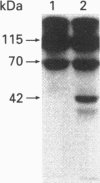
Abstract
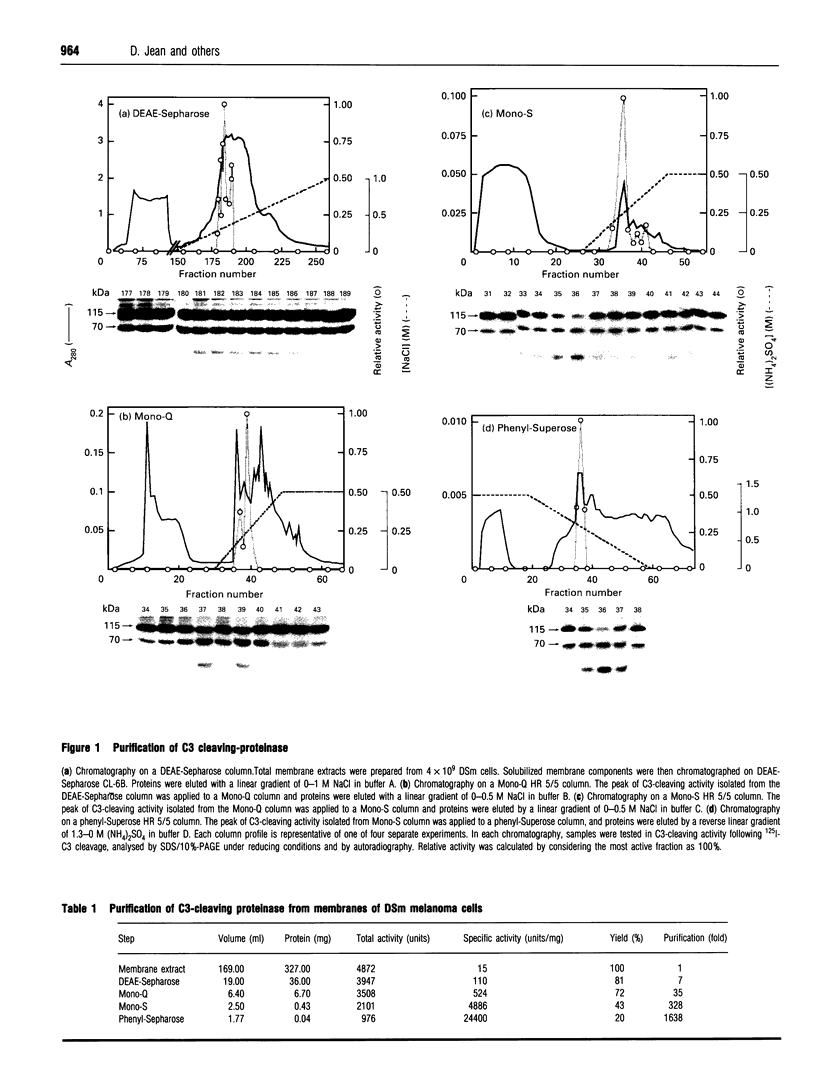
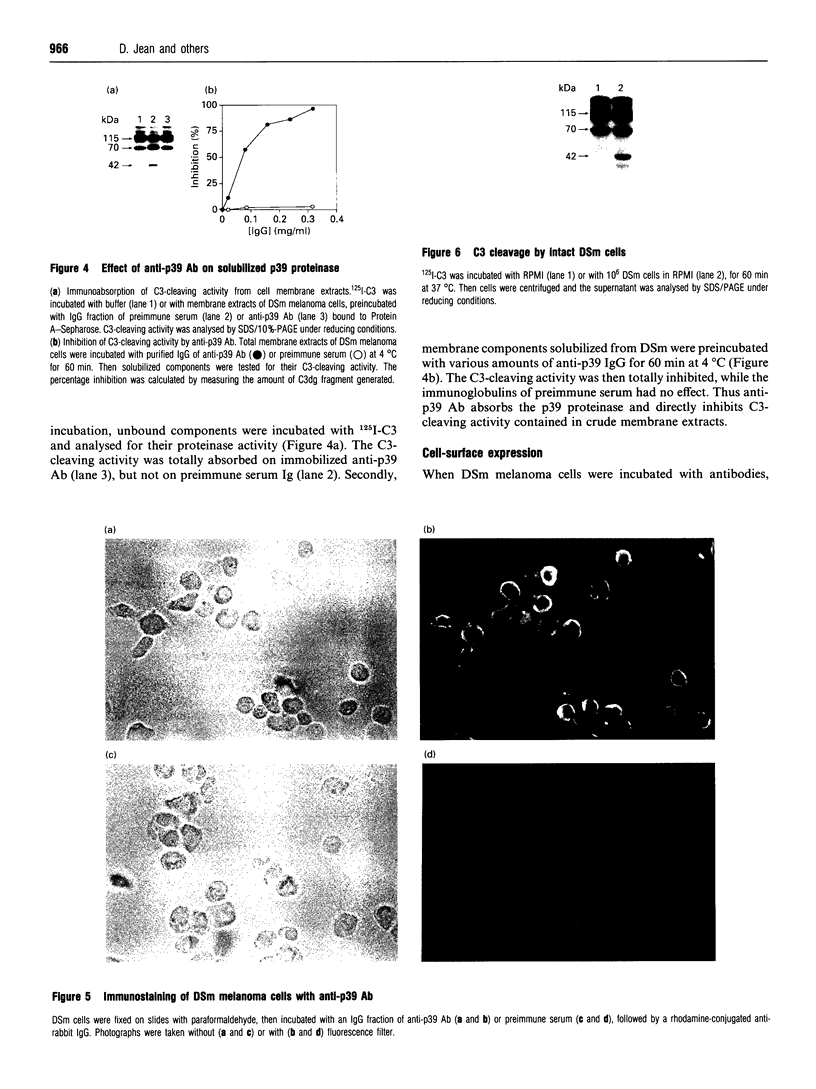
We previously identified, on normal or tumour cells, two membrane proteinases, p57 and p65, that cleave human C3, the third component of complement, thus regulating C3's biological properties. Whereas p57 was purified from human erythrocytes, p65 was identified using polyclonal anti-p57 antibodies on a human melanoma cell line resistant to complement lysis. Analysis of cell distribution of C3-cleaving proteinases established that DSm, a murine melanoma cell line, expressed a C3-cleaving proteinase distinct from p57 and p65 proteinases. Thus we purified the C3-cleaving proteinase solubilized from membranes of DSm cells. The purified proteinase, termed 'p39' on the basis of its molecular mass of 39 kDa, was identified, using specific proteinase inhibitors, as a cysteine proteinase. Anti-p39 antibodies, prepared against highly purified p39, localized the p39 C3-cleaving proteinase mainly at the cell surface and demonstrated that p39 is also secreted. Anti-p39 antibodies inhibited solubilized C3-cleaving activity. Preincubation of DSm cells with anti-p39 F(ab')2 fragments increased up to 60% complement cell susceptibility. Amino acid analysis of N-terminal and three other regions of p39 demonstrated that this C3-cleaving proteinase carries 100% identity within four regions of procathepsin L. This is the first demonstration that a melanoma cell line expresses on its surface and secretes a p39 C3-cleaving cysteine proteinase that shares sequence identities with procathepsin L. Thus the p39 cysteine proteinase represents a new member of the C3-cleaving proteinase family associated with, and/or expressed on, the cell surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barel M., Fiandino A., Lyamani F., Frade R. Epstein-Barr virus/complement fragment C3d receptor (CR2) reacts with p53, a cellular antioncogene-encoded membrane phosphoprotein: detection by polyclonal anti-idiotypic anti-CR2 antibodies. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10054–10058. doi: 10.1073/pnas.86.24.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. Ankyrins. Adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992 May 5;267(13):8703–8706. [PubMed] [Google Scholar]
