Abstract
Naked mole rats (Heterocephalus galber) are eusocial mammals from East Africa. Their extraordinary social organisation is accompanied by remarkable adaptations to an underground lifestyle, extreme longevity and resistance to many diseases, making naked mole rats a highly relevant model for biological research. However, their living conditions in controlled environments do not allow them to express fundamental behaviours: digging galleries and exploring. This gap probably constitutes a bias to any behavioural or even medical study, because it represents a potential obstacle to their well-being. In this article, we tested the effects of the introduction of a diggable substrate on the behaviour of a colony of naked mole rats at the Menagerie, le Zoo du Jardin des Plantes, Paris. We measured individual exploratory latencies, the number of entries per minute and the frequency with which naked mole rats gnawed tunnels during observation trials. We found that: (i) young individuals explore more quickly, (ii) the introduction of a diggable substrate encourages exploration and digging behaviour, and (iii) could therefore be a relevant element to introduce under human care. This new environmental design could improve the welfare of naked mole rats by creating opportunities for cognitive challenges such as exploration and environmental control.
Subject terms: Zoology, Animal behaviour, Ecology, Behavioural ecology
Introduction
Exploratory behaviours allow animals to gather information about their environment and reduce uncertainty within it1. Exploration can be influenced by the physical environment, but also by how it is perceived by the animal. For example, social organisation, motivation, cognition, memory2 and internal states such as anxiety can influence exploration. Indeed, less stressed animals have a lower exploratory latency (the time required for an animal to begin exploring a new area or object) than those more stressed ones in a wide variety of situations3. Thus, a reduction in exploratory latency after the introduction of a change in the environment could indicate a reduction in stress3. By measuring exploratory behaviour, individual personalities can be characterised, emotions assessed and the links between personality and morphology studied. Here we have focused on an animal model that is relevant to many medical studies, but for which the application of behavioural knowledge to management is still inadequate: naked mole rats.
Naked mole rats (Heterocephalus galber) are eusocial mammals from East Africa. They exhibit eusocial behaviour by living in castes consisting of fertile and non-fertile individuals, including a reproductive queen and generally one reproductive male, although sometimes multiple males can reproduce4. Unlike many eusocial Hymenoptera, they do not exhibit morphologically distinct casts among the non-breeding workers5,6. All specialisations are behavioural and vary along a continuum, from defence to occupational activities and offspring care7–10. Recent in-depth studies have revealed that tasks such as pup care, colony defence and dispersal behaviour are unequally distributed10,11. Age emerges as a key factor in the distribution of tasks, with older individuals being involved in colony defence and younger ones in pup transport10 and burrowing12. Overall, non-breeding individuals vary in their cooperative investment but do not specialise in specific tasks. Their behavioural organisation is closer to other cooperatively breeding vertebrates such as meerkats than to eusocial insect species12.
Although their extraordinary social organisation is accompanied by remarkable adaptations to the subterranean lifestyle13–15, their living conditions in captivity often do not allow them to express a fundamental behaviour: digging galleries and exploring, which could jeopardise their welfare. Even though digging substrate as enrichment for captive mole-rats has a long history, it is still not generalized enough. Back in the 1980s, Brett et al. introduced a burrow that returned the sand dug out of the tunnels to a different tunnel, and Braude et al. used cork for digging substrate because it did not create mud that would scratch or stick to the clear plastic tubing16. Other researchers used peat as a substrate17. Nevertheless, besides sticks, packages, or thin pieces of clay used as enrichment in zoos, the use of a permanent digging substrate providing frequent digging opportunities and allowing naked mole rats to explore and dig shape their environment is not mandatory, nor common. The impossibility of digging could be a source of frustration and possibly stress. Stress, including that caused by new odours or handling, can in turn be a source of conflict in the colony, leading to putsches in which the queen is killed by another female (observed by the veterinary team) or to a peak in mortality18. It is therefore essential to find ways of reducing stress and frustration in order to promote the well-being of naked mole rats in captivity.
As stated by Poole in 199719, « happy animals make good science ». Indeed, if standardisation and impoverishment of laboratory animals’ living conditions was an initial measure to lower data variability, several studies have showed that enriched or heterogenized environments decrease variability and improve data reproducibility20, as well as the poor translational rate of preclinical research21,22. However, many laboratory animals used in fundamental and medical research like rats and zebrafish are still denied fundamental behaviours like hiding or burrowing no matter how crucial these behaviours are23.
The concept of animal welfare encompasses both the physiological and psychological health of animals, as set out in the Five Domains model24. In this framework, good welfare conditions can be achieved when an animal is free to adapt to external or internal factors and constraints by adopting species-specific behaviours and achieving a positive mental state24. For example, giving captive animals control over their environment, a property known as 'agency', and the choice to adopt species-specific behaviours can trigger positive mental states and promote well-being. However, performing a species-specific behaviour does not mean reproducing all the natural behaviours exhibited by a species in the wild. Indeed, natural behaviours are not always relevant in captivity (e.g. being chased by a predator), and they cannot be used as a measure of well-being since they fail to take into account what animals want, which is ultimately what motivates their behaviour25. As a result, animals may adopt unnatural behaviours to obtain a similar reward. For example, when given the choice between an automatic brush and trees to rub against, cows systematically chose the automatic brush and were willing to open much heavier doors to reach the automatic brushes than to reach the trees26. What matters is not the rubbing behaviour, but the result of the rubbing, i.e. the associated reward of touch.
Naked mole rats are rodents that have gained importance as a biomedical research model for various conditions including hypoxic brain injury, cancer and nociception27, and much is unknown about how to optimise housing conditions in captivity and rather little, is done to provide them with the opportunity to engage in species-specific behaviours, like digging, which could be detrimental to their welfare. We decided to investigate the issue of lacking digging opportunities by studying a captive colony of naked mole rats at La Ménagerie, le Zoo du Jardin des Plantes, Paris. This colony had never interacted with a diggable substrate before.
