Supplementary Tables 1 (cell and clonotype counts in total T cell dataset) and 2 (cell and clonotype counts in TFH/Treg subset dataset).
Sequences of TFH TCRs chosen for specificity screening.
Study participant HLA typing.
TFH subset module gene sets.
Source data
Source data for Fig. 7.
Acknowledgements
We thank E. Lantelme for facilitating sorting, and L. Kessels, M. Royal and the staff of the Infectious Diseases Clinical Research Unit at Washington University School of Medicine for assistance with vaccination and sample collection. The Thomas Laboratory was funded by American Lebanese Syrian Associated Charities at St. Jude, the Center for Influenza Vaccine Research for High-Risk Populations (CIVR-HRP; contract 75N93019C00052), the St. Jude Center of Excellence for Influenza Research and Surveillance (contract HHSN272201400006C to P.G.T.), the St. Jude Center of Excellence for Influenza Research and Response (contract 75N93021C00016 to P.G.T.) and grants U01-AI150747, U01-AI144616 and R01-AI136514. R.C.M. is funded by a National Institute of Allergy and Infectious Diseases (NIAID) Ruth L. Kirschstein National Research Service Award Individual Postdoctoral Fellowship (award F32-AI157296). The Ellebedy Laboratory was supported by NIAID (grants R21-AI139813 and U01-AI141990) and NIAID Centers of Excellence for Influenza Research and Surveillance (contract HHSN272201400006C). The WU321 study was reviewed and approved by the Washington University IRB (approval 201808171). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Extended data
Author contributions
S.A.S. analyzed scGEX and TCR sequencing data, designed experiments, generated libraries, processed data for bulk TCR profiling and wrote the manuscript. J.S.T. collected and analyzed the flow cytometry data and performed fluorescence-activated cell sorting. M.G. generated TCR and aAPC cell lines and performed antigen-stimulation experiments. J.C.C. and H.K. parsed and analyzed scGEX and TCR data. W.A. performed single-cell TCR sequencing. R.C.M. wrote and edited the manuscript and aided in figure design. A.J.S. and K.M.M. helped in the processing of blood and draining LN samples. W.K. prepared the 10x libraries for the single-cell RNA-sequencing analyses. W.M. assisted in sample collection from draining LNs. A.H.E., R.M.P., M.K.K. and A.H. wrote and maintained the IRB protocol, recruited, vaccinated and phlebotomized participants, and coordinated sample collection. T.S. performed the FNA under the supervision of S.T. J.S.T., J.Q.Z. and A.H.E. conceived, designed and supervised the seasonal influenza vaccination study. P.G.T. supervised this study and wrote the manuscript. All authors reviewed the manuscript.
Peer review
Peer review information
Nature Immunology thanks Peter T. Sage, Michelle Linterman and E. John Wherry for their contribution to the peer review of this work. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team.
Data availability
The processed total T cell and TFH/Treg scGEX and TCR datasets, as well as single-cell and bulk TCR data, are available on Zenodo (10.5281/zenodo.6476022). Reads for samples from year 2 for 321-05, year 1 and year 2 for 312-04, all samples from 321-07 and all samples from 321-08 are available on the NCBI Sequence Read Archive (SRA) under BioProject accession PRJNA1132302. Reads for year 1 samples for 321-05 are available on SRA under BioProject PRJNA610059. Source data are provided with this paper.
Code availability
The code for running the analyses is available at https://github.com/sschattgen/Flu_TFH_paper.
Competing interests
A.H.E. is a consultant for InBios and Fimbrion Therapeutics. The Ellebedy Laboratory received funding under sponsored research agreements from Emergent BioSolutions. P.G.T. has consulted and/or received honoraria and travel support from Pfizer, Merck, Illumina, Johnson and Johnson and 10x Genomics, and serves on the Scientific Advisory Board of Immunoscape, Shennon Bio and Cytoagents. The authors have applied for patents covering some aspects of these studies. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefan A. Schattgen, Jackson S. Turner, Mohamed A. Ghonim.
Contributor Information
Ali H. Ellebedy, Email: ellebedy@wustl.edu
Paul G. Thomas, Email: paul.thomas@stjude.org
Extended data
is available for this paper at 10.1038/s41590-024-01926-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-024-01926-6.
