ABSTRACT
Reconstruction of bone defects or fractures caused by ageing, trauma and tumour resection is still a great challenge in clinical treatment. Although autologous bone graft is considered as gold standard, the source of natural bone is limited. In recent years, regenerative therapy based on bioactive materials has been proposed for bone reconstruction. Specially, numerous studies have indicated that bioactive ceramics including silicate and phosphate bioceramics exhibit excellent osteoinductivity and osteoconductivity, further promote bone regeneration. In addition, magnesium (Mg) element, as an indispensable mineral element, plays a vital role in promoting bone mineralisation and formation. In this review, different types of Mg-containing bioceramics including Mg-containing calcium phosphate-based bioceramics (such as Mg-hydroxyapatite, Mg-biphasic calcium phosphate), Mg-containing calcium silicate-based bioceramics (such as Mg2SiO4, Ca2MgSi2O7 and Mg-doped bioglass), Mg-based biocements, Mg-containing metal/polymer-bioceramic composites were systematacially summarised. Additionally, the fabrication technologies and their materiobiological effects were deeply discussed. Clinical applications and perspectives of magnesium-containing bioceramics for bone repair are highlighted. Overall, Mg-containing bioceramics are regarded as regenerative therapy with their optimised performance. Furthermore, more in-depth two-way researches on their performance and structure are essential to satisfy their clinical needs.
Keywords: bioactive ions, bioceramics, bone repair, magnesium, osteoconductivity
Introduction
Bone is a hard connective tissue that serves as an important component of the human body to support the body and protect various internal organs.1 Despite the potential of bone remodelling and repair, large bone defects produced by trauma and tumour resection are frequently difficult to self-heal.2 The goal of bone defect treatment is to restore structural integrity and their biological functions via a combination of surgical intervention, medication, and rehabilitation. Autologous bone grafting, as the gold standard, is the most effective method of repairing large bone defects, but there are many drawbacks, including the need for opening a second operative zone and the limited amount of available bone.3 There is a growing demand for bone defect repair, and the superior properties of bioceramic materials make them indispensable in the field of bone tissue replacement.
Conventional synthetic materials for bone repair aim to match the material’s physical and chemical properties to those of the bone being restored while minimising harmful effects in the body. While novel bioceramics possess excellent biocompatibility, bioactive traits for bonding with bone, and potential antibacterial properties, rendering them suitable as scaffolding materials of bone regeneration, as well as aiding in the healing and recovery of impaired or absent bone tissue.4, 5 An optimal bioceramic for bone repair should promote new bone formation, while undergoing biodegradation without releasing harmful substances or inducing an immune system response. These characteristics not only facilitate successful restoration of compromised function but also reduce the need for subsequent operations to remove the implanted material. In conclusion, the development of bioceramic materials for bone repair and regeneration requires careful consideration of biocompatibility, bioactivity, adequate mechanical properties, and appropriate degradation rate. Meeting these requirements is essential to ensure the restoration of shape of bone defect and the facilitation of cell proliferation and migration to achieve new bone formation.
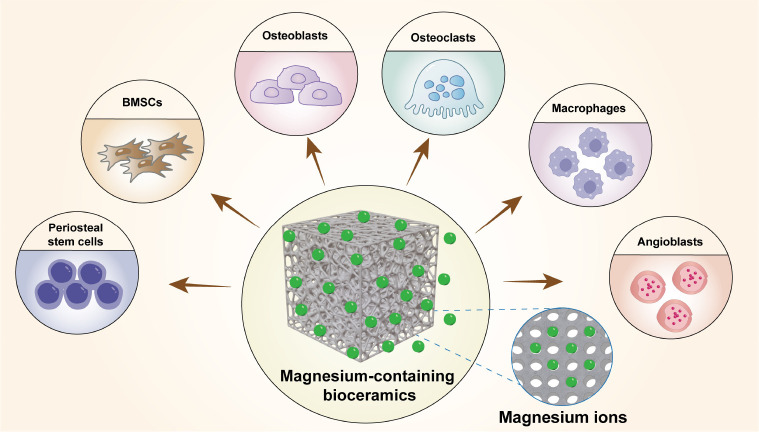
It is reported that the incorporation of specific ions and the optimisation of physicochemical properties in bioceramic materials can enhance their osteoinductive potential.6 Among these elements, magnesium (Mg) plays a crucial role in various physiological processes of the human body and is essential for bone metabolism.7 Mg is an indispensable mineral element intricately involved in bone repair, exerting a pivotal influence on various physiological processes, including bone remodelling, immune modulation, angiogenesis, and mineralisation (Figure 1). Mesenchymal stem cells (MSCs) play a crucial role in bone regeneration, as they can differentiate into different types of cells, such as osteoblasts, involved in bone tissue regeneration. Mg regulates the proliferation and osteogenic differentiation of MSCs.8, 9 Additionally, Mg significantly impacts on the activity of osteoblasts and osteoclasts. Osteoblasts are responsible for the synthesis, secretion, and mineralisation of bone matrix. Insufficient levels of Mg have been associated with impaired bone formation and dysregulated bone resorption.10 Elevated Mg levels have shown therapeutic potential in ameliorating osteoporosis.11 Mg-containing biomaterials influence endogenous periosteal cell-induced cortical bone growth by releasing Mg.12
Figure 1. Cellular biological behaviour of magnesium ions released from magnesium-containing bioceramics. Created with Adobe Illustrator 2022. BMSCs: bone marrow stem cells.
Mg also plays a vital role in regulating a range of immune responses after the implantation of biomaterial. Mg deficiency has been proved to impair the functionality of adaptive immune cells while concurrently activating the innate immune system and promoting inflammatory processes.13 It has been reported that Mg2+ can modulate cellular crosstalk between macrophages and MSCs by synergising with ferric ions (Fe3+), thereby reducing macrophage secretion of pro-inflammatory factors and promoting MSC osteogenic differentiation.14 However, recent studies have shown that the influence of Mg2+ on bone regeneration is dose- and time-dependent. During the early stages of repair, Mg2+ stimulates mononuclear macrophages to support bone formation, while in the later stages of remodelling, prolonged exposure to high levels of Mg2+ in the later stages of remodelling can lead to excessive activation of nuclear factor kappa B in macrophages and an upregulation of osteoclast activity.10 Moreover, excessive concentrations of Mg2+ can inhibit collagen and calcium phosphate (CaP) formation, thereby reducing the degree of hydroxyapatite (HA) crystallisation and adversely affecting mineralisation.15
Mg has been utilized as a dopant in various bioceramic materials to enhance mechanical strength and biocompatibility. An increasing number of studies are focused on Mg-containing bioceramic materials and their effects on bone remodelling and repair. This review examines the progress of Mg-containing bioceramics for bone regeneration, including fabrication methods, composite types and their biological functions, and clinical applications via using specific keywords. Initially, relevant keywords related to Mg containing bioceramics were searched in Web of Science. Subsequently, keywords associated with bone repair were used to search for related articles. All terms were analyzed using medical subject headings (MeSH), and the final list was complied and modified in Table 1.