We asked a simple question: does the introduction of a diggable substrate modify the behaviour and organisation of the colony, and is it beneficial to their well-being? To answer this question, we compared exploratory behaviours and gnawing frequencies of the colony before and after the introduction of substrates designed to promote digging in reptiles. During our experiments, we guaranteed individuals a permanent contact with their colony, and allowed them to choose to participate in or retreat from the experiment by only adding new tunnels parallel to their usual circuit. Their usual circuit consisted of two central tunnels of different diameters (5 cm and 8 cm). Before introducing new substrates, in what we called “Phase A”, we added downward or upward one-way tunnels (respectively conditions “no substrate, upward angle” and “no substrate, upward angle”) and measured individual exploratory latencies. Then, we introduced new substrates (“New substrates” phase) by filling downward tunnels to observe first interactions with the reptile substrates, and check if everything went well. The colony then had several days of free access to the substrates, and we proceeded to “Phase B” where we measured exploratory latencies again, either with an empty upward tunnel (condition “post substrate, upward angle”) or a half full tunnel to investigate the immediate effect of the substrate (“substrate, upward angle”). Additionally, naked mole rats were observed to repeatedly destroy their boxes by gnawing on the tunnel walls and box angles, which sometime jeopardise their safety. This behaviour is essential for wearing down incisors28, but might be a redirected behaviour and reflect a lack of digging opportunities. In order to assess whether gnawing on the walls was affected by a longer-term opportunity to dig into a substrate, we compared observational sessions of behaviours in the two main tunnels of the colony, before (“Phase A”) and after (“Phase B”) exposition to the substrate. In the latter case, a third wide and horizontal tunnel full of substrate was added during the observational session. Our hypothesis was that the introduction of the substrate would decrease exploratory latencies and increase activity of individuals in the colony. We also expected gnawing on the walls behaviour to decrease.
Results
Direct observations: interactions with the substrate
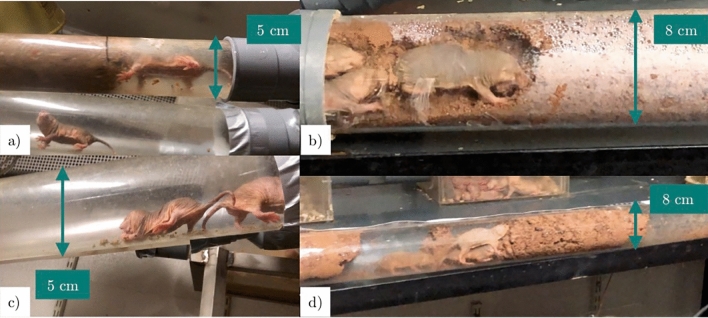
When exposed to tunnels filled with a diggable substrate, naked mole rats quickly started to gnaw, and did so continuously until they finished the tube, or until the end of the session. They dug alone or several at a time (Fig. 1). In the adjacent tunnels, other colony members were sweeping the wastes or transporting the debris to the toilet chamber. Occasionally, they nibbled the desiccated clay.
Figure 1.
Extracts from the films of the introduction of the new substrates (a and c) and the time budget sessions (b and d). Naked mole rats were observed to dig alone (a) or several at a time (b, d) and to nibble or sweep the debris (c).
Characterising the most exploratory individuals: age and mass effect on presence in the tunnels
The first analysis compared the presence of individuals in each test phase with a GLMM model (Table 1). We found that the number of sessions in which an individual was present was affected by the variables mass (ß = 0.057 ± 0.023, P = 0.0121), age (ß = − 0.024 ± 0.0054, P < 0.01), and the conditions “no substrate, upward angle” (ß = − 2.00 ± 0.31, P < 0.01), “substrate, upward angle” (ß = 0.54 ± 0.24, P = 0.027) and “no substrate, downward angle” (Intercept = − 1.81 ± 0.75, P = 0.015). Our model was significantly better than the null model (Chi-square test: X26 = 100.6, P < 2.2e−16) and obtained a marginal R2 of 0.252.
Table 1.
Results of the global linear model with mixed effect of presence during tests.
| Response variable | AIC | Predictor variable | Estimate ± SE | df | P |
|---|---|---|---|---|---|
| Presence (0 or 1) | 885.4 | – | |||
| Presence (0 or 1) | 796.8 | Intercept (“no substrate, downward angle” for females) | − 1.81 ± 0.75 | 0.0154 | |
| Mass | 0.057 ± 0.023 | 0.0121 | |||
| Sex (male) | 0.40 ± 0.35 | 0.2428 | |||
| age | − 0.024 ± 0.0054 | < 0.001 | |||
| Condition “no substrate, upward angle” | − 2.00 ± 0.31 | < 0.001 | |||
| Condition “post substrate, upward angle” | 0.23 ± 0.25 | 0.3507 | |||
| Condition “substrate, upward angle” | 0.54 ± 0.24 | 0.0265 |
Bold results are significant results with alpha = 0.05.
Number of explorers
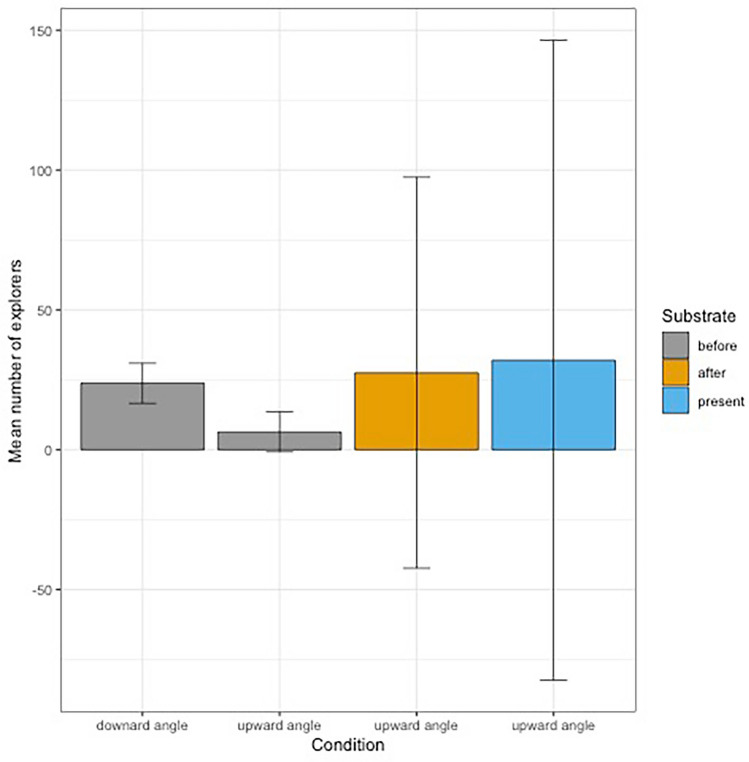
Based on the total number of explorers present during each session, we conducted a GLM analysis (Table 2) to test the effect of tunnel condition on the number of explorers. We found a significant effect of the condition “no substrate, downward angle” (Intercept = 3.16 ± 0.10, P < 0.001), and a negative effect of the condition “no substrate, upward angle” (ß = − 1.32 ± 0.25, P < 0.001). The other conditions did not significantly differ from the condition “no substrate, downward angle”. Our model was significantly better than the null model (Chi-square test: X23 = 57.392, P = 2.119e−12) and obtained a marginal R2 of 0.996. The mean entries and size effects are reported in Table 3 and presented in Fig. 2.
