Abstract
The Water Framework Directive (WFD) requires member states to routinely assess the river ecological status using community-based indices. However, there is still a lack of published WFD-compliant methods for the French West Indies, especially using diatom-based indices. Martinique and Guadeloupe exhibit diverse landscapes shaped by their complex geological history and tropical climatic conditions. These strong particularities make the existing indices developed for the European mainland unusable. Based on diatom sampling from to 2013 (607 samples) and through multivariate analyses, we developed the Indice Diatomique des Antilles (IDA). We first identified the key abiotic factors influencing diatom communities on both islands, and then characterized taxon sensitivity by considering their presence probability along a pressure gradient.. The index was based on the presence and relative abundance of these taxa in each sample. The last step consisted of using new data from the 2014–2022 sampling surveys (457 samples) as a validation dataset to verify IDA accuracy. Our results suggest that the IDA methodology is well designed to assess the ecological status of rivers in the West Indies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10661-024-12980-w.
Keywords: Water Framework Directive, River ecological status, Diatoms, Biotic indices, French West Indies
Introduction
The Caribbean islands are recognized as one of the most important global biodiversity hotspots, as they concentrate on numerous endemic species while undergoing exceptional habitat loss (Myers et al., 2000). These Islands contain at least 2% of the world’s endemic plant and vertebrate species on only 0.4% of the Earth’s land surface, and any damage to biodiversity in this region has accelerated global biodiversity erosion.
Among these islands, Martinique and Guadeloupe are particularly remarkable. As part of the West Indies, they have been shaped by intense volcanic activity since the Middle Eocene (49 Ma) (Graham, 2003). Composed of sedimentary limestone or magmatic rocks formed by the cooling of volcanic magma, the relief is highly variable. High, recently formed volcanoes give way to older reliefs and lowlands. This particular geography highly influences precipitation, as mountainous areas are exposed to intense rainfall, whereas coastal areas are drier. The complex geological origin of the Martinique and Guadeloupe islands, coupled with a tropical climate, created ecosystems with contrasting relief and pluviometry, leading to diverse habitats that support high biodiversity and endemism.
However, the expansion of agriculture, cities, and tourism has endangered this exceptional heritage. Freshwater habitats, including rivers, streams, lakes, and wetlands are particularly threatened by water pollution, flow modifications, species invasions, and over-exploitation of living resources (Lugo et al., 2012; Dudgeon, 2006; Dromard et al., 2016). To maintain or restore the ecological status of water bodies, and preserve the biodiversity they host, the development of aquatic ecosystem monitoring tools dedicated to these specific regions is crucial.
As part of the French overseas territories, the objectives of the Water Framework Directive (WFD, European Union, 2000) must be met in Martinique and Guadeloupe. However, there is still a lack of published WFD-compliant methods for the French West Indies, especially for community-based assessments of river quality (Bernadet et al., 2013). Existing indices dedicated to the European continent cannot be directly transposed due to biogeographic differences in community composition, as the development of bioassessment methods overseas has long been hampered by the lack of knowledge on exotic fauna and flora (Carayon et al., 2019). Despite the fact that microalgae are recognized worldwide as relevant bioindicators (Leboucher et al., 2019; Soininen, 2007), diatom flora from the West Indies has remained particularly overlooked. Initial work was carried out by Bourrelly and Manguin (1952) who sampled rivers, mangrove areas, waterfalls, ponds, mosses, and seeps in Guadeloupe. Eight hundred species of algae have been described, including 124 diatom taxa from freshwater streams and waterfalls. No further investigation was undertaken until Coste and Dauta (1997) made the first freshwater diatom inventory of Martinique and Tudesque and Ector (2002) published the first atlas of Guadeloupe diatom flora. In the 2010s, little information on Guadeloupe and Martinique diatoms was available.
In this context, a 5-years research program (2009–2013) was used to establish the first diatom-based index dedicated to the ecological status assessment of rivers in Martinique and Guadeloupe (Lefrancois et al., 2019; JORF, 2023; Eulin Garrigue et al., 2017). Since 2014, this index referred to as Indice Diatomique des Antilles (IDA) has been routinely used to assess river water quality over the West Indies, particularly during the second WFD management plan (JORF, 2023) (an application to calculate the index is available on the website https://seee.eaufrance.fr/). In this study, we present the initial data acquisition context and the methodology developed for the elaboration of IDA. We then discuss its relevance in monitoring pressure gradients and make recommendations on the improvements that are needed today in light of its routine use.
Materials and methods
Sampling sites
The Martinique’s hydrographic network is dense, with 70 rivers, 40 of them perennial. However, two major hydrographic units, or hydroecoregions, can be distinguished. Northern Martinique (“Pitons du Nord” hydroecoregion) is characterized by prominent mountains, including the Pelée Mountain and the “Pitons” (“Pitons du Carbet”). The rivers flowing from the Pelée Mountain have a relatively straight course due to steep slopes, while those flowing from the Pitons show a more diversified morphology, with meanders in the downstream section. In both cases, short watersheds (mainly < 15 km2) and steep gradients (4% and more) generate torrential flows. The southern part of the island features gentler hills and fewer perennial rivers (“Mornes du Sud” hydroecoregion). The valleys widen due to low slopes that reach zero in the mangrove swamp zone. The clayey nature of the soils, combined with low rainfall, results in frequently low water levels. Guadeloupe is formed by two main islands separated by a marine inlet. The Eastern part, known as Grande Terre, is predominantly flat and characterized by limestone soils. Basse Terre, situated in the Western part, is a mountainous volcanic island featuring a north-to-south chain of volcanoes that defines its landscape. The highest peak is “La Soufrière,” an active volcano standing at 1467 m high, in the south. Basse-Terre is drained by more than fifty permanently flowing rivers, characterized by their short lengths, shallow depth, and small catchment areas (10 to 30 km2). This hydrographic network is fed mainly by runoff, but is also supported by small aquifers. Its hydrological regime is torrential. Grande Terre in the east lacks perennial streams. Due to these contrasting, both territories are separated into two distinct hydroecoregions (HERs).
Martinique as well as Guadeloupe experiences a tropical climate with distinct dry (February to April) and wet (July to October) seasons. Sampling efforts were designed to best capture these climatic and geographical conditions and potential pollution sources that mainly originate from organic sources and pesticides related to the presence of crops, sugar factories, distilleries, slaughterhouses, and sewage treatment plants. Within the 2009–2013 period, 130 sites were sampled (70 from Martinique and 60 from Guadeloupe—Basse Terre—Fig. 1a and b, and Appendix 1), resulting in 607 samples with physicochemical and floristic data.
Fig. 1.