Table 1. Search terms in the review.
| Primary keywords | Secondary keywords |
|---|---|
| Magnesium | Bone |
| Magnesium oxide | Bone regeneration |
| MgO | Osteogenesis |
| Magnesium peroxide | Bone tissue engineering |
| Hydroxyapatite | |
| HA | |
| Beta-tricalcium phosphate | |
| TCP | |
| Magnesium phosphate | |
| Magnesium phosphate cement | |
| Akermanite | |
| AKT | |
| Magnesium silicate | |
| Forsterite | |
| Magnesium alloy | |
| Magnesium-based |
Fabrication Technologies of Magnesium-Containing Bioceramics
The preparation of ceramic powders involves various methods such as solid phase, combustion, and wet chemical methods. Among the wet chemical methods, sol-gel, coprecipitation, hydrothermal synthesis, and microemulsion techniques are prominent. The sol-gel method utilises compounds containing highly active chemical components as precursors, which are uniformly mixed in a liquid phase. A stable transparent sol system forms via hydrolysis and condensation reactions, and then slowly polymerises to create a gel. Finally, the gel is dried and cured by sintering to produce powders.16 In the coprecipitation method, inorganic salt components containing cations are dissolved in a solvent, and the cations are stirred to reach a supersaturated state, leading to the formation of insoluble precipitation. After washing and calcining, powder is obtained.17 Hydrothermal synthesis involves single-phase or multiphase reactions of a solvent in a closed system with a pressure exceeding 1 atm (1 atm = 101,325 Pa) above room temperature.18 The microemulsion method entails the formation of an emulsion solution of two immiscible liquids under the action of surfactants, followed by powder acquisition through nucleation and heat treatment. This method lowers the sintering temperature and yields higher purity powder.19 The spray pyrolysis method employs a spray generator to convert a precursor solution containing the target solute into small droplets, which chemically decompose into powder under high temperature conditions.20 Similar to spray pyrolysis, the spray and microemulsion method differ in the absence of a chemical decomposition process and utilize lower drying temperatures.21 Flame‐spray pyrolysis involves dissolving the precursor of the desired compound in a flammable solvent, igniting it, and complete combustion resulting in powder formation.22 The freeze-drying method solidifies the liquid medium in the ceramic slurry at low temperatures, followed by crystal growth under specific conditions and sublimation under low pressure or vacuum to create pores with crystal morphology. Eventually, porous ceramics with a unique microstructure are obtained through high-temperature treatment.23
After obtaining ceramic powders, it undergoes various processing to be applied in different clinical application scenarios. The powder is mixed with binding agent to create printing ink and then constructed porous supporting scaffolds with load-bearing capablities via three-dimensional (3D) printing technology.24, 25 Additionally, powder is mixed with polycaprolactone (PCL) and other materials to produce an electrospun film utilising electrospinning technology, delaying ceramic corrosion and facilitating slow release.12, 26 Combining ceramic material with the hydrogel increases fluidity, creating injectable material.27 The addition of pore-forming agents before ceramic firing enhances scaffold material porosity and strengthens cell adhesion.28
Composite Types and Materiobiological Effects of Magnesium-Containing Bioceramics
Bone tissue, a specialised and dynamic form of connective tissue, serves crucial functions such as providing structural support, protecting vital organs, maintaining mineral homeostasis, and facilitating haematopoiesis. It comprises various cellular components (e.g., osteoblasts, osteocytes, and osteoclasts) and extracellular matrix components that work synergistically to maintain bone integrity and strength. The main components of the bone extracellular matrix include type I collagen, along with various non-collagenous proteins, and inorganic mineral crystals. Bone remodelling, a dynamic process, involves the continuous turnover of bone tissue, intricately governed by interplay of diverse factors and mechanical stimuli.29
Bioceramic materials should be osteoinductive and biodegradable, enabling the restoration of bone defects while promoting osteogenic differentiation of MSCs and bone formation.4, 30 After implantation, bone tissue grows into the scaffold, accompanied by neovascularisation, while the implant device gradually degrades, leading to the formation of new bone tissue in the implanted area. Research on Mg-containing biomaterials for use in hard tissue implant devices has been extensive. Mg2+ has been shown to regulate cellular activity, enhance osteoblast proliferation and mineralisation, and improve endothelial cell angiogenic function and cell-material adhesion.31, 32 Mg2+ can replace calcium ions (Ca2+) in the crystalline structure of HA, enhancing the physical and bioactive properties of biomaterials.32 Mg-based bioceramic materials encompass a range of Mg compounds, including Mg-doped calcium and phosphate (e.g., Mg3(PO4)2), Mg-containing calcium silicate-based bioceramics (e.g., Mg2SiO4, Ca2MgSi2O7, and Mg-doped bioglass (BG)), magnesium oxide (MgO), magnesium hydroxide (Mg(OH)2), and other biocompatible ceramics.33 Mg-based bioceramics offer a combination of biodegradability, osteoconductivity, antibacterial properties, and mechanical compatibility, making them promising materials for various biomedical applications, especially in bone-related fields.34, 35
Mg-containing CaP based bioceramics
In recent years, the incorporation of Mg2+ into CaP has emerged as a promising method for producing orthopaedic bioceramics. These composites exhibit enhanced mechanical and tribological properties, including increased strength and toughness, reduced wear rate, and friction coefficient compared to pure CaP ceramics.36, 37
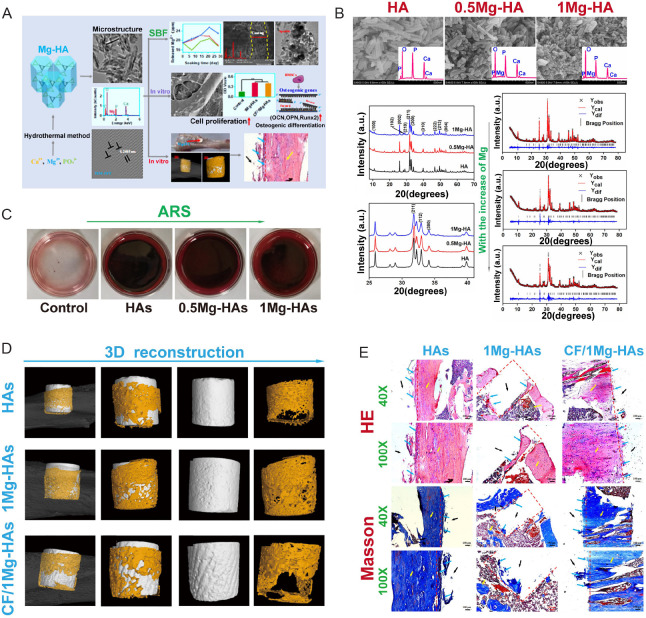