Table 2.
Results of the global linear model number of explorers per test.
| Response variable | AIC | Predictor variable | Estimate ± SE | df | P |
|---|---|---|---|---|---|
| Number of explorers | 123.95 | – | |||
| Number of explorers | 84.082 | Intercept (“no substrate, downward angle” for females) | 3.16 ± 0.10 | < 0.001 | |
| Condition “no substrate, upward angle” | − 1.32 ± 0.25 | < 0.001 | |||
| Condition “post substrate, upward angle” | 0.15 ± 0.17 | 0.3869 | |||
| Condition “substrate, upward angle” | 0.30 ± 0.16 | 0.0652 |
Bold results are significant results with alpha = 0.05.
Table 3.
Mean number of explorers during the different test conditions.
| Condition | Substrate | Mean number of explorers±SE | Size effect compared to condition “no substrate, downward angle” |
|---|---|---|---|
| “no substrate, downward angle” | before | 23.75 ± 2.25 | 0.00 |
| “no substrate, upward angle” | before | 6.33 ± 1.67 | 4.42 |
| “post substrate, upward angle” | after | 27.50 ± 5.5 | 0.68 |
| “substrate, upward angle” | present | 32.00 ± 9.00 | 1.10 |
Figure 2.
Barplot of the mean number of explorers per condition. Error bars represent 95% confidence intervals.
Entry flux (number of entries per minute)
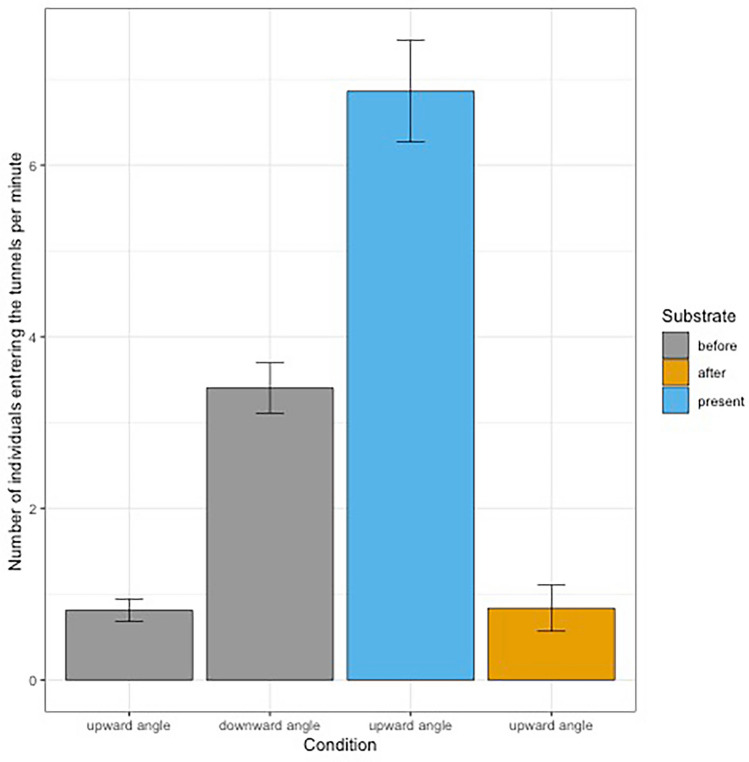
We collected data from our one-hour-long exploratory sessions and counted the number of entries by time intervals of 60 s. This led to a number of 1967 observed entries from at least 68 different individuals (4 sessions of “no substrate, downward angle”: 830 entries; 4 sessions “no substrate, upward angle”: 198 entries; 2 sessions “post substrate, upward angle”: 102 entries; 2 sessions “substrate, upward angle”: 837 entries). To test the effect of the substrate introduction on the entry flux of naked mole rats in the exploration tunnel, we conducted a GLMM analysis with a Poisson distribution (Table 4) with the tunnel condition as a fixed effect and including day, sessions, time interval and observation number (to correct for over-dispersion) as random effects. We found a significant effect of the condition “no substrate, upward angle” (Intercept = − 0.33 ± 0.13, P < 0.001), and positive effects of “no substrate, downward angle” (ß = 1.46 ± 0.18, P < 0.001) and “substrate, upward angle” (ß = 2.17 ± 0.22, P < 0.001). Our model was better than the null model (Chi-square test: X25 = 30.448, P < 0.001) and had a marginal R2 of 0.530. The mean number of entries per minute are presented in Table 5 and Fig. 3.
Table 4.
Results of the global linear model with mixed effect of entry flux tests.
| Response variable | AIC | Predictor variable | Estimate ± SE | df | P |
|---|---|---|---|---|---|
| Number of entries per minute | 2701.7 | – | |||
| Number of entries | 2677.3 | Intercept (“no substrate, upward angle | − 0.33 ± 0.13 | 0.0163 | |
| Condition “no substrate, downward angle” | 1.46 ± 0.18 | < 0.001 | |||
| Condition “post substrate, upward angle” | 0.06 ± 0.24 | 0.8087 | |||
| Condition “substrate, upward angle” | 2.17 ± 0.22 | < 0.001 |
Bold results are significant results with alpha = 0.05.
Table 5.