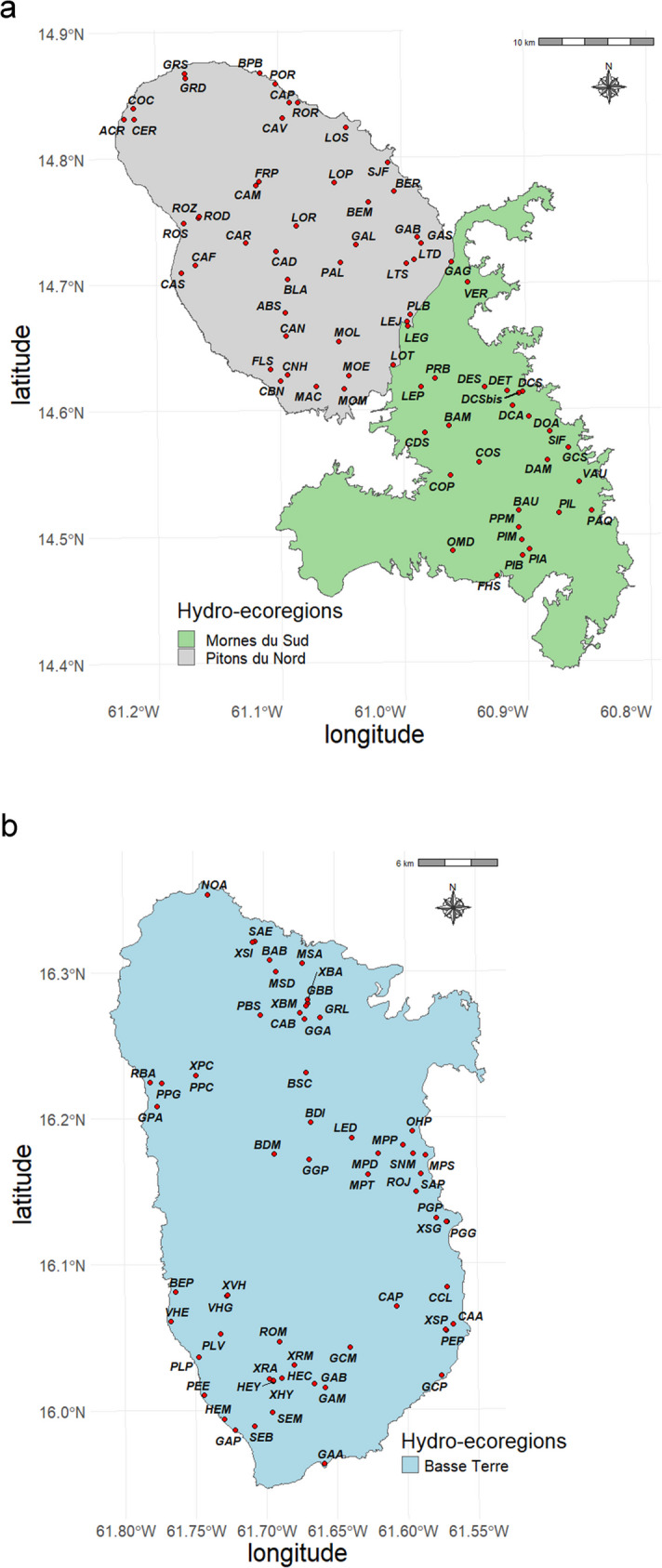
a Map of sampling sites from Martinique with hydroecoregions (projection system WGS 84). b Map of sampling sites from the Basse Terre in Guadeloupe (projection system WGS 84)
Physicochemical data
Environmental conditions in watercourses were estimated using 10 physicochemical variables: suspended matter (SM), biological oxygen demand (BOD5), nitrites (NO2), nitrates (NO3), orthophosphates (PO4), ammonium (NH4), Kjeldahl nitrogen (NKj), oxygen saturation (O2.Sat), total phosphorus (Tot.P), and dissolved organic carbon (Org.C). All analyses were carried out by six laboratories (departmental analysis laboratories—Pyrénées, Landes, Martinique, and Drôme; Pasteur Institute; and Lyon Carso Hygiene and Safety Laboratory), based on the same European standards (available on the European Committee for Standardization website: https://standards.iteh.ai/catalog/tc/cen/fb2ac0f7-0811-458a-a4b3-b06e5456a865/cen-tc-230).
For further analysis, all abiotic variables that were not normally distributed were standardized (i.e., reduced and rescaled) using the Yeo-Johnson method as it takes into account null values (Riani et al., 2023; Yeo & Johnson, 2000). Potential autocorrelations among variables were checked using the Spearman method (De Winter et al., 2016).
Diatom data
Diatoms were sampled by Asconit Consultants, from hard substrates (pebbles and cobbles) under both dry and wet conditions according to the NF T90-354 protocol (AFNOR, 2016), in line with the European standards (EN 13946, CEN, 2003). Valves were identified at 1000 × magnification by examining permanent slides of cleaned diatom frustules, digested in boiling H2O2 (30%) and mounted in a high refractive index medium (Naphrax, Northern Biological Supplies Ltd., UK; RI = 1.74) (EN 13946, CEN, 2003). Four hundred valves per slide were counted and identified to obtain relative abundances for each taxon. Literature related to diatom taxonomy from the Caribbean islands is very sparse (but see Tudesque & Ector, 2002); some series must be cited in particular as they provide valuable drawings and micrographs to identify the diatom flora collected: Iconographia Diatomologica, Diatoms of Europe, Sübwasserflora von Mitteleuropa. Taxonomic homogenization was performed to avoid synonymy, as different appellations may coexist for the same taxa.
Thresholds were applied to retain only the taxa that provide relevant ecological information. First, taxa occurring in fewer than five samples were removed from the database. Among the remaining ones, only those showing at least one abundance data > 2.5% within all samples (Lavoie et al., 2009) were kept (hereafter referred to as “contributive taxa”). To capture the information provided by the less abundant taxa, relative abundances were log-transformed (log(x + 1)).
Data analysis and index settlement
General approach
IDA calculation is based on the presence and abundance of contributive taxa (i.e., taxa for which an ecological profile was defined) in the sample considered. Five major steps are necessary. First, the environmental gradients driving sites and diatom communities were determined using multivariate analysis, following the concept of indirect gradient analysis explained by Ter Braak (2004). Two gradients were determined, one derived from principal component analysis (PCA) (Wold et al., 1987) applied to physicochemical data, and the other derived from canonical correspondence analysis (CCA) (Ter Braak & Verdonschot, 1995) applied to physicochemical and floristic data. The combination of both gradients led to settlement of a multimetric alteration gradient (MAG). Using this gradient, samples were distributed into distinct quality classes. Taxa ecological profiles were based on their presence probability along these classes, allowing us to determine an index score for all samples, based on floristic lists. All analyses were performed using R version 4.1.2 (2021–11-01) (R Core Team, 2021).
Multivariate analyses
A two-step analytical approach was used to gain comprehensive insights into the relationships between physicochemical variables and diatom data. As Martinique and Guadeloupe are part of the West Indies hydrosystem, merging data from both islands for analysis was prompted by the similarity in diatom communities. First, PCA was performed on the physicochemical data to provide a synthetic overview of environmental conditions at the study sites. CCA was subsequently performed to integrate both abiotic and floristic information and determine the ecological gradients that drive community composition. Analyses were based on log(x + 1) abundance data and centered and reduced physicochemical data. The statistical significance of the CCA was tested using ANOVA (Girden et al., 1992). Both analyses were performed using the “ade4” package (Chessel et al., 1995).
Multimetric alteration gradient (MAG)
Two gradients were first determined based on PCA and CCA results. The position of each sample in the gradient was estimated using the following equation:
where i corresponds to the axis number and j to the sample.
Building a single gradient from PCA and CCA results enabled the integration of both physicochemical and biological information while enhancing the weight of environmental conditions. Both gradients were first normalized along a 0 to 1 axis, where samples closer to 0 indicated highly degraded environmental conditions.
Axis normalization was performed using the following formula:
with maxA and minA corresponding to the maximum and minimum values of the desired range (in our case 0 to 1), maxVar and minVar to the maximum and minimum values of the vector to be scaled, i.e., the PCA or CCA gradient, and Var to the vector value to be scaled.
The multimetric alteration gradient was then written as the sum of the PCA and CCA gradient positions of all samples, leading to a single gradient scaled from 0 to 2.