HA possesses a chemical formula of Ca10 (PO4)6 (OH)2 and exhibits a composition similar to that of natural bones and teeth, thereby demonstrating favourable biocompatibility. The advantageous biodegradability of HA contributes to an increase in the concentration of Ca2+ ion at the site of bone defects, promoting the creation of stronger bonds between the implant and the surrounding bone tissue. Furthermore, HA exhibits notable osteoconductivity, making it a viable candidate for incorporation into biodegradable polymer-based composites.38 However, HA is limited by its poor fracture toughness and brittleness. To address these concerns, strategies such as ion substitution of the HA structure or compounding with other materials have been explored to improve both the physical capabilities and biological compatibility of HA.39 The composition of Mg and HA, often in the forms of Mg-HA and MgO-HA, had become increasingly popular in the bone tissue engineering due to its chemical and biological advantages. Zhao et al.40 demonstrated the exceptional mechanical abilities and customized biological characteristics of carbon fibre-reinforced Mg-doped HA composite material as a potential load-bearing bone substitute and repair material (Figure 2). Coelho et al.41 developed an MgO-HA bone substitute and optimised its antibacterial and angiogenic function in bone regenerative applications. Their study further confirmed the antibacterial property of the material in preventing dental and orthopaedic infections from Staphylococcus aureus and Escherichia coli.42
Figure 2. Carbon-fibre reinforced Mg-doped HA composites promote bone regeneration. (A) Schematic illustration of preparation of CF/Mg-HAs composites and their biological functions. Data are expreesed as mean ± SD. (B) Characterization of Mg-HA. (C) ARS of Mg-HA. (D) Micro-CT of the HAs, 1Mg-HAs and CF/1Mg-HAs 4 weeks post-surgery. (E) HE and Masson staining of the rat tibial defect 4 weeks post-surgery. The red dashed line represents the defect area. The yellow arrow represents the host bone. The blue arrow represents the new bone. Scale bars: 200 μm. Reprinted with permission from Zhao et al.40 Copyright 2022, American Chemical Society. 0.5Mg-HAs: 5% Mg-doped hydroxyapatite; 1Mg-HAs: 10% Mg-doped hydroxyapatite; 3D: three-dimensional; a.u.: absorbance unit; ARS: atomic absorption spectrometer; BMSC: bone marrow mesenchymal stem cell; CF: carbon fibre; CF/1Mg-HAs: CF-reinforced 1Mg-HAs; CT: computed tomography; HA: hydroxyapatite; HE: haematoxylin and eosin; Mg: magnesium; OCN: osteopontin; OPN: osteocalcin; Runx2: runt-related transcription factor 2; SBF: simulated body fluid.
The addition of Mg2+ to biphasic calcium phosphate (BCP) materials yields favourable physicochemical properties compared to pure CaP ceramics. In vitro experiments indicate the bioactivity and non-toxicity of Mg-BCP to cells, and the successful implantation of Mg-BCP in vivo models provides further evidence of its biocompatibility and efficacy as a bone substitute.43 Frasnelli et al.44 introduced the effect of Mg2+ doping on beta-alpha phase transition in tricalcium phosphate (TCP) bioceramics, emphasising the contribution and promotion of Mg2+ doping to the spontaneous α→β reconversion upon fast cooling. In terms of 3D-printed practice of biomaterials, the Mg-doped CaP-based bioceramics also hve potential orthopaedic applications. In He et al.’s study,45 Mg-CaP composite bioceramic scaffolds based on Ca3Mg3(PO4)4 were manufactured using Mg3(PO4)2 and β-Ca3(PO4)2 as initial materials, with their structure formed through 3D printing technology. The Ca3Mg3(PO4)4-based bioceramic scaffolds showed adaptability as bone regeneration materials and to the needs of bone defects. Ge et al.46 introduced the physical features of the 3D-printed high-melting-point-difference MgO/CaP composite bioceramic scaffold based on Ca3(PO4)2 and MgO. In addition to emphasising the potential use of high-density composite ceramic scaffolds in the field of bone repair, they further found the bioceramic scaffolds with 80 wt% MgO exhibited the best mechanical performance. Zhang et al.47 showed the great potential as bone graft alternatives of 3D gel-printed porous Mg scaffold coated with dibasic CaP dihydrate. Besides, a latest work from Cao et al.48 generated several tilapia bone-derived Mg-rich CaP bioceramics, including HA, BCP, and commercial HA using the gradient thermal treatment approach. They discovered the specific processing temperatures for generation and confirmed the considerable quantities of Mg in both HA and BCP compared to commercial HA. As a result, the HA and BCP materials showed remarkable formation of bone-like apatite, stronger osteoconductive activity and nontoxicity.
Other studies have demonstrated various methods of using Mg CaP for bone regeneration, such as injectable cement,49, 50 Mg-releasing,51 and incorporating exogenous citrate.52 Additionally, Mg phosphate-based cements (MPCs) are notable for their effectiveness in promoting bone regrowth. Kanter et al.53 shown the bone regeneration potential of MgNH4PO4·6H2O cements in two different orthotopic ovine implantation models. Kaiser et al.54 found that MPCs fostered faster bone regeneration compared to CaP by reducing the powder-liquid ratio in cements containing struvite and K-struvite. Interestingly, Brückner et al.55 identified the potential of mineral MPCs as bone adhesives, using cements derived from farringtonite (Mg3(PO4)2).
Mg-containing calcium silicate-based bioceramics
In contrast to CaP based materials with minimal osteoinductive properties, silicon (Si) in Si-based materials actively triggers bone formation and participates in the initial mineralisation of the bone matrix.56 Si plays a crucial role in metabolic processes, affecting protein secretion, cell survival, and apoptosis.57 Additionally, Si fosters bone development, calcification, and regulates the production of type I collagen, demonstrating superior activity in promoting bone repair and regeneration.58 Silicate bioceramics have gained widespread use in bone regeneration due to their favourable properties. In this section, we would discuss the effects of Mg-containing calcium silicate (CaSi) based bioceramics including akermanite (Akt), forsterite and Mg-containing BG on bone regeneration.