Mean number of entries per minute depending on the different test conditions.
| Condition | Substrate | Mean number of entries per minute ± SE | Size effect compared to condition “no substrate, downward angle” |
|---|---|---|---|
| “no substrate, downward angle” | before | 3.40 ± 0.15 | 0.00 |
| “no substrate, upward angle” | before | 0.81 ± 0.067 | 1.44 |
| “post substrate, upward angle” | after | 6.86 ± 0.30 | 1.22 |
| “substrate, upward angle” | present | 0.84 ± 0.14 | 1.29 |
Figure 3.
Barplot of the mean number of individuals entering the tunnel per minute and condition. Error bars represent 95% confidence intervals.
Exploratory latencies
We collected the time of first entry for each session of each individual that participated in the test, leading to a total of 252 entries (4 sessions “no substrate, downward angle”: 85 entries; 3 sessions “no substrate, upward angle”: 38 entries; 2 sessions “post substrate, upward angle”: 55 entries; 2 sessions “substrate, upward angle”: 64 entries). By performing a GLMM with a Gaussian family, with mass, age, tunnel as fixed effects and individual identity as a random effect, we found that younger individuals had lower exploration latencies than older individuals (ß = 0.0088 ± 0.033, P = 0.008) and lighter individuals had higher latencies than heavier ones with other parameters fixed (ß = − 0.03 ± 0.16, P = 0.04). Exploratory latencies were longer in condition “no substrate, downward angle” than in condition “no substrate, upward angle” (ß = 0.56 ± 0.21, P = 0.009) and condition “substrate, upward angle” (ß = − 0.58 ± 0.23, P = 0.011) (see Table 6). Our model was better than the null model (Chi-square test: X25 = 25.465, P < 0.001) and performed with a marginal R2 of 0.164.
Table 6.
Results of the global linear model with mixed effect of the exploratory latencies during the test conditions. Significant values are in bold.
| Response variable | AIC | Predictor variable | Estimate ± SE | P |
|---|---|---|---|---|
| log(latency + 0.5) | 838.5 | — | ||
| log(latency + 0.5) | 823.1 | Intercept (Condition “no substrate, upward angle”) | 7.49 ± 0.54 | < 0.01 |
| Mass | − 0.033 ± 0.016 | 0.041 | ||
| Age | 0.0088 ± 0.033 | < 0.01 | ||
| Condition “no substrate, downward angle” | 0.56 ± 0.21 | < 0.01 | ||
| Condition “post substrate, upward angle” | − 0.34 ± 0.23 | 0.15 | ||
| Condition “substrate, upward angle” | − 0.58 ± 0.23 | 0.011 |
Bold results are significant results with alpha = 0.05.
Gnawing frequency
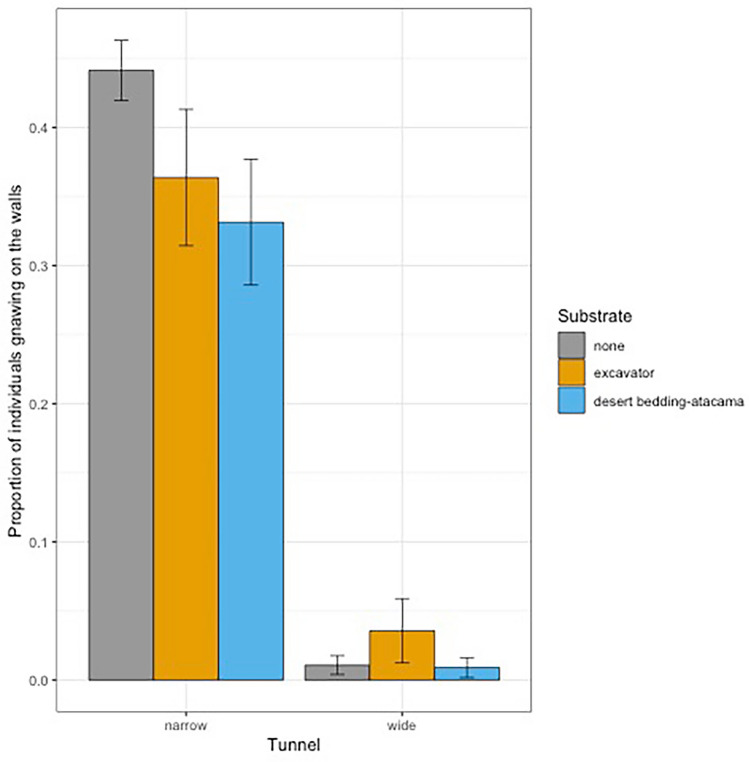
During the two-hour-long observational sessions, we counted the number of individuals gnawing on the walls every minute, leading to a total of 1936 observations, 242 by session. We fitted global linear model with mixed effects with substrate condition (before or after substrate introduction) and tunnel condition as fixed effects, day and observation number (to correct for over-dispersion) as random effects, and a binomial family. Our model was better than the null model (Chi-square: X23 = 1310, P < 0.01) and performed with a marginal R2 of 0.570. The number of individuals gnawing on the tunnel walls was higher in the narrow tunnel than in the wider tunnel (ß = − 4.32 ± 0.18, P < 0.001) and lower in presence of substrate (tendency for Excavator: ß = − 0.29 ± 0.27, P < 0.28; Excavator-Atacama: ß = − 0.55 ± 0.27, P = 0.0392) (Table 7). The mean proportions of individuals gnawing on the tunnel walls depending on the substrate exposition and the tunnel considered are presented in Table 8 and Fig. 4.
Table 7.
Results of the global linear model with mixed effect of the proportion of individuals gnawing on the walls of the tunnels. Significant values are in bold.
| Response variable | AIC | Predictor variable | Estimate ± SE | P |
|---|---|---|---|---|
| Proportion of individuals gnawing | 4225.9 | – | ||
| Proportion of individuals gnawing on the walls | 2921.9 | Intercept (no substrate in the narrow tunnel) | − 0.25 ± 0.15 | 0.0925 |
| Wide tunnel | − 4.32 ± 0.18 | < 0.001 | ||
| Excavator | − 0.29 ± 0.27 | 0.28 | ||
| Excavator-Atacama | − 0.55 ± 0.27 | 0.0392 |
Bold results are significant results with alpha = 0.05.
Table 8.
