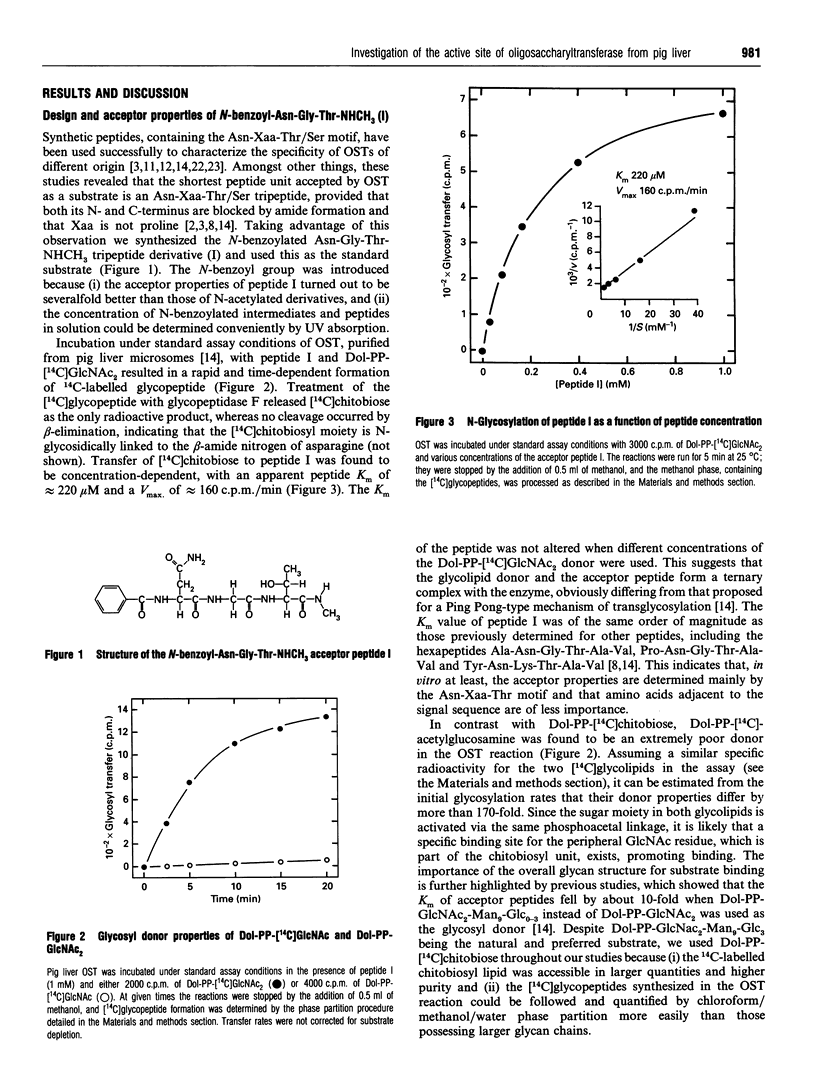
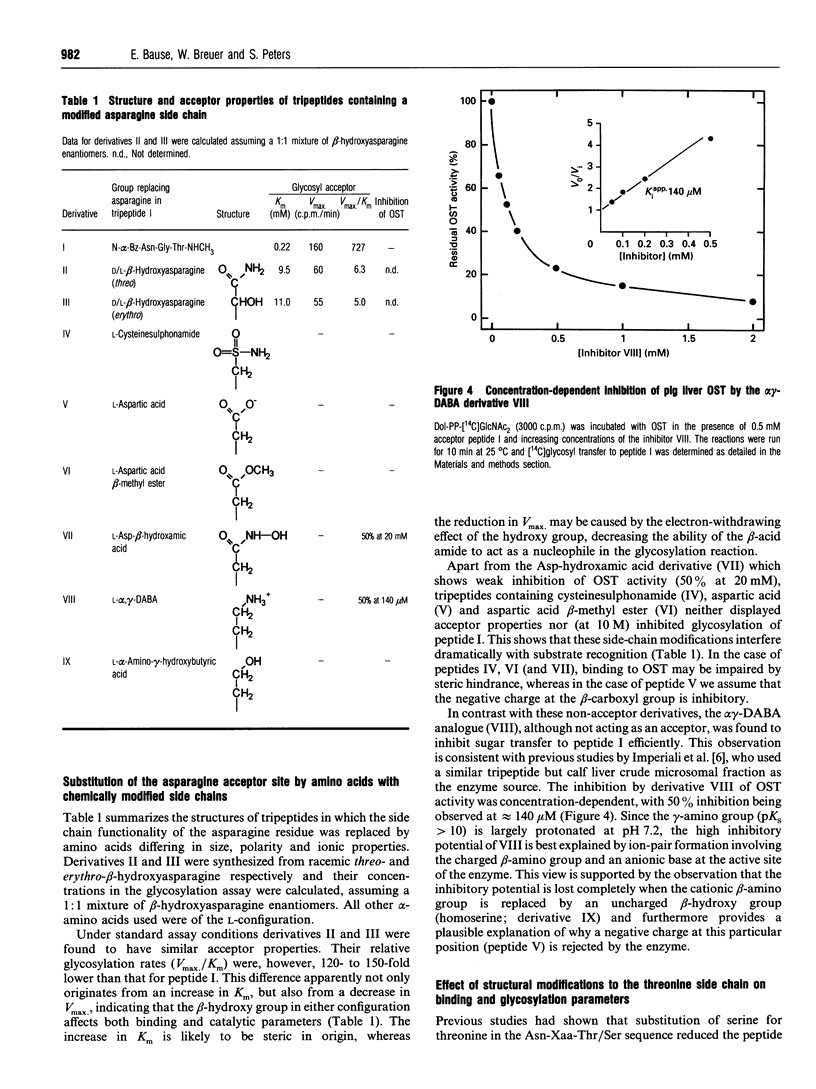
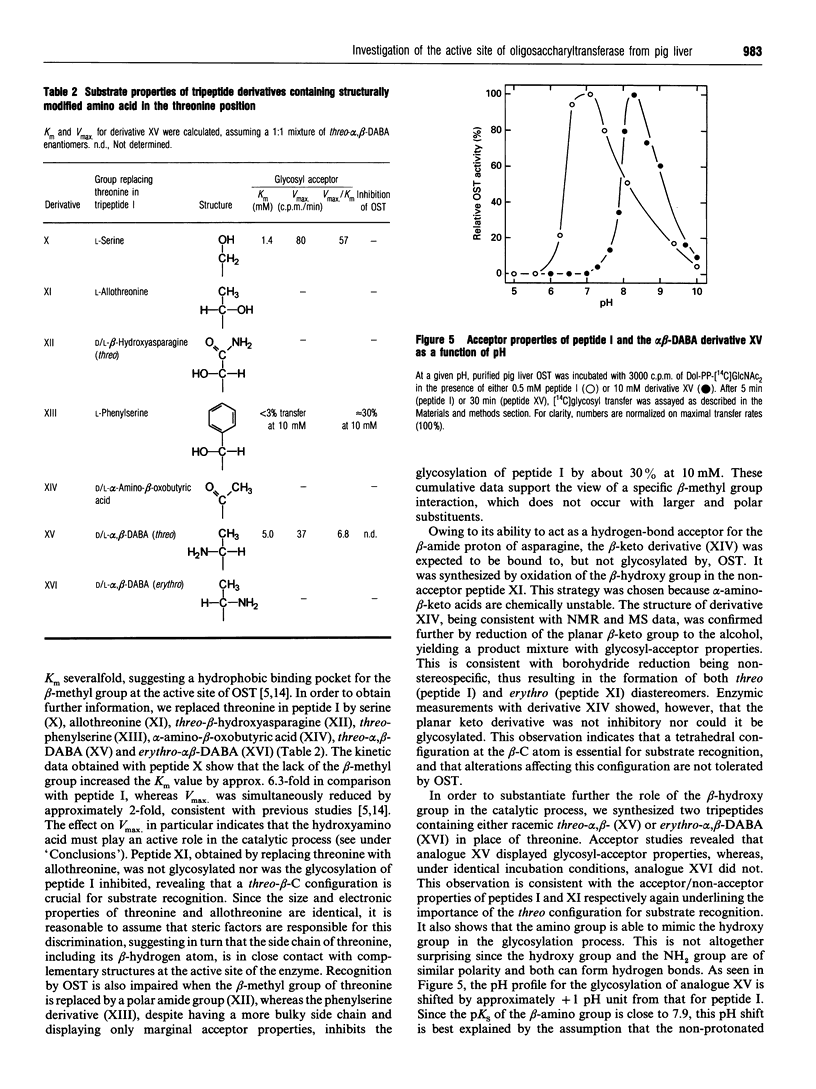
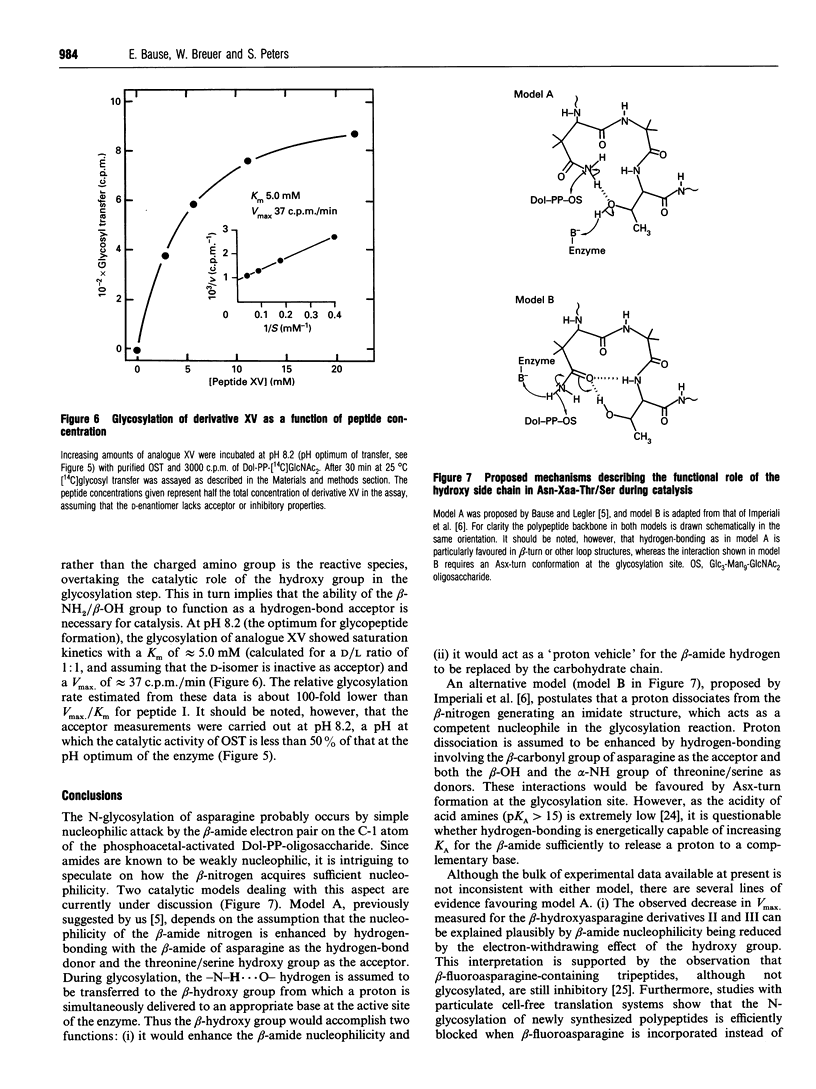
Abstract
Oligosaccharyltransferase (OST), an integral component of the endoplasmic-reticulum membrane, catalyses the transfer of dolichyl diphosphate-linked oligosaccharides to specific asparagine residues forming part of the Asn-Xaa-Thr/Ser sequence. We have studied the binding and catalytic properties of the enzyme from pig liver using peptide analogues derived from the acceptor peptide N-benzoyl-Asn-Gly-Thr-NHCH3 by replacing either asparagine or threonine with amino acids differing in size, stereochemistry, polarity and ionic properties. Acceptor studies showed that analogues of asparagine and threonine with bulkier side chains impaired recognition by OST. Reduction of the beta-amide carbonyl group of asparagine yielded a derivative that, although not glycosylated, was strongly inhibitory (50% inhibition at approximately 140 microM). This inhibition may be due to ion-pair formation involving the NH3+ group and a negatively charged base at the active site. Hydroxylation of asparagine at the beta-C position increased Km and decreased Vmax, indicating an effect on both binding and catalysis. The threo configuration at the beta-C atom of the hydroxyamino acid was essential for substrate binding. A peptide derivative obtained by replacement of the threonine beta-hydroxy group