Akt
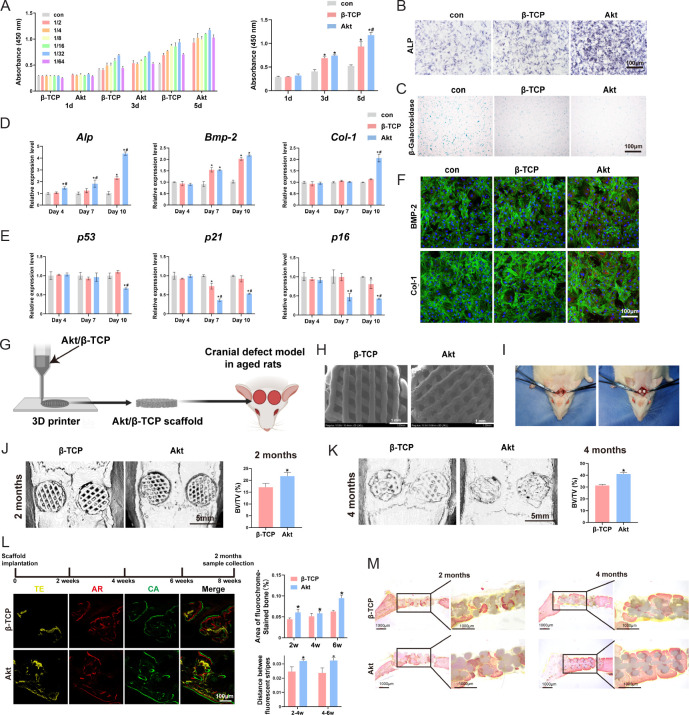
Akt, a CaSi mineral (Ca2MgSi2O7), exhibits biological activity and osteogenic properties, making it a promising material for diverse biomedical applications. Studies indicate that Akt is more biocompatible and degrades at a higher rate than β-TCP, thereby enhancing its potential for bone regeneration.59 Patients with osteoporosisoften experience a noticeable decline in bone mineral density and strength, increasing their risk of fracture. In such cases, Akt has been shown to increase the proliferation and osteogenic differentiation of bone marrow derived MSCs (BMSCs), while also suppressing osteoclastogenesis,60 offering a theoretical basis for its use in bone regeneration for osteoporotic patients. Our recent research has demonstrated that Akt promotes the proliferation and osteogenic differentiation of senescent BMSCs via the mitogen-activated protein kinase signalling pathway61 (Figure 3). Angiogenesis and osteogenesis mutually reinforce each other, promoting the formation of new bone. Vascular endothelial growth factor, a crucial angiogenic factor, is necessary for promoting endothelial progenitor cell proliferation and development, as well as promoting osteoblast chemotactic migration.62-64 Recent studies have indicated that Mg2+ and Si2+ stimulate vascular endothelial growth factor expression in human umbilical vein endothelial cells.63 Akt, which is rich in Mg (8.92 wt.%) and Si (20.6 wt.%),65 exhibits excellent osteogenic and angiogenic properties, making it a promising biomaterial for bone regeneration. In view of these points, Akt bioceramic promotes angiogenesis, which is essential for bone defect repair and regeneration processes.63, 66 Akt bioceramics also have garnered significant attention for their versatile applications in bone tissue engineering, including scaffold development, coatings over implants, and drug delivery systems.65, 67
Figure 3. Akt promote senescent bone regeneration in vitro and in vivo. (A–F) The effects of Akt onproliferation (A), ALP staining (B), β-galactosidase staining (C), osteogenic-related genes (D), senescent-related genes (E) and osteogenic-related proteins (F) in O-BMSCs. (G) The schematic diagram of 3D printed Akt/β-TCP scaffold. (H) The SEM images of Akt/β-TCP scaffold. (I) Critical skull bone defects model of senescent rats. (J–M) The bone repair evaluation of 3D printed Akt/β-TCP scaffold by micro-CT, sequential fluorescence and VG staining. Data are expreesed as mean ± SD. *P < 0.05, vs. β-TCP; #P < 0.05, vs. α-MEM without extracts. Scale bars: 100 μm (B, C, F, L), 1 mm (H), 5 mm (J, K). Reprinted from Qi et al.61 3D: three-dimensional; Akt: akermanite; Alp: alkaline phosphatase; AR: alizarin red; Bmp-2: bone morphogenetic protein 2; BV/TV: bone volume fraction; CA: calcein; Col-1: type I collagen; CT: computed tomography; O-BMSCs: aged bone marrow mesenchymal stem cells; SEM: scanning electron microscopy; TE: tetracycline; VG: Van Gieson; α-MEM: α-minimal essential medium; β-TCP: β-tricalcium phosphate.
Forsterite
Forsterite, with the chemical formula Mg2SiO4, is a well-known Mg silicate bioceramic used in bone regeneration. It exhibits superior mechanical properties compared to HA and BG.68 Human bone tissue typically shows an average compressive strength ranging from 130 to 190 MPa for cortical bone and 3.6 to 9.3 MPa for cancellous bone. Forsterite demonstrates mechanical properties that closely resemble those of natural bone, making it advantageous over other bioceramic materials.58 This similarity in mechanical behaviour can mitigate stress shielding and enhance the long-term stability of implants. Forsterite scaffolds, fabricated using glycine as a fuel, have been reported to surpass cortical bone strength, potentially reaching up to 200 MPa.58 In contrast, CaSiO3 bioceramics have shown the ability to promote apatite formation and stacking in simulated body fluid, while coarse-grained forsterite has not achieved similar success.69
Fathi and Kharaziha70 utilized a two-step sintering technique to create nanostructured forsterite, which demonstrated enhanced properties. This nanostructured variant exhibits superior fracture toughness and microhardness compared to its coarse-grained counterpart and promotes apatite deposition on surfaces in simulated body fluid. Additionally, nanopowders of forsterite offer a more controlled release of Mg and Si into the biological environment compared to bulk forsterite.71 Nanostructured forsterite bioceramics enhance the attachment and proliferation of osteoblasts, including osteoblast-like G292 and human osteoblasts U20S, rendering them promising biomaterials for bone repair.71, 72
When forsterite is doped with trace elements like zinc (Zn) and strontium (Sr), the degradation and porosity of the doped sample increase as the dopant concentration rises, potentially enhancing the biological response of forsterite.73, 74 It has been reported that magnesia olivine ceramic scaffolds prepared through sol-gel combustion can adjust the pH value, leading to the development of antimicrobial properties.75 Forsterite is currently utilized in scaffolds for bone engineering and implant coatings,76 although research in this field is still in its early stages, necessitating further studies to assess its suitability for bone repair and regeneration.