Mean proportions of individuals gnawing on the tunnel walls depending on the substrate exposition and the tunnel considered.
| Tunnel | Substrate | Mean proportion of individuals gnawing on the walls ± SE | Size effect compared to each tunnel without substrate | Mean number of individuals gnawing on the walls ± SE | Size effect compared to each tunnel without substrate |
|---|---|---|---|---|---|
| Narrow | None | 0.44 ± 0.1 | 0 | 2.58 ± 0.07 | 0.00 |
| Excavator | 0.36 ± 0.03 | 0.27 | 1.01 ± 0.07 | 1.05 | |
| Desert Bedding-Atacama | 0.33 ± 0.02 | 0.38 | 0.80 ± 0.06 | 1.27 | |
| Wide | None | 0.011 ± 0.004 | 0 | 0.033 ± 0.008 | 0.0 |
| Excavator | 0.04 ± 0.01 | 0.26 | 0.05 ± 0.02 | 0.26 | |
| Desert Bedding-Atacama | 0.06 ± 0.004 | 0.03 | 0.02 ± 0.01 | 0.03 | |
| Narrow vs wide | 2.3 | 2.17 |
Figure 4.
Barplot of the mean proportion of individuals gnawing on the tunnel walls per minute and tunnel. Error bars represent 95% confidence intervals.
Discussion
By measuring the number of entries per minute (entry flux) during exploratory tests, we found that entry fluxes are significantly lower in the absence of substrates before their introduction to the colony (condition “no substrate, downward angle” which also had the lowest number of different explorers, condition “no substrate, upward angle”) than in presence of substrate (condition “substrate, upward angle”) or after exposition to the substrate even without substrate (condition “post substrate, upward angle”). We did not find any significant difference between conditions “no substrate, upward angle” and “post substrate, upward angle”. Because both tunnels had the same spatial orientation, we could conclude that presenting the substrate during the preceding weeks, on its own, did not encourage individuals to explore. However, when the substrate was present, the number of individuals to enter the tunnels per minute was higher than in all other conditions but condition “no substrate, downward angle”. We can deduce that naked mole rats choose to explore the new substrate and are more active when it is present.
The downwards tunnel configuration (condition “no substrate, downward angle”) had the highest entry flux. This could be explained by a spatial orientation that is closer to their natural behaviour (digging towards the bottom), or by a least costly effort because of a downhill incline. Testing different inclinations could answer this question.
We also found that exploratory latencies are significantly shorter when the tunnel is half-full (condition “substrate, upward angle”) than when it is empty (condition “post substrate, upward angle”). Moreover, younger individuals are the first to explore, in accordance with a previous study12 in which the authors found that young individuals are the most active.
Our captive colony of naked mole rats had access to two central parallel tunnels differing in diameter. The narrow tunnel was about the width of two individuals, and we observed them to jostle, pass over one another and pull each other’s’ tail. In the wider tunnel, the animals occasionally huddle together and slept in a pile. This size difference thus might have behavioural and biological implications that should be tested in the future. We expected naked mole rats to be more drawn to gnawing on the walls. We did find a higher frequency of gnawing on the walls in the narrow tunnel than in the wider one, and we did find a significant decrease in the gnawing on the wall frequency in presence of the substrate. Gnawing the substrate could thus compensate for gnawing on the wall behaviour. It would be interesting to keep track of the long-term rate of damages on the walls of the tunnels and chambers to see whether the presence of the substrate did decrease the rate of gnawing behaviour. Indeed, three months after the end of the study, the keepers had already observed a decrease in such damages.
Our study enabled us to show a decrease in a naked mole rat colony means exploratory latency in presence of a diggable substrate. We suggest the colony showed an increased motivation to be active since: (i) it organised around the task with individuals digging and others sweeping the debris, (ii) it increased the behavioural diversity of the colony—which has been pointed out as a potential indicator of welfare29—by providing opportunities to dig, (iii) many individuals chose to dig on their own will, and providing the opportunity to choose has been shown to be valued by animals30.
However, our study presents limitations. First, we conducted our experiment on only one captive colony, and we would need to replicate such experiments on other colonies. This raises the question of generality of our results, and limits the inference of our statistics. Second, we were not able to identify individuals at all times, which reduced the potential scope of our study. For example, since we could not observe the whole colony, nor a small sample of it to conduct a real time budget, we had to focus on the central tunnels only. When we introduced the third tunnel so that the animals would dig in it, we could not see how many of them, and which of them, were actually digging. We only had access to the number of individuals in the other two tunnels, and how many of them were gnawing on the wall. Since there were fewer individuals in those tunnels when the substrate was present, even a few digging events made the frequency rise. To confirm by observation the effects of individual characteristics, and deepen our understanding of the colony organisation, a better tracking system could be used. For example, smaller microchip scanners could be stuck inside the tunnels and connected to a computer to automatically record entries and exits of individuals. This could even allow us to gather information on duration of exploration.
It would be relevant to collect reproductive success data and behavioural data such as conflicts on the long term, since previous litters did not survive (probably killed by the colony members) and recurrent injuries happen. Our results could also be discussed with other researchers who have been managing captive colony with other kinds of digging enrichment, on the long term.
When assessing the value of an enrichment, it is important to see if animals choose to use an enrichment and measure how much they value it in terms of effort they are willing to invest to gain access to it. Here, the colony had the choice to explore tunnels, whether empty or full of substrate, and they chose to do so continuously until they had dug through the tunnel. They did so even though digging and sweeping debris is costly in itself. To prove that naked mole rats value the digging opportunity, we should aim to measure the effort they are willing to invest to dig. For example, we could use substrates with drastically different hardness, or play on the tunnel’s incline. Naked mole rats could be intrinsically motivated by digging since as they go, they modify their environment and receive feedback that could be perceived as a positive reward. It would also be of great interest to investigate what substrate naked mole rats would choose to dig in if they had the choice. Cork, as used by Braude28, might also be a good compromise to make it easier for the care-staff to clean the tunnels and avoid skin desiccation. Permanently exposing naked mole rats to a diggable substrate could thus be a realistic solution to favour their exploratory and digging behaviours in captivity, and improve their welfare conditions. Such insight could also be beneficial for medical and fundamental studies by improving reproducibility and translation rate to humans through better welfare of naked mole rat colonies raised in laboratories.