Taxa ecological profiles
After obtaining the MAG positions of all samples, a hierarchical classification (Husson et al., 2010) was performed to cluster them according to their scores. The relevance of the clusters obtained was tested using pairwise Wilcoxon tests (Wilcox, 2001) with a Bonferroni correction for multiple comparisons (Weisstein, 2004). Ecological profiles were then determined for each taxon, based on their presence probability across the different classes obtained.
where, for the class considered, corresponds to the taxon occurrence in the class, to the relative abundance of the taxon in the class considered, and to the number of sites in this class.
Three types of ecological profiles were determined: “2 − ,” “1 − ,” and “1 + .”
Taxa with high presence probabilities in bad quality classes were called “alert taxa” and were defined as “2 − ” or “1 − ” depending on these probabilities (if we consider that the hierarchical analysis resulted in five classes):
Taxa with the highest presence probabilities in the worst quality classes were defined as “2 − ”:
Taxa rather present in moderate quality classes were defined as “1 − ”:
All other taxa were not considered as “alert taxa” and were therefore classified as “1 + .”
Index calculation
Based on taxa ecological profiles and abundance data, the Indice Diatomique des Antilles (IDA) was computed as follows:
where SR corresponds to the specific richness of all contributive taxa, to the number of taxa classified as “1 + ,” to the number of taxa classified as “1 − ,” and to the number of taxa classified as “2 − .” corresponds to the relative abundance of “1 + ” taxa, to the relative abundance of “1 − ” taxa, and corresponds to the relative abundance of “2 − ” taxa.
A greater weight (− 3) was assigned to taxa present in highly impacted environments. The more “2 − ” taxa in the sample, the lower the IDA score. The worst-case scenario for alteration in a survey thus involves only “2 − ” taxa, each with an alteration coefficient of − 3, resulting in a final score of − 300. Scores could initially range from + 100 (optimal conditions) to − 300 (worst conditions), forming a 400-unit scale. Although the best conditions can be found in some sites, the worst theoretical conditions of only “2 − ” taxa never occur in reality. Even in severely altered areas, watercourses still carry positive ( +) and negative ( −) taxa, preventing the score from dropping to − 300. In practice, even in the most polluted watercourses in the Antilles, the lowest recorded score is − 148, observed downstream of a highly polluting STEP discharge, and it is unlikely that a lower score will ever be encountered. For this reason, we decided to set the lowest possible IDA score to − 150. Then, the scores were normalized to a [0]–[+ 20] scale as follows:
where − 150 represents the lowest possible value of IDA, 100 is the highest, and − 3 is the weight attributed to “2 − ” taxa.
Index validation
We used a set of new samples collected from Martinique and Guadeloupe between 2014 and 2022 for index validation. In total, 457 new samples, all characterized by complete physicochemical and biological data, were used as a validation matrix. First, we projected the new data onto the three PCA and CCA dimensions to calculate the new MAG scores. To do so, biological and physicochemical matrices underwent several modifications.
Only taxa from the new community matrix that were present in the initial one were considered, and then (log + 1) transformed as previously;
For a few samples, not all physicochemical variable values were recorded. To make sure we had a complete set to work on, we decided to predict those missing values using the “MICE” package in R (R Development Core Team, 2021).
Samples’ positions on the PCA were obtained by displaying the new physicochemical data on the different dimensions using the “suprow” function from the “ade4” package (Chessel et al., 1995). Samples’ positions on the CCA were obtained for the new community matrix using weighted constrained averages of the initial species scores (WA scores) using the “vegan” package (predict.cca function, Dixon, 2003). Finally, we calculated and normalized the new MAG values and performed a Spearman correlation test (De Winter et al., 2016) between new IDA scores (computed from new data abundances) and MAG values.
In the second step, we performed a multiple linear regression model to investigate the potential relationships between IDA scores and physicochemical variables using the new dataset. The best model was selected based on Akaike’s information criterion (AIC) (Akaike, 1974), performed with the “stepAIC” function from the R package MASS (Ripley et al., 2013). We then tested the correlation between IDA scores predicted by the selected model and the real scores using the Spearman method.
Results
Biological and physicochemical data
The 607 biological surveys carried out between 2009 and 2013 have made it possible to identify and inventory 512 different taxa that can now be recognized and counted, among which 60% have been described as new.