References
- 1.Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity41, 529–542 (2014). 10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevalier, N. et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol.186, 5556–5568 (2011). 10.4049/jimmunol.1002828 [DOI] [PubMed] [Google Scholar]
- 3.He, J. et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate TFH cell activity and promote antibody responses upon antigen reexposure. Immunity39, 770–781 (2013). 10.1016/j.immuni.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 4.Herati, R. S. et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J. Immunol.193, 3528–3537 (2014). 10.4049/jimmunol.1302503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mettelman, R. C. et al. Baseline innate and T cell populations are correlates of protection against symptomatic influenza virus infection independent of serology. Nat. Immunol.24, 1511–1526 (2023). 10.1038/s41590-023-01590-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obeng-Adjei, N. et al. Circulating TH1-cell-type TFH cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep.13, 425–439 (2015). 10.1016/j.celrep.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locci, M. et al. Human circulating PD-1+CXCR3−CXCR5+ memory TFH cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity39, 758–769 (2013). 10.1016/j.immuni.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity34, 108–121 (2011). 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heit, A. et al. Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J. Exp. Med.214, 2139–2152 (2017). 10.1084/jem.20161794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vella, L. A. et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J. Clin. Invest.129, 3185–3200 (2019). 10.1172/JCI125628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenna, E. et al. CD4+ T follicular helper cells in human tonsils and blood are clonally convergent but divergent from non-TFH CD4+ cells. Cell Rep.30, 137–152 (2020). 10.1016/j.celrep.2019.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner, J. S. et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature586, 127–132 (2020). 10.1038/s41586-020-2711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cañete, P. F. et al. Regulatory roles of IL-10-producing human follicular T cells. J. Exp. Med.216, 1843–1856 (2019). 10.1084/jem.20190493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar, S. et al. Developmental bifurcation of human T follicular regulatory cells. Sci. Immunol.6, eabd8411 (2021). 10.1126/sciimmunol.abd8411 [DOI] [PubMed] [Google Scholar]
- 15.Khatun, A. et al. Single-cell lineage mapping of a diverse virus-specific naive CD4 T cell repertoire. J. Exp. Med.218, e20200650 (2021). 10.1084/jem.20200650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu, W. et al. The transcription factor Tox2 drives T follicular helper cell development via regulating chromatin accessibility. Immunity51, 826–839 (2019). 10.1016/j.immuni.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 17.Lee, J.-Y. et al. The transcription factor KLF2 restrains CD4+ T follicular helper cell differentiation. Immunity42, 252–264 (2015). 10.1016/j.immuni.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu, T. et al. TCF1 is required for the T follicular helper cell response to viral infection. Cell Rep.12, 2099–2110 (2015). 10.1016/j.celrep.2015.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podestà, M. A. et al. Stepwise differentiation of follicular helper T cells reveals distinct developmental and functional states. Nat. Commun.14, 7712 (2023). 10.1038/s41467-023-43427-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song, W. & Craft, J. T follicular helper cell heterogeneity: time, space, and function. Immunol. Rev.288, 85–96 (2019). 10.1111/imr.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol.8, 753–761 (2007). 10.1038/ni1472 [DOI] [PubMed] [Google Scholar]
- 22.Künzli, M. et al. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci. Immunol.5, eaay5552 (2020). 10.1126/sciimmunol.aay5552 [DOI] [PubMed] [Google Scholar]
- 23.Hill, D. L. et al. The adjuvant GLA-SE promotes human TFH cell expansion and emergence of public TCRβ clonotypes. J. Exp. Med.216, 1857–1873 (2019). 10.1084/jem.20190301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kon, T. C. et al. Influenza vaccine manufacturing: effect of inactivation, splitting and site of manufacturing. Comparison of influenza vaccine production processes. PLoS ONE11, e0150700 (2016). 10.1371/journal.pone.0150700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koroleva, M. et al. Heterologous viral protein interactions within licensed seasonal influenza virus vaccines. NPJ Vaccines5, 3 (2020). 10.1038/s41541-019-0153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sette, A. et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity28, 847–858 (2008). 10.1016/j.immuni.2008.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards, K. A. et al. Recombinant HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody responses in humans. NPJ Vaccines5, 77 (2020). 10.1038/s41541-020-00227-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dash, P. et al. Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J. Clin. Invest.121, 288–295 (2011). 10.1172/JCI44752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dash, P. et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature547, 89–93 (2017). 10.1038/nature22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egorov, E. S. et al. Quantitative profiling of immune repertoires for minor lymphocyte counts using unique molecular identifiers. J. Immunol.194, 6155–6163 (2015). 10.4049/jimmunol.1500215 [DOI] [PubMed] [Google Scholar]
- 31.Shugay, M. et al. Towards error-free profiling of immune repertoires. Nat. Methods11, 653–655 (2014). 10.1038/nmeth.2960 [DOI] [PubMed] [Google Scholar]
- 32.Shugay, M. et al. VDJtools: unifying post-analysis of T cell receptor repertoires. PLoS Comput. Biol.11, e1004503 (2015). 10.1371/journal.pcbi.1004503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ImmunoMind Team. Immunarch: an R package for painless bioinformatics analysis of T-cell and B-cell immune repertoires. Zenodo10.5281/zenodo.3367200 (2019).
- 34.McGinnis, C. S. et al. MULTI-seq: sample multiplexing for single-cell RNA sequencing using lipid-tagged indices. Nat. Methods16, 619–626 (2019). 10.1038/s41592-019-0433-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol.20, 296 (2019). 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods16, 1289–1296 (2019). 10.1038/s41592-019-0619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol.32, 381–386 (2014). 10.1038/nbt.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods14, 309–315 (2017). 10.1038/nmeth.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods14, 979–982 (2017). 10.1038/nmeth.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillje, R., Pelicci, P. G. & Luzi, L. Cerebro: interactive visualization of scRNA-seq data. Bioinformatics36, 2311–2313 (2020). 10.1093/bioinformatics/btz877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform.14, 7 (2013). 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heather, J. M. et al. Stitchr: stitching coding TCR nucleotide sequences from V/J/CDR3 information. Nucleic Acids Res.50, e68 (2022). 10.1093/nar/gkac190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran, E. et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science350, 1387–1390 (2015). 10.1126/science.aad1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gros, A. et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med.22, 433–438 (2016). 10.1038/nm.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1 (cell and clonotype counts in total T cell dataset) and 2 (cell and clonotype counts in TFH/Treg subset dataset).
Sequences of TFH TCRs chosen for specificity screening.
Study participant HLA typing.
TFH subset module gene sets.
Source data for Fig. 7.
Data Availability Statement
The processed total T cell and TFH/Treg scGEX and TCR datasets, as well as single-cell and bulk TCR data, are available on Zenodo (10.5281/zenodo.6476022). Reads for samples from year 2 for 321-05, year 1 and year 2 for 312-04, all samples from 321-07 and all samples from 321-08 are available on the NCBI Sequence Read Archive (SRA) under BioProject accession PRJNA1132302. Reads for year 1 samples for 321-05 are available on SRA under BioProject PRJNA610059. Source data are provided with this paper.
The code for running the analyses is available at https://github.com/sschattgen/Flu_TFH_paper.