Additionally, a similar experiment had been conducted by Sherman and Lacey16. They introduced three captive naked mole rat colonies with fresh dirt packed into tunnels so they could observe and score digging behaviour. Their focus was on workload distribution and they measured which individuals were digging every 30 s. Like us, they found that digging began as soon as the substrate was discovered, and that the animals worked quickly. No difference between males and females was observed, no more than two mole rats could stand side-by-side and dig simultaneously and conflict occurred during digging rotation. Task rotation was quick, dirt was swept to the toilet and not to the active nest box. However, we did observe them to sweep the debris to the food chamber, probably because of the spatial orientation. They found that larger individuals were the primary participants in volcanoing, which may reflect the positive effect of weight on exploration we observed. Moreover, because Lacey and Sherman did not have access to the accurate age of most colony members, they only used a relationship between mass, and age observed on captive individuals, or growth rate. We, on the other hand, had direct access to age for all colony members. The inverse effect of age and mass reveals two morphs participating in exploration/digging: young individuals and massive individuals. This supports the age polyethism hypothesis where animals specialize with age5, with young individuals involved in colony maintenance, and older (massive one) in colony defence. Indeed, we found that with all other parameters equal, younger individuals explored more, and for the same age, the heavier ones were the first to explore. This could be further explored in the future, as well as the effect of such behavioural modification on the disperser morph. Our experimental design could help explore how exploratory behaviour relates to the degree to which an animal expresses the disperser morph7 since all animals will express this morph at some point, but in captivity lose it and return to worker status.
The task of exploring could also be fulfilled by the young individuals, as part of their development, or due to their temperament31. Finally, data from laboratory settings may differ from field study as well, primarily because we cannot observe animals digging while they are underground. Our setting might thus be beneficial for future studies.
In conclusion, we found that the introduction of a diggable substrate increased the behavioural diversity and activity of one captive naked mole rat colony, as well as providing individuals with new decisions (digging or not). The use of such substrates could improve the welfare of naked mole rats under human care.
Methods
Ethical note
This study was approved by the Comité Cuvier of the MNHN (Muséum national d’Histoire naturelle, reference 2023-68-122) as a justified scientific project following the 3R principles. The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations. This study was conducted in coordination with the animal care staff in order to respect the colony's behaviour and welfare, and to adapt our procedures, if needed, during the duration of the experiments. We were careful to minimise loud noises and vibrations that could cause disturbance to the colony. The captive colony was assembled in a box during the annual inventory, under the supervision of several veterinarians (Authorisation number: DTPP 2009-1002, Certificate of qualification no.: DTPP 2021-018 dated 8 January 2021) and the animal care staff. The individuals were captured by hand and handled individually for a health check (weighing, individual observations and injection of an RFID chip). The authors complied with the ARRIVE guidelines [].
Subjects and housing
We conducted our study on a captive colony of naked mole rats coming from the École nationale vétérinaire d’Alfort (EnvA), originating from Cape Nature, South Africa and which arrived in September 2021 at La Ménagerie, Zoo du Jardin des Plantes. An inventory of the colony was carried out on February 30th, 2023. The colony is composed of 79 individuals among which 40 males, 35 females, and 4 individuals whose sex could not be identified. All were more than a year old. During the inventory procedure, the veterinarians and curators weighed and identified individuals, and inserted radio-frequency identification (RFID) transponders in individuals that did not already possess one. The colony lives in a network of chambers interconnected by tunnels in Plexiglas®, with wood shaving bedding and kitchen paper. The light was on during the experiments since the colony is used to light on a daily basis during maintenance. The temperature is maintained at 28 °C with a 35% humidity so that temperature in the chambers is maintained between 28 and 30 °C, and 80% humidity (Fig. 5). Animals have access to food ad libitum with a base of tubers such as sweet potatoes at a rate of 6 g per individual and a mixture of vegetables at a rate of 3 g per individual (carrots, black radish, parsnip, pink radish, celeriac, red beetroot, black salsify, rutabaga, potato peas (Jicama), corn on the cob, green vegetables, carrot tops, butternut squash with seeds, aubergine; and in limited quantities: lettuce, cabbage, soya and beans).The environment is enriched with cardboard packaging to gnaw on. Each afternoon, keepers remove dirty bedding and occasionally wash the whole network with water. A radio is constantly playing in order to accustom animals to the sound of speech.
Figure 5.
Housing of the naked mole rat colony. The naked mole rat colony was housed in a system of four boxes connected to one another directly, or through two central parallel tunnels, Tunnel 1 which was narrow, and Tunnel 2 which was wider.
Phase A: before substrate exposition
Exploratory behaviour
We chose not to test individuals separately from others in order to avoid the stress associated with isolation32. Rather, we let them choose whether or not they would participate and guaranteed them access to their habitat and other colony members. The exploration task consisted in introducing a 54 cm long and 5 cm diameter tunnel, parallel to their usual circuits with 17° inclination from the horizontal (Fig. 6). In order to limit the number of individuals using the tunnel, we chose a 5 cm diameter (to which they were already used to), and blocked one of the issues so that naked mole rats could only enter from one side: down-up (condition 1) or up-down (condition 2.a). We alternated between both conditions during one week, 1 h a day, 1 h per condition and per day. At the end of each test session, the tunnel was washed with water and black soap to remove the colony’s scent.
Figure 6.
Tunnel installation for (a) exploration sessions, (b) observational sessions. The exploration tunnel was 58.5 cm long and the tunnel used during observational sessions was 140 cm long.
After the installation of the tunnel, each individual entering the tunnel was identified with a microchip reader. We considered an individual to have entered the tunnel when their four legs were inside the tunnel. The experiment was recorded with a Sony HD cam, and the entry times were taken into account by 2 different experimenters.
Gnawing frequency and entry flux of individuals
We performed 2 h long observations during which we counted the number of individuals in tunnel 1 and tunnel 2 each minute, and how many of them were gnawing on the tunnels.