Mg-containing BG
BG surpasses ceramic materials in bone regeneration, typically composed of Ca-containing silicates resembling bone composition, thus serving as a highly compatible substitute for bone tissue. A well-known bioactive glass, 45S5, attracts calcium and phosphorus ions from the environment, forming a colloidal layer, that enables the deposition and stacking of HA. This HA layer then captures collagen, mucopolysaccharides, and glycoproteins, forming an inorganic-organic interface that facilitates new bone formation.77 Beyond establishing robust connections with both hard and soft tissues, BG enhances gene expression and fosters angiogenesis in osteoblasts through its bioactive dissolution products. Compared to conventional bioactive glasses, Mg-doped bioactive glass exhibits unique pore structures and increased specific surface areas, enhancing its bioactivity. Interconnected macropores play a pivotal role in promoting cell migration, nutrient transport, and bone tissue growth. Furthermore, rich nanostructures on the biomaterial’s surface facilitate cell adhesion and stable contact between the implant and the surrounding bone tissue.78, 79 Addressing bacterial infections is a significant challenge in bone repair, and Mg-containing BG possesses inherent antimicrobial properties. Another demonstrated commercially available BG owned antibacterial activity and could be applied in treating osteomyelitis and bone deformities resulting from bacterial infections.80
During heat treatment, conventional BG materials often undergo crystallisation, leading to the preferential degradation of residual glass phases. This can potentially reduce the biological activity of the final system and introduce instability to the implant.81 Therefore, there is a need to develop novel BG compositions with reduced propensity for crystallisation. Verné et al.82 found that higher crystallisation temperatures in Mg2+-containing BG resulted in greater stability and reduced crystallisation tendency. It has been reported that as the MgO content in BG increases, the crystallisation temperature also tends to increase. Conversely, the glass transition temperature exhibits an inverse relationship with MgO content, decreasing as the level of MgO in the BG rises. This leads to a widening numerical difference between crystallisation temperature and transition temperature, making it more challenging for BG with higher MgO levels to form crystal structures during the sintering process, which is crucial for processing BG.83 Numerous studies have shown that adding MgO to silicate BG can alter the physical, thermal, and mechanical properties of the materials. MgO can function as a glass network modifier in BG materials.84 Mg2+ has a greater Dieztel ion field strength compared to Ca2+ and Na+, resulting in an increase in the material’s elastic modulus with higher cation’s Dieztel ion field strength. Therefore, BG with higher MgO content tend to have a higher elastic modulus.85 However, according to a study from Hand’s team,86 adding MgO to the BG system reduces its hardness, brittleness, and elastic modulus while increasing its fracture toughness with higher MgO percentages. These discrepancies may arise because the mechanical properties of BG are influenced by both the glass network modifier and the glass network connectivity and structure.87 Thus, studying the effect of MgO on the mechanical properties of BG requires consideration of the entire BG composition. Furthermore, the thickness of the apatite-like layer formed on silicate BG is influenced by the level of MgO, where higher released Mg2+ into solution leads to increased thickness of the apatite layer.83
BG is extensively utilised, particularly in scaffold form, which can be produced through advanced techniques like 3D printing, indirect selective laser sintering, slip casting, and freeze extrusion fabrication. However, BG does have limitations that need to be acknowledged. Due to its brittleness,77 BG may be restricted in load-bearing applications that demand high mechanical strength. Additionally, due to processing constraints of BG materials, the manufacture of BG implants with intricate shapes or large sizes could pose challenges, making it challenging to emply BG materials for treating large bone defects.
MgO
MgO incorporated into bioceramic materials offers potential advantages in terms of mechanical strength, bioactivity, antibacterial properties, and osteogenic effects, making it a valuable component for Mg -based bioceramic materials. Chen et al.88 reported the MgO content significantly influence the mechanical characteristics and biological functionalities of bioceramics. When integrated into bioceramic scaffolds or coatings, MgO facilitates cellular attachment, proliferation, and differentiation, thereby enhancing osteogenic potential and promoting the formation of new bone tissue. Additionally, MgO exhibits bioactive behaviour by facilitating the release of Mg2+ ions, which can modulate cellular responses and promote tissue regeneration. Its antibacterial properties further enhance its usefulness in preventing infections associated with implants.89, 90
MgO plays a crucial role in enhancing osteogenesis and bone repair by stimulating osteoblastic activity and facilitating the differentiation of precursor cells into mature bone-forming cells. Its controlled degradation properties enable the gradual release of Mg2+ ions, which regulate cellular processes such as inflammation and angiogenesis, creating a conductive environment for bone healing.91, 92 Nandi et al.93 assessed the biocompatibility and functions of SrO- and MgO-doped brushite cement (BrC) in bone repair using a rabbit model. The results showed that the addition of small amounts of MgO dopants into BrC significantly promoted new bone formation compared to BrC.
To overcome the limitations of conventional polymethylmethacrylate (PMMA) bone cement in bone healing, Li et al.94 developed PMMA bone cements containing active nano-MgO particles (nano-MgO/PMMA). Measurements revealed that nano-MgO/PMMA exhibited improved biocompatibility, enhanced osteogenic potential, and superior bone-bonding strength compared to PMMA alone. Ke et al.95 discovered that the addition of MgO into TCP scaffolds had beneficial effects on osteoblastic viability and differentiation in vitro, as well as osteogenesis in vivo. Furthermore, the MgO-containing nanomaterials have been extensively investigated in bone regeneration, including nanoparticles, nanocrystals, and nanoscrolls.26, 27, 96
Mg(OH)2
Yuan et al.97 demonstrated that other forms of Mg binary compounds, such as Mg(OH)2, exhibit good corrosion resistance, antibacterial properties, and osteogenic activity, making them promising coatings for biomedical implants. Wang et al.98 fabricated Mg(OH)2 nanosheet coatings in-situ on the surface of pure Mg through alkali heat treatment, significantly enhancing the corrosion resistance and antibacterial efficacy of the substrate. The presence of Mg(OH)2 is reported to enhance osteoblast activity while reducing the involvement of osteoclasts in peri-implant bone remodelling.99 Pinho et al.100 discovered that Mg(OH)2RH nanoparticles, synthesized from Mg(OH)2 and rosehip (RH) extract, could stimulate osteoblastic differentiation to promote bone formation. RH possesses potent antioxidant properties, potentially modulating bone cell metabolism by regulating oxidative stress in the context of elevated oxidative burden.
Mg-containing bioceramic composites
Achieving optimal properties for a bone replacement material, including remarkable physical, chemical, and biological capabilities, poses a challenge as a single material often struggles to meet all these requirements simultaneously. Consequently, scholars have focused their efforts on the development of composite materials.
Mg-containing polymer-bioceramic composites
Natural bone tissue is a polymer composite material. Following the principle of bionics, this polymer material is combined with HA to form a composite material that mimics the properties of type I collagen in the extracellular matrix, providing elasticity and toughness. Synthetic polymers such as polylactic acid (PLA) and PCL are also commonly used in composites due to their favourable mechanical properties, biodegradability, and ease of processing. PLA, a natural biodegradable material, possesses excellent biological properties.101 However, the mechanical properties of pure PLA may not meet the requirements of loaded fracture fixation devices. Therefore, materials such as alloys and bioceramics can be incorporated into PLA to enhance its mechanical properties.102 MgO nanoparticles were doped into PLA/gelatin membranes and reconstructed into 3D scaffolds featuring interconnecting pores.103 The material had superior elasticity, and the incorporation of MgO mitigated the acidic degradation products of the PLA/gelatin scaffolds. In addition, Mg2+ released from this composite material benefited pre-osteoblasts by promoting proliferation and upregulating osteogenic differentiation. Ji et al.104 produced bioactive composites of ordered mesoporous calcium Mg silicate and poly(L-lactide) through the melt blending process. It was demonstrated that adding a dose of mesoporous calcium Mg silicate reduced the crystallinity of poly(L-lactide) but had little effect on the composites biomaterials’ thermal stability. The mesoporous calcium Mg silicate content was also associated with optimal hydrophilicity and in vitro degradability of the composites.104
PCL is another commonly used polymer composite in bone regeneration, known for its slow degradation rate, which allows sufficient time for bone regeneration.105 Electrospun PCL nanofibres have attracted a lot of attention in bone tissue engineering applications due to their non-toxicity, good biocompatibility, and resorbability properties. MgO nanoparticles are essential for bone regeneration, and incorporating them into PCL nanofibres to create electrospun MgO/PCL scaffolds can improve cell adhesion and survival, thereby increasing bioactivity of PCL. Besides, MgO/PCL scaffolds have a lower contact angle than pure electrospun PCL scaffolds, increasing the its hydrophilicity.106 It has been demonstrated that electrospun MgO/PCL scaffolds can significantly boost the expression of osteogenesis-related genes in rat adipose-derived MSCs, accelerate calcium deposition, and hence improve the osteogenic differentiation of adipose-derived MSCs.106 Salaris et al.107 enhanced PCL nanofibre-based mats with Mg(OH)2 and MgO using the electrostatic spinning technique. Mg(OH)2, being more stable for osteoinduction than Mg particles, promotes bone formation of implants due to lower hydrogen (H2) formation when in contact with physiological media. MgO, serving as a reinforcing agent, exhibits excellent chemical and thermal stability, as well as antibacterial properties.107, 108 Moreover, Dong et al.109 created PCL/β-TCP/MgO2 scaffolds through 3D printing technology successfully treated significant bone deformities in rats. They demonstrated that PCL/β-TCP/MgO2 composite scaffolds could enhance the survival and proliferation of exogenous BMSCs by releasing oxygen, hence speeding up bone regeneration.