Few taxa were abundant (e.g., Achnanthidium minutissimum, Mayamaea permitis, Nitzschia tripunctata, Nitschia gregaria), whereas 435 were present in less than 10% of the samples. After implementing the criteria for selecting the most contributive ones, 178 taxa were included in the dataset (Appendix 2).
Concerning physicochemical data (Table 1), the value distribution ranges for oxygen saturation, suspended matter, organic carbon, and BDO5 were particularly wide (and to a lower extent for NH4, NO3, and KjN). Some sampling sites were located directly at the effluent discharge points of wastewater treatment plants (BAB, GBB, HEY, OHP, PGG, PEP, XHY, XSG, and XSP for Guadeloupe and CAS, CDS, DCA, FHS, FLS, GCS, and LTS for Martinique), leading to high nutrient and organic matter loads. The maximum autocorrelation between variables was 0.627, and concerned NH4 and N02. We considered that this value was not sufficiently high to remove any variable from the ten listed above.
Table 1.
Physicochemical data available for analyses
| Variable | Units | Minimum | 25th Percentile | Median | 75th percentile | Maximum | Mean | Standard deviation |
|---|---|---|---|---|---|---|---|---|
| Oxygen saturation (O2.Sat) | % | 2.190 | 91.000 | 97.500 | 101.400 | 157.5 | 92.278 | 19.507 |
| Suspended matter (SM) | mg/L | 0.330 | 2.600 | 8.200 | 24.500 | 289.0 | 18.773 | 28.382 |
| Total phosphorus (tot.P) | mg/L | 0.007 | 0.017 | 0.040 | 0.140 | 7.3 | 0.143 | 0.397 |
| Orthophosphate (PO4) | mg (PO4)/L | 0.000 | 0.030 | 0.050 | 0.125 | 16.0 | 0.229 | 0.887 |
| Organic carbon (Org.C) | mg/L | 0.067 | 0.800 | 1.220 | 2.055 | 200.0 | 2.689 | 12.148 |
| Biological oxygen demand (BDO5) | mg O2/L | 0.170 | 0.170 | 0.700 | 1.045 | 410.0 | 2.495 | 22.875 |
| Kjeldahl nitrogen (KjN) | mg N/L | 0.130 | 0.167 | 0.333 | 0.500 | 61.1 | 0.876 | 3.331 |
| Ammonium (NH4) | mg (NH4) + /L | 0.008 | 0.017 | 0.020 | 0.030 | 67.0 | 0.607 | 3.856 |
| Nitrites (NO2) | mg (NO2) − /L | 0.007 | 0.008 | 0.010 | 0.020 | 12.0 | 0.084 | 0.569 |
| Nitrates (NO3) | mg (NO3) − /L | 0.030 | 0.330 | 0.620 | 2.900 | 25.5 | 2.166 | 3.322 |
Multivariate analyses
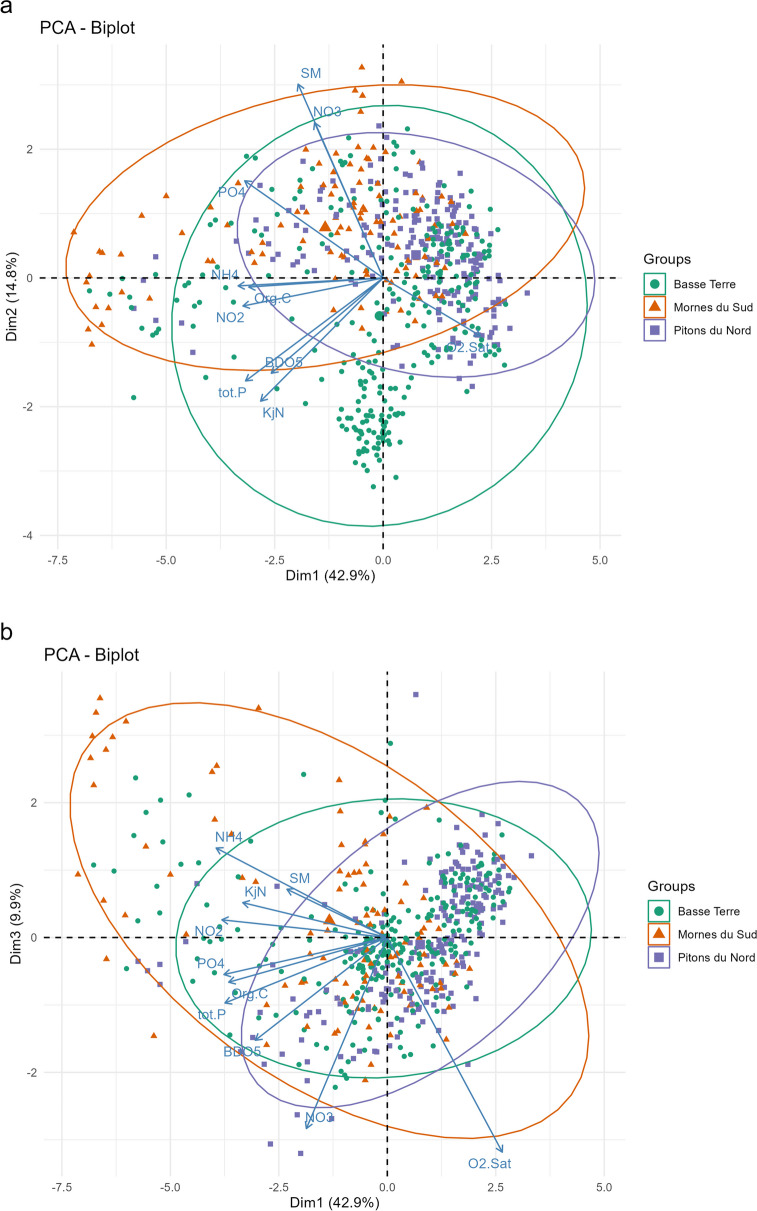
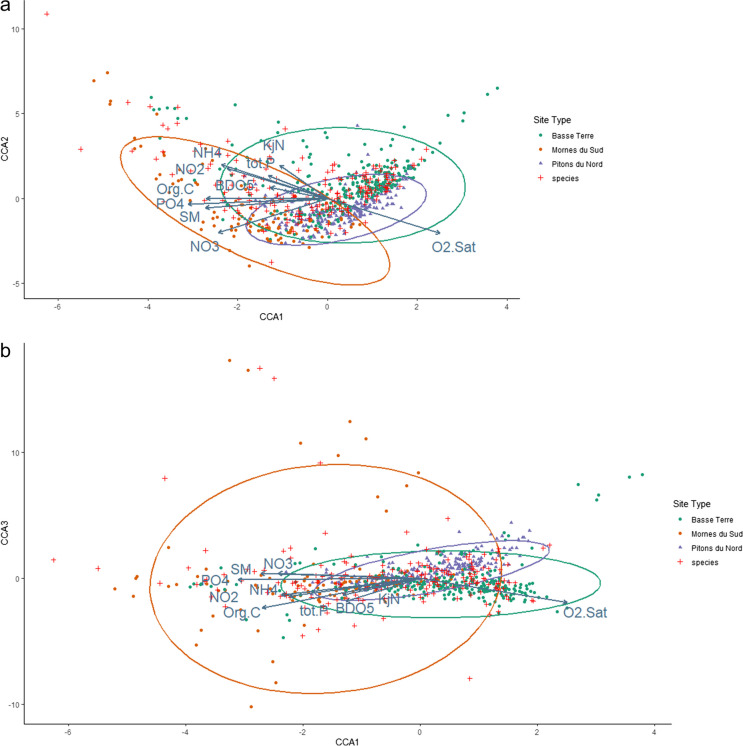
Only the first three axes of PCA (Fig. 2a and b) and CCA (Fig. 3) analyses were considered, as the amount of information carried by other dimensions was negligible (less than 10%).
Fig. 2.
Correlation plots of the first three PCA components: (a) axes 1 and 2, (b) axes 1 and 3
Fig. 3.
Ordination plots of the three first CCA axes using WA scores (taxa scores): (a) axes 1 and 2, (b) axes 1 and 3
Concerning PCA, axis 1 explained nearly 43% of the total variance and axes 2 and 3 respectively 14.9% and 9.9% (Fig. 2). Oxygen saturation, primarily driven by the third axis (42%), appeared opposed to the other variables. Axes 1 and 2 captured most other variable variances except NO3, rather linked to the second and third axes (Table 2).
Table 2.
Variable contributions on PCA axis
| Parameter | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| Org.C | 12.39 | 0.12 | 1.55 |
| BDO5 | 8.63 | 8.00 | 9.62 |
| SM | 4.87 | 34.05 | 2.13 |
| NH4 | 14.47 | 0.09 | 7.27 |
| KjN | 10.36 | 13.14 | 0.72 |
| NO2 | 13.49 | 0.84 | 0.39 |
| NO3 | 3.13 | 23.10 | 30.49 |
| PO4 | 13.05 | 9.24 | 1.29 |
| tot.P | 13.07 | 8.82 | 4.53 |
| O2.Sat | 6.53 | 2.58 | 42.02 |
Concerning the CCA, axis 1 carries the most important part of the variance, i.e., 40.3%, and axes 2 and 3 respectively 17.6% and 11.2% (Fig. 3). The total constrained inertia was 7.2, in line with the classical values obtained when working on diatom flora (Vyverman et al., 2007). The ANOVA p-value was < 0.001, indicating a significant effect of the environmental conditions on the communities. Again, oxygen saturation was opposed to all other variables. Diatom communities appeared significantly driven by variables linked to eutrophication and organic pollution. The importance of O2.Sat in the spatial distribution of the data reflects the geographical influence of hydroecoregions. Samples from Pitons du Nord for example, characterized by prominent mountains and fast-flowing streams, present high levels of O2.Sat and are consequently grouped in the right part of axis 1 on the CCA plots.
Ecological profiles settlement
The hierarchical classification of the MAG scores resulted in five distinct water quality classes (Table 3).
Table 3.
Water quality classes estimated from MAG scores
| Class | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Quality level | Very bad | Bad | Medium | Good | Very good |
| MAG score | (0.182–0.611] | (0.611–0.903] | (0.903–1.11] | (1.11–1.3] | (1.3–1.94] |
| Number of samples | 62 | 101 | 183 | 159 | 102 |
Forty-four percent of the samples were assigned to the good or very good quality classes, 30% to the medium quality class, and 26% to the bad and very bad quality classes. Environmental conditions in each quality class are given in Table 4. The statistical Wilcoxon pairwise comparison results between classes are available in Appendix 3.
Table 4.