New substrates
For the first time, we introduced three different diggable substrates in our captive. We did not use a control colony, but rather compared the colony behaviour before and after exposition to these substrates. Like in the exploration tests, we used an up-down parallel tunnel filled with each kind of substrate. During these first two weeks of introduction, we observed one hour of interaction per substrates per week, for a total of 2 h per substrate. The order of exposition was randomly chosen and repeated two times. We used the reptile substrates described in Table S1. These first two weeks allowed us to make sure there was no issue with the substrate. Then, we continued the exposition to the substrates by adding tunnels of various lengths and diameters to allow the animals to dig for at least one hour a day. We adapted the duration of exposition and the type of substrate according to the time needed for the substrate to dry (see Table S2 for the planning). We made sure there were no problems introducing the substrate by talking to the animal care staff. On one occasion, they had to add water spray to increase the ambient humidity in the chambers. We also had to interrupt our sessions for a fortnight due to the birth of a litter by the queen. Unfortunately, a first session of "no substrate, upward angle" (the first test session) could not be analysed because data collection failure. We do not believe this session to be problematic since it does not drastically change our sample size and the behaviours observed during the 4 other sessions were quite homogenous.
Phase B: after substrate exposition
We repeated the exploratory tests with an empty tunnel upwards (condition “post substrate, upward angle”), and a half-empty tunnel upwards (condition “substrate, upward angle”) to test the attractiveness of the substrate. We alternated with observation sessions as before, adding a third tunnel filled with substrate to test the effect of the presence of substrate on the colony behaviour.
Data analysis
During one trial of condition “no substrate, upward angle”, we could not have access to the microchip identity number of explorers. We thus excluded this trial from analyses where individual identity was needed (i.e. Presence in the tunnels and Exploratory Latencies). All videos were analysed with knowledge of the condition tested.
Presence in the tunnels
We ran a GLMM model with a logit link function and binomial distribution (function “glmer”, package ‘lme4’ version 1.1-3233 to measure the effect of individual characteristics on presence during the different test phases. Fixed effects were mass, sex, age and condition, with an individual random effect. We checked that the model was correctly applied using the function check_model() designed for visual check of model assumption from the package ‘performance’ version 0.10.434. As the sex of two individuals was unknown, they were excluded from this analysis for the generation of the null model only. We tested our model against the null model with a likelihood ratio test.
Number of explorers
We conducted a GLM analysis (function “glm”, package ‘stats’ version 4.2.335), with the tunnel condition as a fixed effect, and using a Poisson family.
We checked that the model was correctly applied using the function check_model() from the package ‘performance’ version 0.10.434. We tested our model against the null model with a likelihood ratio test.
Entry flux
We conducted a GLMM analysis (function “glmer”, package ‘lme4’ version 1.1-3233), with the tunnel condition as a fixed effect, including day, sessions, time interval and observation number (to correct for over-dispersion) as random effects, and using a Poisson family.
We checked that the model was correctly applied using the function check_model() from the package ‘performance’ version 0.10.434. We tested our model against the null model with a likelihood ratio test.
Exploratory latencies
We adjusted a GLMM model with an identity link function and a gaussian distribution (function “lmer”, package ‘lme4’ version 1.1-3233) to measure the effect of individual characteristics on exploratory log transformed latencies during the different test phases. Fixed effects were mass, sex, age and test, with an individual random effect. “Sex” was then excluded because the effect was not significant and the model had a lower AIC. We checked that the model was correctly applied using the function check_model() from the package ‘performance’ version 0.10.434. We tested our model against the null model with a likelihood ratio test.
Gnawing frequencies
We fitted a GLMM (function “glmer”, package ‘lme4’ version 1.1-3233) to model the number of individuals gnawing on the tunnel walls. Fixed effects were substrate exposition and tunnel condition. We included substrate condition (before or after substrate introduction) and tunnel condition as fixed effects, day and observation number (to correct for over-dispersion) as random effects, and used a binomial family. We checked that the model was correctly applied using the function check_model() from the package ‘performance’ version 0.10.434.
All data analysis was conducted with R version 4.2.3.
Supplementary Information
Acknowledgements
We would like to thank la Ménagerie, le Zoo du Jardin des Plantes for letting us access their naked mole rat colony and helping us with the experimental design. Thanks to the veterinary staff and the animal care staff, especially Christelle Hano, Clémence Verguin, Claire Réjaud, Aurélie Magnier, Jennifer Rodrigues, Michaël Magnier, and Patrick Martin. We also thank the anonymous reviewers who reviewed our paper for their constructive comments.
Author contributions
M.A contributed to the conceptualisation, data curing, investigation, formal analysis, and wrote the original draft. A.M contributed to the conceptualisation, data curing, investigation, writing—review and editing. P.ZT contributed to the supervision, formal analysis, writing—review and editing. A.B contributed to the conceptualisation, writing—review and editing. E. P contributed to the coordination, conceptualisation, writing—review and editing.
Data availability
Tables containing exploratory latencies and observation sessions are available as supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64146-w.
References
- 1.VandenBroecke, B. et al. Does exploratory behavior or activity in a wild mouse explain susceptibility to virus infection?. Curr. Zool.64, 585–592 (2018). 10.1093/cz/zox053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehlhorn, K. et al. Unpacking the exploration–exploitation tradeoff: A synthesis of human and animal literatures. Decision2, 191–215 (2015). 10.1037/dec0000033 [DOI] [Google Scholar]
- 3.Oosthuizen, M. K. Exploratory behaviour, memory and neurogenesis in the social Damaraland mole-rat (Fukomysdamarensis). J. Exp. Biol.223, jeb221093 (2020). 10.1242/jeb.221093 [DOI] [PubMed] [Google Scholar]
- 4.Faulkes, C. G. et al. Micro- and macrogeographical genetic structure of colonies of naked mole-rats Heterocephalus glaber. Mol. Ecol.6, 615–628 (1997). 10.1046/j.1365-294X.1997.00227.x [DOI] [PubMed] [Google Scholar]
- 5.Jarvis, J. U. M. Eusociality in a mammal: Cooperative breeding in naked mole-rat colonies. Science212, 571–573 (1981). 10.1126/science.7209555 [DOI] [PubMed] [Google Scholar]
- 6.Gilbert, J. D., Rossiter, S. J. & Faulkes, C. G. The relationship between individual phenotype and the division of labour in naked mole-rats: It’s complicated. PeerJ8, e9891 (2020). 10.7717/peerj.9891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Riain, M. J., Jarvis, J. U. M. & Faulkes, C. G. A dispersive morph in the naked mole-rat. Nature380, 619–621 (1996). 10.1038/380619a0 [DOI] [PubMed] [Google Scholar]
- 8.O’Riain, M. J. & Faulkes, C. G. African mole-rats: Eusociality, relatedness and ecological constraints. in (eds. Korb, J. & Heinze, J.) 207–223 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2008). 10.1007/978-3-540-75957-7_10.