In conclusion, the incorporation of Mg-containing bioceramics into polymers can enhance their mechanical properties, biological effects and degradation properties to further improve regeneration.
Mg-containing metal-bioceramic composites
Recently, metallic materials have attracted much attention in bone tissue engineering for repairing bone defects caused by trauma or diseases because of their favourable mechanical properties, biocompatibility, and manufacturing processes. However, the elastic modulus of metal is higher than that of the human cortical bone, resulting in a stress-shielding effect.110 To address the issues, metal alloys have been extensively used as excellent bone implant materials owing to their corrosion resistance, relatively low elastic modulus, and low density.111 Mg is an essential element in the human body as it plays important role in bone metabolism, DNA stabilisation, and skeletal development.112 Mg-containing bioceramic are renowned in the field of bone repair as revolutionary biodegradable metallic materials owing to their excellent biodegradability, mechanical properties, and biocompatibility.34 Therefore, Mg-containing metal-bioceramic composites were expected to intergate the advantages of Mg-containing bioceramic and metallic materials to further improve the efficiency of bone regeneration.
Fe35Mn, a type of biodegradable iron (Fe)-manganese (Mn) alloy, has been considered as a possible biodegradable metallic biomaterial. The development of composites of biomedical metals and bioceramics is an excellent technique to capitalize on the complimentary benefits of each in bone implant applications. The incorporation of biodegradable ceramics into metal matrices has been reported to accelerate corrosion rates and improve the biocompatibility of metals.113, 114 Zhang et al.115 found that incorporating Mg-containing bioceramic Akt into the Fe35Mn expedited its breakdown while increasing its biocompatibility. The addition of Akt increased the compressive strength and microhardness compared with pure Fe35Mn. Besides, the inclusion of Akt enhances Fe35Mn’s biocompatibility by promoting the formation of human osteoblasts on the material surface. Furthermore, it has been shown that Akt can induce surface mineralisation of Fe-Akt composites and stimulate the adhesion, proliferation, and differentiation of bone progenitor cells. In addition, forsterite-coating was coated to an AZ91 Mg alloy using sol-gel technology to develop a biodegradable composite.116 The forsterite-coated samples lost little weight, demonstrating the coating’s protective effect, which decreased the corrosion rate of the AZ91 Mg alloy. The composite was found to improve corrosion resistance and biocompatibility and reduce metal ion cytotoxicity and promote bone repair.
Applications of Magnesium-Containing Bioceramics
Due to the diversity in the location and morphology of bone defects, various types of bone defects often necessitate materials with distinct characteristics to achieve optimal osteogenic effects based on treatment requirements. Mg-containing bioceramics used in the clinical treatment of bone defects can be primarily classified into the following three categories: 1) Mg-containing bioceramic scaffolds: Scaffolds can offer mechanical support for damaged or diseased bones and deliver bioactive molecules during the bone repair and regeneration process.117 2) Injectable Mg-containing bioceramic materials: This category includes CaP cement, microspheres, hydrogels, etc. Injectable materials like hydrogels can encapsulate cells, ensuring high cell viability and creating a dynamic microenvironment niche to support, stimulate, and guide bone formation and remodelling.118 3) Mg-containing bioceramic coatings: Coatings are mainly applied to the surface of implants, slowing down the degradation rate of implants and enhancing the direct attachment of living tissues, thereby promoting bone conduction and regeneration.119 Considering the distinct advantages of these materials, different materials can be selected for specific application scenarios, and they can be applied in various fields related to bone regeneration such as inflammation, infection, osteoporosis, tumours, and osteomyelitis.
Application of Mg-containing bioceramic scaffolds for bone defect repair
Clinical causes of bone defects are varied, including trauma, tumours, congenital abnormalities, and osteoarthritis. Despite the common use of bone grafts from various sources for bone repair, biological implants may still encounter challenges.120 Due to their exceptional biocompatibility, osteoconductivity, and osteoinductivity, a wide range of bioceramics have been extensively employed for bone defect repair and regenerative medicine applications. Among these, the bioceramic-based scaffoldshavegarneredsignificantattentioninboneregeneration and tissue engineering under many various physiological and pathological conditions, including inflammation, infection, tumour, ageing, diabetes, osteomyelitis, and nonunion.121-123 To address the challenges encountered in clinical practice, bioceramics scaffolds are designed with representative advantages such as a 3D porous structure, mechanical support, tissue integration, revascularisation, and bioactive ion release. He et al.124 synthesized lithium Mg phosphate (LMP) biomaterials using a solid-phase reaction method and utilized 3D printing to fabricate the Li2Mg2(PO4)2 bioceramic scaffolds. In vitro experiments have demonstrated that these LMP bioceramic scaffolds had low porosity, resulting in high compressive strength. Compared with β-TCP bioceramics, LMP bioceramics exhibited higher compressive strength and better cell proliferation. Additionally, the release of Lithium and Mg from LMP stimulated osteogenesis and angiogenesis, enhancing the osteogenic differentiation and pro-angiogenic activity of this novel bioceramic scaffold. In vivo experiments revealed that the Li2Mg2(PO4)2 bioceramic scaffolds effectively healed bone defects and promoted the regeneration of new bone tissue and blood vessels in the innermost region, making them promising biomaterials for the effective repair of challenging bone defects.
Infectious diseases can lead to severe inflammation and compromised healing of bone tissue. Bioceramic-based scaffolds have been shown potential as a strategy for treating osteomyelitis by combating inflammation and promoting bone regeneration.125, 126 Radwan et al.127 demonstrated that CaP scaffolds could reduce bacterial burden and inflammation in the bones of animals with chronic osteomyelitis. Specifically, bioactive scaffolds are being optimised as drug delivery systems for osteomyelitis treatment in preclinical research.128-131 Moreover, 3D printed bioscaffolds with antioxidative properties have been shown to promote scavenging of reactive oxygen species scavenging and osteogenic differentiation of BMSCs for osteochondral regeneration in osteoarthritis treatment.132 Another significant property is the antibacterial ability against potential pathogenic infections. Mg2 SiO4 ceramic powders generated by sol-gel combustion were used to fabricate scaffolds. The scaffolds, with a high surface area, exhibited greater degradability, mechanical strength, and antibacterial activity when immersed in simulated body fluid.75 Therefore, Mg2SiO4 scaffolds are considered as antibacterial ceramic materials with high bactericidal activity suitable for load-bearing applications.