Environmental conditions in the 5 quality classes ([minimum, maximum]; median)
| Group | O2.Sat | SM | tot.P | PO4 | Org.C | BDO5 | KjN | NH4 | NO2 | NO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [2.19, 157.5]; 61.75 | [5.2, 184]; 30.5 | [0.04, 7.3]; 0.305 | [0.06, 16]; 0.48 | [1.4, 200]; 3.9 | [0.5, 410]; 1.7 | [0.167, 61.1]; 1.85 | [0.017, 67]; 0.77 | [0.01, 12]; 0.17 | [0.033, 25.5]; 1.85 |
| 2 | [24, 124.5]; 96 | [0.67, 246.8]; 20 | [0.007, 1.79]; 0.11 | [0.005, 1.36]; 0.17 | [0.6, 17.17]; 2.05 | [0.17, 19]; 0.9 | [0.13, 3]; 0.333 | [0.01, 3.6]; 0.02 | [0.007, 1]; 0.02 | [0.1, 12.9]; 3 |
| 3 | [47.8, 142.3]; 98.5 | [0.67, 289]; 5.4 | [0.007, 1.63]; 0.06 | [0, 0.27]; 0.03 | [0.4, 13.73]; 1.4 | [0.17, 7]; 0.9 | [0.13, 1.63]; 0.333 | [0.008, 0.96]; 0.02 | [0.007, 1.2]; 0.017 | [0.03, 14.3]; 0.77 |
| 4 | [31, 127]; 98.5 | [0.33, 53]; 5 | [0.007, 0.62]; 0.017 | [0, 1.29]; 0.03 | [0.1, 5.165]; 0.9 | [0.17, 4.2]; 0.5 | [0.13, 0.92]; 0.22 | [0.008, 0.14]; 0.017 | [0.007, 0.08]; 0.01 | [0.03, 14.3]; 0.41 |
| 5 | [8.06, 115.8]; 98.05 | [0.33, 52]; 6.05 | [0.007, 0.06]; 0.01 | [0, 0.1]; 0.02 | [0.067, 2.56]; 0.6 | [0.17, 1.97]; 0.17 | [0.13, 0.5]; 0.17 | [0.01, 0.19]; 0.02 | [0.007, 0.14]; 0.01 | [0.03, 1.8]; 0.33 |
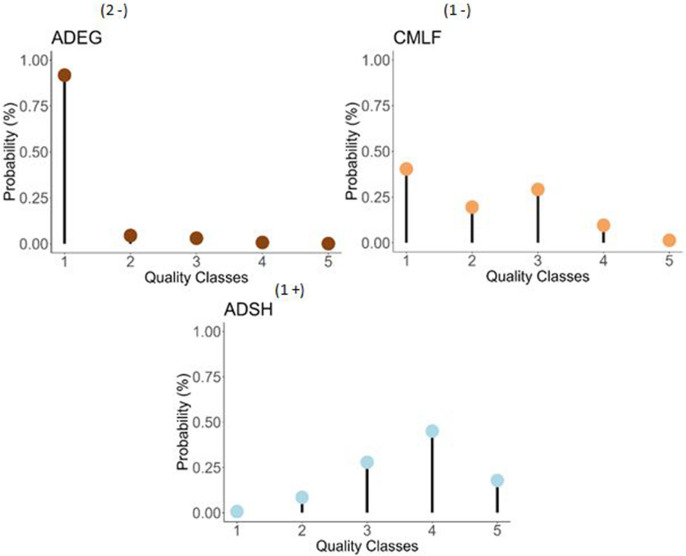
The a priori partition into five classes appeared relevant, with each class being significantly different from the others for at least one variable (see Appendix 3). Based on these results, ecological profiles were calculated for each taxa, which were subsequently classified into alert taxa (“2–” or “1 − ”) and “1 + ” taxa (Appendix 2). Finally, 131 taxa were associated with a “1 + ” profile, 25 with a “1 − ” profile, and 22 with a “2 − ” profile. Examples are given in Fig. 4: ADEG (Achnanthidium exiguum) is specific to very bad quality classes (“2 − ” profile) while CMLF (Craticula molestiformis) exhibits greater tolerance for classes 1 and 3 (“ − 1” profile), and ADSH (Achnanthidium subhudsonis) thrives in good conditions (“ + 1” profile).
Fig. 4.
Examples of ecological profiles (i.e., presence probability of the taxon considered among the five quality classes). ADEG, Achnanthidium exiguum (now Gogorevia exilis (Kützing), Kulikovskiy et al., 2020); ADSH, Achnanthidium subhudsonis (Hustedt) (Kobayasi, 2006); CMLF, Craticula molestiformis (Hustedt) (Mayama, 1999)
Index calculation
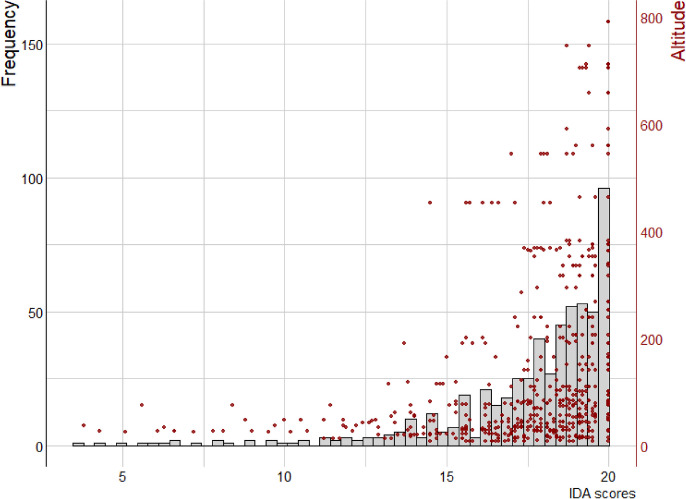
Globally, most IDA scores ranged from 15/20 to 20/20 and indicated good to very good water quality conditions, at least for the variables taken into account in the MAG; a few samples were characterized by low scores (Fig. 5). The average score across all the samples was 17.81/20, with a low standard deviation (2.93). No scores below 15–20 were found at sites above 200 m high.
Fig. 5.
Two-axis histogram showing IDA score’ frequency and altitude
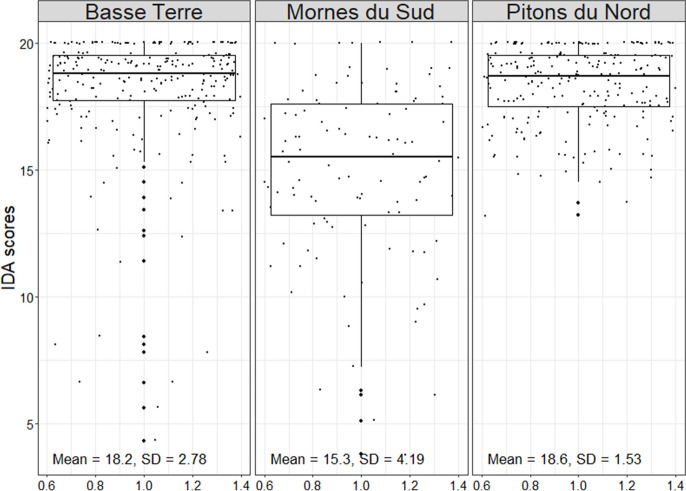
Consequently, global IDA scores were greater in the mountainous HER (Basse Terre in Guadeloupe and Pitons du Nord in Martinique) than in the lowlands (Mornes du Sud in Martinique) (Fig. 6). Median scores were significantly different according to the HER in Martinique (p-value < 2.2 × 10−16; Wilcoxon test). Samples with the lowest scores were all located downstream of wastewater treatment stations or slaughterhouses.
Fig. 6.