- Charriaut-Marlangue C., Barel M., Frade R. Identification of P-57, a serine proteinase, from human erythrocyte membranes, which cleaves both chains of human third component (C3) of complement. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1113–1120. doi: 10.1016/0006-291x(86)90750-3. [DOI] [PubMed] [Google Scholar]
- Charriaut C., Barel M., Frade R. Behavior of soluble human 125I-labeled C3b, the third component of complement, after binding to human cells. Eur J Immunol. 1982 Apr;12(4):289–294. doi: 10.1002/eji.1830120407. [DOI] [PubMed] [Google Scholar]
- Delaria K., Fiorentino L., Wallace L., Tamburini P., Brownell E., Muller D. Inhibition of cathepsin L-like cysteine proteases by cytotoxic T-lymphocyte antigen-2 beta. J Biol Chem. 1994 Oct 7;269(40):25172–25177. [PubMed] [Google Scholar]
- Frade R., Hermann J., Barel M. A 16 amino acid synthetic peptide derived from human C3d triggers proliferation and specific tyrosine phosphorylation of transformed CR2-positive human lymphocytes and of normal resting B lymphocytes. Biochem Biophys Res Commun. 1992 Oct 30;188(2):833–842. doi: 10.1016/0006-291x(92)91132-a. [DOI] [PubMed] [Google Scholar]
- Frade R., Myones B. L., Barel M., Krikorian L., Charriaut C., Ross G. D. gp140, a C3b-binding membrane component of lymphocytes, is the B cell C3dg/C3d receptor (CR2) and is distinct from the neutrophil C3dg receptor (CR4). Eur J Immunol. 1985 Dec;15(12):1192–1197. doi: 10.1002/eji.1830151210. [DOI] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein of transformed fibroblasts is an activable acid-protease. J Biol Chem. 1986 Feb 5;261(4):1760–1765. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Hatzfeld J., Charriaut-Marlangue C., Levesque J. P., Barel M., Stancou R., Krikorian L., Hatzfeld A., Frade R. Le C3 stimule la prolifération des cellules humaines pré-B de la lignée Raji. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):451–455. doi: 10.1016/s0769-2625(87)80055-7. [DOI] [PubMed] [Google Scholar]
- Hermann J., Barel M., Frade R. Human erythrocyte ankyrin, a cytoskeleton component, generates the p57 membrane proteinase which cleaves C3, the third component of complement. Biochem Biophys Res Commun. 1994 Oct 28;204(2):453–460. doi: 10.1006/bbrc.1994.2481. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Barrett A. J., Mason R. W. Cathepsin L inactivates alpha 1-proteinase inhibitor by cleavage in the reactive site region. J Biol Chem. 1986 Nov 5;261(31):14748–14751. [PubMed] [Google Scholar]