Application of injectable Mg-containing bioceramic materials for bone filling
Bone deformities resulting from trauma, cancer, congenital anomalies, and osteoarthritis have significantly impacted human life and well-being.4 Finding ideal bone graft materials to repair bone defects remains a challenge for clinicians. Currently, Mg-containing bioceramic materials have emerged as promising biomaterials for bone repair. Variety of Mg-based material scaffolds, including porous biodegradable metallic scaffolds, porous bioceramics, polymer-based scaffolds, and hydrogel scaffolds, have been developed for repairing bone defects.27 However, scaffold-form materials with often require a large space when implanted, which can cause secondary damage to the body and increase the risk of inflammation, infection, and poor bone healing.133 Moreover, preformed Mg-based material scaffolds may not be effective for irregular or narrow bone defects, as well as the bone defects with special anatomical structures.134 Therefore, developing a bioactive material with superior bone properties, morphological plasticity, and minimal invasiveness is a major challenge for achieving minimally invasive and precise treatment of bone defects.133 Injectable biomaterials, such as injectable bone cements, injectable hydrogels, and injectable core-shell microspheres, have shown great promise for bone repair in various pathological environments. These injectable biomaterials offer the advantage of adaptability to irregular or narrow bone defects and special anatomical structures, thereby providing a more precise and less invasive treatment option.
MPCs
Due to its chemical similarity to bone, outstanding bioactivity, osteoconductivity, injectable cement form, high plasticity, and self-setting properties,135 CaP cements (CPCs) have been widely adopted for bone tissue engineering,135, 136 especially in complex bone cavities or a narrow defect sites. However, existing CPCs still have drawbacks, including low strength, slow solidification time, and sluggish absorption in vivo.135 In comparison, studies have demonstrated that MPCs exhibit higher strength, shorter setting time, more favourable solidification time, and absorption rate than CPCs. Therefore, MPCs may represent a more optimal bone substitute material than CPCs for certain applications in bone defect repair.135 Mg-containing cement systems are typically composed of a solid phase such as MgO or trimagnesium phosphate anhydrous (Mg3(PO4)2) and various soluble phosphate solutions, including phosphoric acid (H3PO4), monoammonium hydrogen phosphate (NH4H2PO4), disodium hydrogen phosphate (Na2HPO4), and dipotassium hydrogen phosphate (K2HPO4).135 Several studies have reported that MPCs demonstrate excellent biocompatibility and osteoconductivity,54 which, unlike CPCs, can accelerate bone defect repair and rarely induce foreign body responses or inflammatory infections.135
Yu et al.137 conducted implantation of fully reacted cement plugs composed of MgO and monoammonium hydrogen phosphate within the muscle and femur condyle of rabbits. After 6 months, they observed that the implanted material was gradually underwent substitution by newly formed woven bone, which subsequently transformed partially into lamellar bone. The high solubility of MPC allows it to offer superior regenerative capacity.54 Kaiser et al.54 increased the content of highly dissolved phases such as struvite (MgNH4PO4·6H2O) and K-struvite (MgKPO4·6H2O) by reducing the powder ratio of cement and then separately injected them into the partially loaded tibia defects in sheep. The results suggested that both cements were partially degraded and replaced by bone tissue after 4 months, indicating their potential as substitutes for slowly degrading CPCs in cases where the defect size and degradation rate are limited. Wu et al.138 incorporated MPCs into CPCs to develop a new injectable and degradable Ca-Mg-P cement and implanted Ca-Mg-P cement into holes drilled in the femur of rabbits. They reported that compared to CPCs, Ca-Mg-P cement exhibited greater degradability and enhanced efficiency of new bone formation.
Infection constitutes one of the primary causes of bone defects and represents a major complication in implantation and bone regeneration surgeries.139 Three studies have illustrated the inhrinsic antimicrobial properties of specific amorphous, sodium-containing MPCs against various bacterial strains commonly associated with implant infections (e.g., Escherichia coli) or dental plaque (e.g., Streptococcus sanguinis).34, 140, 141 Besides possessing inherent antimicrobial properties, MPCs also exhibit antibiotic delivery and sustained-release capabilities. In the case of CPCs, MPCs demonstrate similar antibacterial efficacy when added antibiotics are added. Cabrejos-Azama et al.139 incorporated vancomycin into Mg-doped brushite cements. In vitro experiments revealed that Mg-doped brushite cements loaded with vancomycin displayed robust antibacterial activity against Staphylococcus aureus strains, with the sustained release of vancomycin from the cement matrix being dependant on the Mg concentration. They concluded that Mg-doped brushite cements loaded with vancomycin could be effective in treating osteomyelitis and preventing of staphylococcal infections during bone regeneration.
Mg-containing bioceramic microspheres
Bone defects resulting from osteoporosis often exhibit characteristics of poor bone quality, slow healing, and high recurrence rates. Traditional systemic drug intervention therapy typically entails a lengthy treatment duration, limited bone targeting, and high side effects. While local bone transplantation can temporarily address bone defects, autologous bone transplantation may lead to secondary injuries for patients, and complications such as infection and absorption in the transplanted new bone, resulting in suboptimal bone repair.133 Hence, there is an urgent need to explore alternative therapeutic strategies with excellent bone-promoting efficacy, minimal invasiveness, and precise bone targeting to enhance bone regeneration.133 Lin et al.142 developed a monodisperse core-shell microsphere delivery system comprising MgO nanoparticles, alginate hydrogel, and poly(lactic-co-glycolic acid). This innovative technology enables precise regulation of in vivo Mg2+ supply at specific concentrations, enhancing osteogenic activity both in vitro and in vivo, and efficiently promoting in-situ bone repair. However, due to the lack of bone-targeting properties and the limited availability of the Mg2+, this system still has some limitations.
Mg-containing injectable hydrogel composites
Hydrogel is a water-swollen polymeric biomaterial consisting of a 3D hydrophilic network with large amounts of water.136 It shares physical and chemical components with the extracellular matrix, rendering it superior in biosafety, biocompatibility, and biodegradability. Its porous structure and high water absorption rate facilitated cell adhesion, proliferation, migration, growth, nutrition transport, and metabolic waste removal.27 A study has demonstrated that Mg-rich hydrogel scaffolds can enhance the local inflammatory microenvironment, promote neo-angiogenesis, and accelerate local bone healing.136 However, while Mg-enriched hydrogel scaffolds are suitable for regular bone defect repair with ample space for implantation, they are less effective for regenerating irregular narrow defects (e.g., lacunar defects) or critical-sized bone defects.134 Consequently, injectable Mg-enriched hydrogel has garnered significant attention for minimally invasive treatment of irregular bone defects in tissue regeneration.