Boxplots of IDA scores in Guadeloupe (Basse Terre) and Martinique (Mornes du Sud, Pitons du Nord) hydroecoregions (HER)
Index validation
New MAG computation
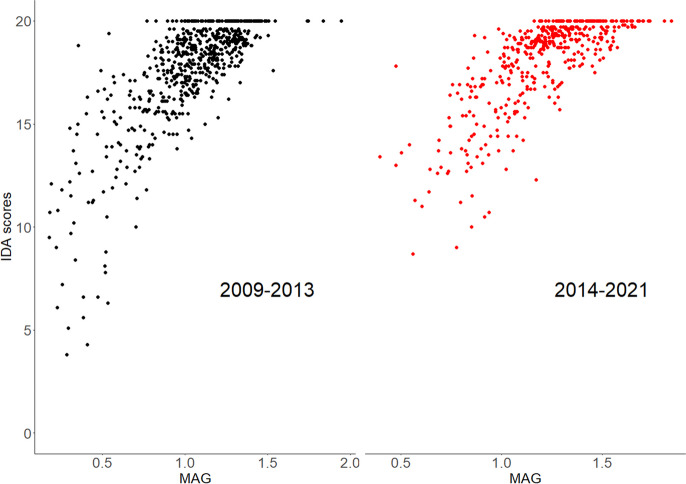
Strong positive and significant correlations between MAG and IDA scores were obtained for both the 2009–2013 (correlation coefficient = 0.74; p-value < 2.2 × 10−16) and the 2014 − 2022 (correlation coefficient = 0.75; p-value < 2.2 × 10−16) datasets (see Fig. 7).
Fig. 7.
Relationships between IDA and MAG scores for the 2009–2013 and 2014–2022 datasets
Pressure-impact approach
The best-selected model, with an AIC of 139.51, showed significant negative relationships between new IDA scores and suspended matter (SM), nitrates (NO3), ammonium (NH4), and dissolved organic carbon (Org.C) values (R2 = 0.51, p-value < 2.2 × 10−16). Based on this model, predicted IDA scores (from SM, NO3, NH4, and C.org) were well correlated with the observed IDA scores (Spearman correlation coefficient = 0.48, p-value < 2.2 × 10−16).
Discussion
European overseas regions such as the Caribbean islands have been widely overlooked until recently because of their particular bioclimatic and geomorphological conditions (Dedieu et al., 2014). This paper introduces the West Indies Diatom Index (IDA) which meets WFD requirements, addressing the region’s need for a suitable index to monitor aquatic ecosystem health.
Through multivariate analyses, we identified the key abiotic factors influencing diatom communities in Martinique and Guadeloupe. Contributive taxa were assigned to a specific ecological profile, based on their presence probability along a pressure gradient. Three types of profiles were determined: “2 − ” for most tolerant taxa occurring in most altered conditions, “1 − ”, and “1 + ” for sensitive taxa occurring in good water quality sites.
Utilizing taxon abundance data and ecological profiles from the samples, we calculated the West Indies Diatom Index and verified its effectiveness using data from 2014 to 2022 as a validation dataset.
Index accuracy
Results revealed a globally good water quality for most water bodies (about 88% of the samples). A posteriori validation of this index demonstrated however its ability to reveal water quality loss due to an overload of nutrients or organic matter of anthropogenic origin. In freshwater biomonitoring, diatom-based indices are commonly used to capture species and community responses to environmental conditions, particularly eutrophication and organic pollution (Passy & Bode, 2004). Changes in diatom communities have been proven to be strongly linked to nutrient loads, as nutrient enrichment benefits the colonization of water bodies by generalist taxa and poor competitors for resources (Leboucher et al., 2019). As such, generalist taxa are found worldwide where eutrophication occurs and contribute as key tolerant species to any diatom-based index, in continents or islands. Thus, among taxa classified as “2 − ” or “1 − ” in IDA, some present a typical profile of tolerant species according to the diatom-based index used in continental France (BDI, Biological Diatom Index, Coste et al., 2009) such as Nitzschia amphibia (Geitler, 1969), Sellaphora seminulum (Wetzel et al., 2015), or Eolimna subminuscula (now Craticula subminuscula, Wetzel et al., 2015) among others.
At the same time, the IDA is able to capture local specificities that indices such as the BDI cannot, firstly concerning organic matter, which can have two different origins in the rivers of Martinique and Guadeloupe: anthropogenic and natural. In fact, rivers are often rich in plant litter or fruit, which leads to significant levels of organic matter and, consequently, to a high abundance of taxa such as Fistulifera saprofila (FSAP). This species is considered by the BDI to be one of the indicator species for poor water quality and its high abundance leads to very low index values. IDA, however, does not classify this species as an alert taxon because FSAP is often associated with natural organic matter enrichment. This avoids inappropriate downgrading of very good quality sites. Secondly, given the high number of endemic species in the Caribbean islands, assessing the ecological status of rivers in Martinique and Guadeloupe using BDI is irrelevant because too few taxa are included in the calculation of the score. In fact, the BDI scores for the samples in our dataset were based on an average of only 48% (min = 5.5, max = 100, median = 48) of the taxa from the floristic lists against 99% for IDA (min = 60, max = 100, median = 100).
The main challenge to settle IDA arose from the very particular climate and geomorphological conditions of the West Indies, characterized by a lack of intermediate situations along abiotic gradients, most of which have a near-binary distribution between low and maximal alteration (Table 1). For example, IDA score differences between the two HERs from Martinique are directly linked to their hydrodynamic natures, raising a subsequent proposition of distinct good ecological status thresholds (JORF, 2023). Because most rivers are torrential systems with strong currents and steep slopes, pollution is directly driven downstream. If the majority of existing bioindicators are calculated as an abundance-weighted average of every taxon ecological profile from a sample, this method is far from optimal for datasets showing these specific environmental conditions. In such cases, identification of alert taxa seems more appropriate than considering diatom communities as a whole (Carayon et al., 2020). The use of alert taxa, to which the index formula also gives special weight, increases the alteration signal, making it easier to detect. All samples from sites downstream of sewage treatment plants, farms, or distilleries had low IDA scores. This makes sense, given that subsequent index validations with new data collected between 2014 and 2022 confirmed the significant relationship between anthropogenic variables that contribute to the MAG (in particular suspended matter, nitrates, ammonium, and dissolved organic carbon) and IDA scores.
Future developments
A need for new data…
It is evident that because of the lack of data in the initial dataset used to settle IDA, taxa profiles’ over- or underestimation may have occurred, particularly for taxa that were recorded less than 10 times in our dataset. Furthermore, floristic and physicochemical samples were not sufficiently frequent in some years, and were sometimes not temporally synchronized. New data that will be collected in the coming years must be used to strengthen the robustness of diatom species profiles and consequently, to facilitate the detection of long-term trends and changes in aquatic ecosystems in the West Indies.
… and complementary approaches
We believe that the particular geomorphology of Martinique and Guadeloupe makes it difficult to identify relationships between abiotic variables that are routinely measured and diatom community composition. In this context, how can the assessment of the river ecological status be facilitated?
First, microhabitats should be well-documented. The torrential nature of rivers leads to a mosaic of local conditions where contrasting current speed, light, and temperature can significantly impact diatom community composition (Jamoneau et al., 2022). Investigation of these particular microhabitats, together with a thorough description of the geological substrate and the riparian vegetation, could help improve our knowledge of the relationships between abiotic conditions and diatom species on islands, and design dedicated sampling protocols.