- Joseph L. J., Chang L. C., Stamenkovich D., Sukhatme V. P. Complete nucleotide and deduced amino acid sequences of human and murine preprocathepsin L. An abundant transcript induced by transformation of fibroblasts. J Clin Invest. 1988 May;81(5):1621–1629. doi: 10.1172/JCI113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Tedja I., Atkinson J. P. Membrane cofactor protein (CD46) of complement. Processing differences related to alternatively spliced cytoplasmic domains. J Biol Chem. 1994 Apr 8;269(14):10776–10779. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Maison C. M., Villiers C. L., Colomb M. G. Proteolysis of C3 on U937 cell plasma membranes. Purification of cathepsin G. J Immunol. 1991 Aug 1;147(3):921–926. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Johnson D. A., Barrett A. J., Chapman H. A. Elastinolytic activity of human cathepsin L. Biochem J. 1986 Feb 1;233(3):925–927. doi: 10.1042/bj2330925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Ollert M. W., Frade R., Fiandino A., Panneerselvam M., Petrella E. C., Barel M., Pangburn M. K., Bredehorst R., Vogel C. W. C3-cleaving membrane proteinase. A new complement regulatory protein of human melanoma cells. J Immunol. 1990 May 15;144(10):3862–3867. [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Rey-Millet C. A., Chesne S., Colomb M. G. Associated complement C3b. Towards an understanding of its intracellular modifications. Mol Immunol. 1993 Jul;30(10):855–864. doi: 10.1016/0161-5890(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Rozhin J., Wade R. L., Honn K. V., Sloane B. F. Membrane-associated cathepsin L: a role in metastasis of melanomas. Biochem Biophys Res Commun. 1989 Oct 16;164(1):556–561. doi: 10.1016/0006-291x(89)91755-5. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Troen B. R., Gal S., Gottesman M. M. Sequence and expression of the cDNA for MEP (major excreted protein), a transformation-regulated secreted cathepsin. Biochem J. 1987 Sep 15;246(3):731–735. doi: 10.1042/bj2460731. [DOI] [PMC free article] [PubMed] [Google Scholar]