- 9.Buffenstein, R. et al. The naked truth: A comprehensive clarification and classification of current ‘myths’ in naked mole-rat biology. Biol. Rev. Camb. Philos. Soc.97, 115–140 (2022). 10.1111/brv.12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mooney, S. J., Filice, D. C. S., Douglas, N. R. & Holmes, M. M. Task specialization and task switching in eusocial mammals. Anim. Behav.109, 227–233 (2015). 10.1016/j.anbehav.2015.08.019 [DOI] [Google Scholar]
- 11.Toor, I., Clement, D., Carlson, E. N. & Holmes, M. M. Olfaction and social cognition in eusocial naked mole-rats, Heterocephalusglaber. Anim. Behav.107, 175–181 (2015). 10.1016/j.anbehav.2015.06.015 [DOI] [Google Scholar]
- 12.Siegmann, S. et al. Naked mole-rats (Heterocephalus glaber) do not specialise in cooperative tasks. Ethology127, 850–864 (2021). 10.1111/eth.13160 [DOI] [Google Scholar]
- 13.Braude, S. et al. Surprisingly long survival of premature conclusions about naked mole-rat biology. Biol. Rev.96, 376–393 (2021). 10.1111/brv.12660 [DOI] [PubMed] [Google Scholar]
- 14.Crish, S. D., Rice, F. L., Park, T. J. & Comer, C. M. Somatosensory organization and behavior in naked mole-rats I: Vibrissa-like body hairs comprise a sensory array that mediates orientation to tactile stimuli. Brain Behav. Evol.62, 141–151 (2003). 10.1159/000072723 [DOI] [PubMed] [Google Scholar]
- 15.Lewin, G. R. et al. The somatosensory world of the African naked mole-rat. In The Extraordinary Biology of the Naked Mole-Rat (eds Buffenstein, R. et al.) 197–220 (Springer International Publishing, 2021). 10.1007/978-3-030-65943-1_7. [Google Scholar]
- 16.Lacey, E. A. & Sherman, P. W. 10. Social Organization of Naked Mole-Rat Colonies: Evidence for Divisions of Labor. In 10. Social Organization of Naked Mole-Rat Colonies: Evidence for Divisions of Labor 275–336 (Princeton University Press, 2017). 10.1515/9781400887132-013.
- 17.Vejmělka, F. et al. Heat dissipation in subterranean rodents: The role of body region and social organisation. Sci. Rep.11, 2029 (2021). 10.1038/s41598-021-81404-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper, T. K. et al. Research-relevant conditions and pathology of laboratory mice, rats, gerbils, Guinea pigs, hamsters, naked mole rats, and rabbits. ILAR J.62, 77–132 (2021). 10.1093/ilar/ilab022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole, T. Happy animals make good science. Lab. Anim.31, 116–124 (1997). 10.1258/002367797780600198 [DOI] [PubMed] [Google Scholar]
- 20.Loss, C. M., Melleu, F. F., Domingues, K., Lino-de-Oliveira, C. & Viola, G. G. Combining animal welfare with experimental rigor to improve reproducibility in behavioral neuroscience. Front. Behav. Neurosci.15 (2021). [DOI] [PMC free article] [PubMed]
- 21.Akkerman, S. et al. PDE5 inhibition improves object memory in standard housed rats but not in rats housed in an enriched environment: Implications for memory models?. PLOS ONE9, e111692 (2014). 10.1371/journal.pone.0111692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voelkl, B. et al. Reproducibility of animal research in light of biological variation. Nat. Rev. Neurosci.21, 384–393 (2020). 10.1038/s41583-020-0313-3 [DOI] [PubMed] [Google Scholar]
- 23.Makowska, I. J. & Weary, D. M. The importance of burrowing, climbing and standing upright for laboratory rats. R. Soc. Open Sci.3, 160136 (2016). 10.1098/rsos.160136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellor, D. J. et al. The 2020 five domains model: Including human-animal interactions in assessments of animal welfare. Animals10, 1870 (2020). 10.3390/ani10101870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawkins, M. S. Farm animal welfare: Beyond “natural” behavior. Science379, 326–328 (2023). 10.1126/science.ade5437 [DOI] [PubMed] [Google Scholar]
- 26.McConnachie, E. et al. Cows are highly motivated to access a grooming substrate. Biol. Lett.14, 20180303 (2018). 10.1098/rsbl.2018.0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mwobobia, R., Abelson, K. & Kanui, T. Housing behaviour of the naked mole rat (Heterocephalus glaber) under laboratory conditions. Scand. J. Lab. Anim. Sci. (2020).
- 28.Sherman, P. W., Jarvis, J. U.M. & Alexander, R. D. The Biology of the Naked Mole-Rat. (Princeton University Press, 1991).
- 29.Miller, L. J., Vicino, G. A., Sheftel, J. & Lauderdale, L. K. Behavioral diversity as a potential indicator of positive animal welfare. Animals10, 1211 (2020). 10.3390/ani10071211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littlewood, K. E., Heslop, M. V. & Cobb, M. L. The agency domain and behavioral interactions: assessing positive animal welfare using the Five Domains Model. Front. Vet. Sci.10 (2023). [DOI] [PMC free article] [PubMed]
- 31.Majelantle, T. L., Ganswindt, A., Pirk, C. W. W., Bennett, N. C. & Hart, D. W. Aggression, Boldness, and Exploration Personality Traits in the Subterranean Naked Mole-Rat (Heterocephalus glaber) Disperser Morphs. Animals12, 3083 (2022). 10.3390/ani12223083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blecher, A. S. et al. Effect of colony disruption and social isolation on naked mole-rat endocrine correlates. Gen. Comp. Endocrinol.295, 113520 (2020). 10.1016/j.ygcen.2020.113520 [DOI] [PubMed] [Google Scholar]
- 33.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 1–48 (2015). 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 34.Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P. & Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw.6, 3139 (2021). 10.21105/joss.03139 [DOI] [Google Scholar]
- 35.Team, R. R: A language and environment for statistical computing. MSOR Connect. (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Tables containing exploratory latencies and observation sessions are available as supplementary material.