Second, the Martinique and Guadeloupe rivers flow directly into the surrounding ocean, making coastal ecosystems natural reservoirs of all types of pollution generated upstream on the islands. These coastal zones are monitored by the WFD (based on biological elements such as macroinvertebrates, physicochemical data, and specific pollutants including chlordecone). Therefore, it is particularly relevant in such an island context to take a close look at these assessments, as they shed light on the continental river status. In particular, Desrosiers et al. (2013) mentioned that coastal benthic diatoms could consist in a powerful water quality assessment tool, as they might be directly and strongly affected by continental water quality changes.
Conclusion
IDA fulfills the WFD requirements of considering the abundance and sensitivity of taxa to pollution for the evaluation of river ecological status. Considering that the fast-flowing nature of the rivers makes any index based on macrophytes inapplicable, IDA is a key element for aquatic system monitoring in the West Indies, complementary to the macroinvertebrate-based index (Touron-Poncet et al., 2014).
Nevertheless, further development is required to enhance IDA’s efficiency. Prior studies must include better taxonomic knowledge of diatoms, an improvement in the periodicity and synchronicity of sampling surveys, larger datasets, and a deeper investigation of microhabitats. These improvements will undoubtedly strengthen the ecological profiles of taxa and the robustness of ecological status assessments derived from IDA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1. List of the sites sampled from 2009 to 2013
Appendix 2. List of the 178 taxa contributing to the development of the IDA, with corresponding ecological profiles
Appendix 3. Statistical Wilcoxon pairwise comparisons results between HCPC classes
Appendix 4. Workflow of the methodology
Acknowledgements
We warmly thank the French Biodiversity Agency (OFB) and the West Indies Water and Regional Departments for data sharing and data collection. We also thank the reviewers for their helpful comments on this work.
Author contribution
All authors participated in designing the study and developing aims and research questions. J.G., F.D., L. H. and S.B. designed methodology, extracted data and made the analyses. L.H. led the writing of the manuscript supported by J.T.R. All authors contributed critically to the drafts, contributed to the final version of the manuscript, and gave final approval for publication.
Funding
The research leading to these results received funding from the French Biodiversity Agency and the West Indies Water and Regional Departments.
Data availability
Data that support the findings of this study have been deposited in the “recherche.data.gouv” website:
https://doi.org/10.57745/AEGOGN (physicochemical data)
https://doi.org/10.57745/WSUMAV (biological data)
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AFNOR. (2016). Qualité écologique des milieux aquatiques : norme NF T 90-354 Échantillonnage, traitement et analyse de diatomées benthiques en cours d’eau et canaux, pp.111. ⟨hal-02603502⟩
- Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control,19(6), 716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Bernadet, C., Touron-Poncet, H., Desrosiers, C., Compin, A., Bargier, N., & Céréghino, R. (2013). Invertebrate distribution patterns and river typology for the implementation of the water framework directive in Martinique, French Lesser Antilles. Knowledge and Management of Aquatic Ecosystems,408, 01. 10.1051/kmae/2013036 [DOI] [Google Scholar]
- Bourrelly, P., Manguin, E. (1952). Algues d’eau douce de la Guadeloupe et dépendances. Centre National de la Recherche Scientifique (pp. 281). Société d’Edition d’Enseignement Supérieur
- Carayon, D., Eulin-Garrigue, A., Vigouroux, R., & Delmas, F. (2019). Assessment of ecological status of French Guiana’s rivers: The French Guiana Diatom Index (FGDI).
- Carayon, D., Eulin-Garrigue, A., Vigouroux, R., & Delmas, F. (2020). A new multimetric index for the evaluation of water ecological quality of French Guiana streams based on benthic diatoms. Ecological Indicators,113, 106248. 10.1016/j.ecolind.2020.106248 [DOI] [Google Scholar]
- Chauvin, C., Feret, T., Loriot, S., Bottin, M., Boutry, S., Coste, M., & Delmas, F. (2015). Arrêté national du 27 juillet 2015 relatif aux méthodes et critères d’évaluation de l’état écologique, de l’état chimique et du potentiel écologique des eaux de surface (Journal Officiel de la République Française du 28 Août 2015). Extraits relatifs aux évolutions de contenu concernant les compartiments végétaux des cours d’eau de France métropolitaine et des DOM.
- Chessel, D., Dufour, A., Dray, S., Lobry, J. R., Ollier, S., Pavoine, S., & Thioulouse, J. (1995). ADE-4: Ordination sous contraintes. Universite Lyon, 1.
- Coste, M., Boutry, S., Tison-Rosebery, J., & Delmas, F. (2009). Improvements of the Biological Diatom Index (BDI): Description and efficiency of the new version (BDI-2006). Ecological Indicators,9(4), 621–650. 10.1016/j.ecolind.2008.06.003 [DOI] [Google Scholar]
- Coste, M., & Dauta, A. (1997). Premier inventaire des diatomées des eaux douces de Martinique. Essai de caractérisation de la qualité des eaux en milieu subtropical. Cryptogamie Algologie,18(1), 73–74. [Google Scholar]
- CEN (Comité Européen de Normalisation). (2003). Water quality - Guidance standard for the routine sampling and pretreatment of benthic diatoms from rivers. EN 13946:2003. Comité Européen de Normalisation, Geneva, Switzerland.
- Dedieu, N., Allard, L., Vigouroux, R., Brosse, S., & Céréghino, R. (2014). Physical habitat and water chemistry changes induced by logging and gold mining in French Guiana streams. Knowledge and Management of Aquatic Ecosystems,415, 02. 10.1051/kmae/2014026 [DOI] [Google Scholar]
- Desrosiers, C., Leflaive, J., Eulin, A., & Ten-Hage, L. (2013). Steps towards the development of a Benthic Diatom Index in Reef environment. Phycologia,52(4), 24. [Google Scholar]
- De Winter, J. C., Gosling, S. D., & Potter, J. (2016). Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychological Methods,21(3), 273. 10.1037/met0000079 [DOI] [PubMed] [Google Scholar]
- Dixon, P. (2003). VEGAN, a package of R functions for community ecology. Journal of Vegetation Science,14(6), 927–930. 10.1111/j.1654-1103.2003.tb02228.x [DOI] [Google Scholar]
- Dromard, C. R., Bodiguel, X., Lemoine, S., Bouchon-Navaro, Y., Reynal, L., Thouard, E., & Bouchon, C. (2016). Assessment of the Contamination of Marine Fauna by Chlordecone in Guadeloupe and Martinique (Lesser Antilles). Environmental Science and Pollution Research,23, 73–80. 10.1007/s11356-015-4732-z [DOI] [PubMed] [Google Scholar]
- Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A.-H., Soto, D., Stiassny, M. L. J., & Sullivan, C. A. (2006). Freshwater biodiversity: Importance, threats, status and conservation challenges. Biological Reviews,81(2), 163–182. 10.1017/S1464793105006950 [DOI] [PubMed] [Google Scholar]
- Eulin Garrigue A., Lefrançois E., Tison-Rosebery J., Coste M., Delmas F. (2017). Flore Diatomées des Antilles Françaises : volume introductif + 5 volumes. Observatoire de l’Eau de la Martinique.
- European Union. (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Official Journal of European Communities, European Commission.