with an NH2 group was found to display acceptor activity. This shows that the primary amine is able to mimic the hydroxy group during transglycosylation. The pH optimum with this derivative is shifted by approximately 1 pH unit towards the basic region, indicating that the neutral NH2 group is the reactive species. The various data are discussed in terms of the catalytic mechanism of OST, particular emphasis being placed on the role of threonine/serine in increasing the nucleophilicity of the beta-amide of asparagine through hydrogen-binding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bause E. Active-site-directed inhibition of asparagine N-glycosyltransferases with epoxy-peptide derivatives. Biochem J. 1983 Feb 1;209(2):323–330. doi: 10.1042/bj2090323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E., Hettkamp H., Legler G. Conformational aspects of N-glycosylation of proteins. Studies with linear and cyclic peptides as probes. Biochem J. 1982 Jun 1;203(3):761–768. doi: 10.1042/bj2030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E., Hettkamp H. Primary structural requirements for N-glycosylation of peptides in rat liver. FEBS Lett. 1979 Dec 15;108(2):341–344. doi: 10.1016/0014-5793(79)80559-1. [DOI] [PubMed] [Google Scholar]
- Bause E., Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem J. 1981 Jun 1;195(3):639–644. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E., Lehle L. Enzymatic N-glycosylation and O-glycosylation of synthetic peptide acceptors by dolichol-linked sugar derivatives in yeast. Eur J Biochem. 1979 Nov;101(2):531–540. doi: 10.1111/j.1432-1033.1979.tb19748.x. [DOI] [PubMed] [Google Scholar]
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E. Studies on the acceptor specificity of asparagine-N-glycosyl-transferase from rat liver. FEBS Lett. 1979 Jul 15;103(2):296–299. doi: 10.1016/0014-5793(79)81348-4. [DOI] [PubMed] [Google Scholar]
- Breuer W., Bause E. Oligosaccharyl transferase is a constitutive component of an oligomeric protein complex from pig liver endoplasmic reticulum. Eur J Biochem. 1995 Mar 15;228(3):689–696. [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin G., Stern A. M., Miller B., Abeles R. H., Boime I. DL-threo-beta-fluoroasparagine inhibits asparagine-linked glycosylation in cell-free lysates. J Biol Chem. 1983 Apr 10;258(7):4047–4050. [PubMed] [Google Scholar]
- Imperiali B., Shannon K. L. Differences between Asn-Xaa-Thr-containing peptides: a comparison of solution conformation and substrate behavior with oligosaccharyltransferase. Biochemistry. 1991 May 7;30(18):4374–4380. doi: 10.1021/bi00232a002. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A., Welply J. K., Lennarz W. J. Oligosaccharyl transferase: the central enzyme in the pathway of glycoprotein assembly. Biochim Biophys Acta. 1987 Jun 24;906(2):161–173. doi: 10.1016/0304-4157(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Liwschitz Y., Singerman A. N-carboxy-anhydrides derived from threo- and erythro-beta-hydroxy-aspartic acids and poly-beta-methyl hydrogen threo-beta-methoxyl-DL-aspartate. J Chem Soc Perkin 1. 1967;18:1696–1700. [PubMed] [Google Scholar]
- Rathod P. K., Tashjian A. H., Jr, Abeles R. H. Incorporation of beta-fluoroasparagine into peptides prevents N-linked glycosylation. In vitro studies with synthetic fluoropeptides. J Biol Chem. 1986 May 15;261(14):6461–6469. [PubMed] [Google Scholar]
- Ronin C. Solubilization of oligosaccharide transferase and glucosidase activities from thyroid rough microsomes. FEBS Lett. 1980 May 5;113(2):340–344. doi: 10.1016/0014-5793(80)80623-5. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J., Brew K. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with alpha-lactalbumin. J Biol Chem. 1978 Aug 25;253(16):5786–5794. [PubMed] [Google Scholar]
- Tillmann U., Günther R., Schweden J., Bause E. Subcellular location of enzymes involved in the N-glycosylation and processing of asparagine-linked oligosaccharides in Saccharomyces cerevisiae. Eur J Biochem. 1987 Feb 2;162(3):635–642. doi: 10.1111/j.1432-1033.1987.tb10685.x. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Shenbagamurthi P., Lennarz W. J., Naider F. Substrate recognition by oligosaccharyltransferase. Studies on glycosylation of modified Asn-X-Thr/Ser tripeptides. J Biol Chem. 1983 Oct 10;258(19):11856–11863. [PubMed] [Google Scholar]