- Geitler, L. (1969). Die Auxosporenbildung von Nitzschia amphibia. Oesterreichische botanische Zeitschrift,117, 404–410. 10.1007/BF01377798 [DOI] [Google Scholar]
- Girden, E. R. (1992). ANOVA: Repeated Measures. Sage. 10.4135/9781412983419 [Google Scholar]
- Graham, A. (2003). Geohistory models and Cenozoic paleoenvironments of the Caribbean region. Systematic Botany,28(2), 378–386. [Google Scholar]
- Husson, F., Josse, J., & Pagès, J. (2010). Analyse de données avec R-Complémentarité des méthodes d'analyse factorielle et de classification. In 42èmes Journées de Statistique (p. nc)
- Jamoneau, A., Soininen, J., Tison-Rosebery, J., Boutry, S., Budnick, W., He, S., He, S., Marquié, J., Jyrkänkallio-Mikkola, J., Pajunen, V., Teittinen, A., Tupola, V., Wang, B., Wang, J., Blanco, S., Borrini, A., Cantonati, M., Valente, A. C., Delgado, C., … Passy, S. I. (2022). Stream diatom biodiversity in islands and continents – a global perspective on the effects of area, isolation and the environment. Journal of Biogeography,00, 1–13. [Google Scholar]
- JORF (Journal Officiel de la République Française). (2023). Arrêté du 27 juillet 2015 modifiant l'arrêté du 25 janvier 2010 relatif aux méthodes et critères d'évaluation de l'état écologique, de l'état chimique et du potentiel écologique des eaux de surface pris en application des articles R. 212-10, R. 212-11 et R. 212-18 du code de l'environnement. ELI: https://www.legifrance.gouv.fr/eli/arrete/2015/7/27/
- Kobayasi, H. (2006). H. Kobayasi’s Atlas of Japanese Diatoms based on electron microscopy. Uchida Rokakuho.
- Kulikovskiy, M., Maltsev, Y., Glushchenko, A., Kuznetsova, I., Kapustin, D., Gusev, E., Lange-Bertalot, H., Genkal, S., & Kociolek, J. P. (2020). Gogorevia, a new monoraphid diatom genus for Achnanthes exigua and allied taxa (Achnanthidiaceae) described on the basis of an integrated molecular and morphological approach. Journal of Phycology,56(6), 1601–1613. 10.1111/jpy.13064 [DOI] [PubMed] [Google Scholar]
- Lavoie, I., Dillon, P. J., & Campeau, S. (2009). The effect of excluding diatom taxa and reducing taxonomic resolution on multivariate analyses and stream bioassessment. Ecological Indicators,9(2), 213–225. 10.1016/j.ecolind.2008.04.003 [DOI] [Google Scholar]
- Leboucher, T., Budnick, W. R., Passy, S. I., Boutry, S., Jamoneau, A., Soininen, J., Vyverman, W., & Tison-Rosebery, J. (2019). Diatom β-diversity in streams increases with spatial scale and decreases with nutrient enrichment across regional to sub-continental scales. Journal of Biogeography,46(4), 734–744. 10.1111/jbi.13517 [DOI] [Google Scholar]
- Lefrançois, E., Eulin, A., Guéguen, J., Coste, M., Delmas, F., & Monnier, O. (2019). Guide pour la mise en oeuvre d’indices biologiques en outre-mer. L’indice diatomique antillais – IDA. Agence française pour la biodiversité, collection Guides et protocoles (p. 68) https://www.documentation.eauetbiodiversite.fr/notice/guide-pour-la-mise-en-oeuvre-d-indicesbiologiques-en-outre-mer-l-indice-diatomique-antillais-ida0. [Google Scholar]
- Lugo, A. E., Helmer, E. H., & Valentín, E. S. (2012). Caribbean landscapes and their biodiversity. Interciencia,37(9), 705–710. [Google Scholar]
- Mayama, S. (1999). Taxonomic revisions to the differentiating diatom groups for water quality evaluation and some comments for taxa with new designations. Diatom, the Japanese Journal of Diatomology,15, 1–9. [Google Scholar]
- Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature,403(6772), 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Passy, S. I., & Bode, R. W. (2004). Diatom model affinity (DMA), a new index for water quality assessment. Hydrobiologia,524, 241–252. 10.1023/B:HYDR.0000036143.60578.e0 [DOI] [Google Scholar]
- R Development Core Team. (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
- Riani, M., Atkinson, A. C., & Corbellini, A. (2023). Automatic robust Box-Cox and extended Yeo-Johnson transformations in regression. Statistical Methods and Applications,32, 75–102. 10.1007/s10260-022-00640-7 [DOI] [Google Scholar]
- Ripley, B., Venables, B., Bates, D. M., Hornik, K., Gebhardt, A., Firth, D., & Ripley, M. B. (2013). Package ‘mass.’ Cran r,538, 113–120. [Google Scholar]
- Soininen, J. (2007). Environmental and spatial control of freshwater diatoms—a review. Diatom Research,22(2), 473–490. 10.1080/0269249X.2007.9705724 [DOI] [Google Scholar]
- Ter Braak, C. J. F., & Prentice, I. C. (2004). A theory of gradient analysis. Advances in Ecological Research,34, 235–282. 10.1016/S0065-2504(03)34003-6 [DOI] [Google Scholar]
- Ter Braak, C. J., & Verdonschot, P. F. (1995). Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences,57, 255–289. 10.1007/BF00877430 [DOI] [Google Scholar]
- Touron-Poncet, H., Bernadet, C., Compin, A., Bargier, N., & Cereghino, R. (2014). Implementing the Water Framework Directive in overseas Europe: A multimetric index for river bioassessment in Carribean islands. Limnologica,47, 34–43. 10.1016/j.limno.2014.04.002 [DOI] [Google Scholar]
- Tudesque, L., & Ector, L. (2002). Pré-atlas iconographique des rivières de la Guadeloupe (p. 78). DIREN Guadeloupe. [Google Scholar]
- Vyverman, W., Verleyen, E., Sabbe, K., Vanhoutte, K., Sterken, M., Hodgson, D. A., Mann, D. G., Juggins, S., Van De Vijver, B., Jones, V., Flower, R., Roberts, D., Hepurnov, V. A. C., Kilroy, A., Vanormelingen, P., & Wever, A. D. (2007). Historical processes constrain patterns in global diatom diversity. Ecology,88(8), 1924–1931. 10.1890/06-1564.1 [DOI] [PubMed] [Google Scholar]
- Weisstein, E. W. (2004). Bonferroni correction. https://mathworld.wolfram.com. Accessed 12 Apr 2024
- Wetzel, C., Ector, L., Van de Vijver, B., Compere, P., & Mann, D. G. (2015). Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea/Czech Phycological Society.-Praha, Czech Republic, 2007, currens,15(2), 203–234. [Google Scholar]
- Wilcox, R. R. (2001). Pairwise comparisons of trimmed means for two or more groups. Psychometrika,66, 343–356. 10.1007/BF02294438 [DOI] [Google Scholar]
- Wold, S., Esbensen, K., & Geladi, P. (1987). Principal component analysis. Chemometrics and Intelligent Laboratory Systems,2(1–3), 37–52. 10.1016/0169-7439(87)80084-9 [DOI] [Google Scholar]
- Yeo, I. K., & Johnson, R. A. (2000). A new family of power transformations to improve normality or symmetry. Biometrika,87(4), 954–959. 10.1093/biomet/87.4.954 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. List of the sites sampled from 2009 to 2013
Appendix 2. List of the 178 taxa contributing to the development of the IDA, with corresponding ecological profiles
Appendix 3. Statistical Wilcoxon pairwise comparisons results between HCPC classes
Appendix 4. Workflow of the methodology
Data Availability Statement
Data that support the findings of this study have been deposited in the “recherche.data.gouv” website:
https://doi.org/10.57745/AEGOGN (physicochemical data)
https://doi.org/10.57745/WSUMAV (biological data)