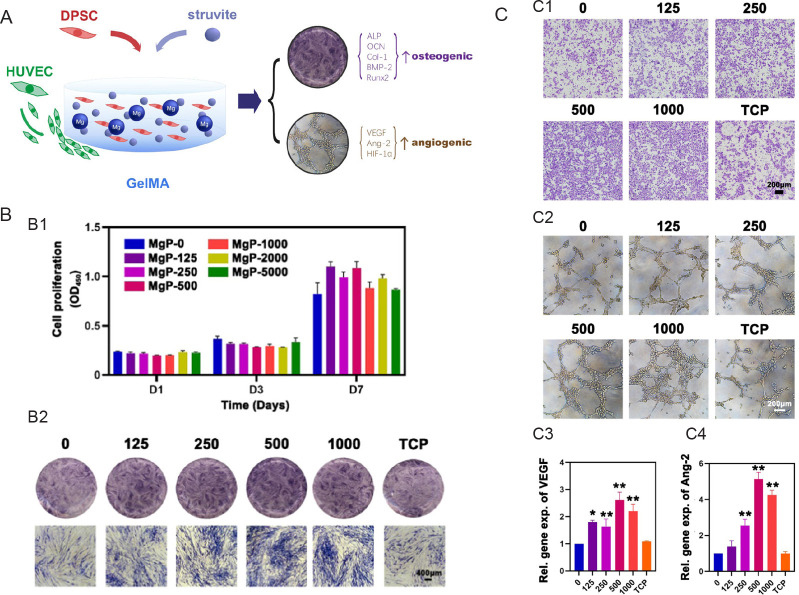
The development of injectable Mg-enriched hydrogel involves incorporating Mg or Mg-containing materials into the basic injectable hydrogel system. Liu et al.143 devised a novel injectable composite hydrogel by incorporating Mg ammonium phosphate hexahydrate (struvite) into gelatin methacrylate. Using this injectable composite hydrogel, they constructed a 3D culture system capable of releasing ionic components to promote vascularized bone formation by dental pulp stem cells. They proposed that this new composite hydrogel based on struvite and gelatin methacrylate may be suitable for minimally invasive treatment of maxillofacial irregular bone defects (Figure 4).
Figure 4. Cell-laden hydrogel with magnesium ammonium phosphate composite promotes angiogenesis and osteogenesis. (A) Magnesium ammonium phosphate composite cell-laden hydrogel promotes osteogenesis and angiogenesis is shown schematically. (B) The struvite extracts’ cell proliferation and osteogenic action. (B1) The proliferation of human DPSCs cultured with varying struvite extract concentrations at various time intervals as assessed using the cell counting kit-8 test (n = 3). (B2) After incubating with struvite and TCP extracts for 7 days, ALP staining was carried out to assess the osteogenic induction capacity of struvite in human DPSCs. Scale bar: 400 μm. (C) Angiogenic effect of the struvite extracts. (C1) Migration assay of HUVECs in response to serial concentrations of struvite and TCP extracts after 12 hours. Scale bar: 200 μm. (C2) Tube formation assay of HUVECs seeded on the gel basement and cultured with the struvite and TCP extracts after 6 hours. Scale bar: 200 μm. (C3) Statistical results for the percentage of HUVECs penetrating the Transwell membranes compared to the control group (n = 5). (C4) Statistical results for the percentage of HUVEC branch points compared to that in the control group (n = 5). Gradient concentration of struvite powder (0–1000 μg/mL) mixed with GelMA solution was designed as the MgP group. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, vs. GelMA solution without magnesium ammonium phosphate (0 group). Reprinted from Liu et al.142 ALP: alkaline phosphatase; Ang-2: angiotensin-2; BMP-2: bone morphogenetic protein 2; COL-1: type I collagen; DPSC: dental pulp stem cell; GelMA: gelatin methacrylate; HIF-1α: hypoxia-inducible factor-1α; HUVEC: human umbilical vein endothelial cell; MgP: magnesium ammonium phosphate powder mixed with GelMA solution; OCN: osteocalcin; Runx2: runt-related transcription factor 2; TCP: tricalcium phosphate; VEGF: vascular endothelial growth factor.
Zhang et al.134 developed an injectable hydrogel called SAG hydrogel, containing sodium alginate (SA), Akt, and glutamic acid, and implanted it into the rabbit nasal bone defect sites. Through in vitro experiment, they evaluated its osteogenic capacity. The results demonstrated that SAG hydrogel not only promoted osteogenic differentiation via the mitogen-activated protein kinase pathway but also enhanced bone regeneration by increasing the recruitment of BMSCs to the defect site through elevated C-X-C motif chemokine receptor 4 levels. This suggests that SAG hydrogel may be suitable for repairing irregular bone cavities. While Zhang et al.134 identified the potential osteogenesis mechanism of SAG hydrogels, the in vivo response of this injectable hydrogel, particularly its inflammatory response, has not been fully elucidated. To investigate the interaction between Akt/SA hydrogel, inflammatory cells, and cells involved in bone regeneration, Zhu et al.144 cultured macrophages with Akt/SA hydrogel in vitro and injected Akt/SA hydrogel subcutaneously in rats. Results indicated that Akt/SA hydrogel activated macrophages towards the M2 phenotype, stimulating them to express anti-inflammatory factors and promoting the recruitment of BMSCs towards the hydrogel.
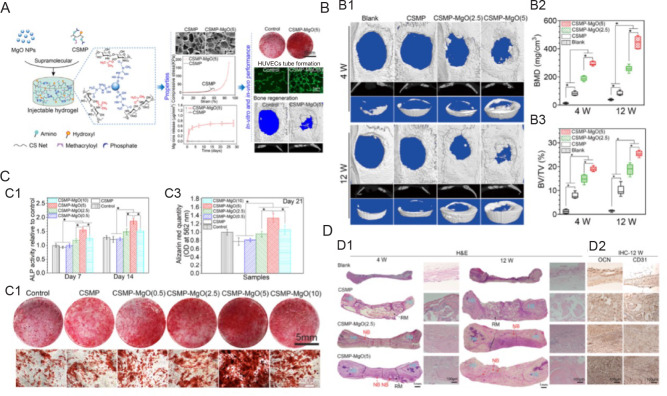
Furthermore, Chen et al.27 developed an injectable hydrogel (soluble chitosan functionalised with phosphocreatine-MgO) by mixing MgO nanoparticles (NPs) in a water-soluble chitosan functionalised with phosphocreatine solution. Tests using a rat critical-sized calvaria defect model demonstrated that soluble chitosan functionalised with phosphocreatine-MgO was beneficial in stimulating the production of new bone (Figure 5).
Figure 5. Magnesium oxide nanoparticle coordinated phosphate functionalised chitosan injectable hydrogel for osteogenesis and angiogenesis in bone regeneration. (A) Schematic illustration of synthesis process of novel injectable supramolecular hydrogel and its in vitro and in vivo experiments. (B) The mechanical properties and micro-CT of the newly produced bone for the hydrogels implanted in the 5 mm critical-sized calvarial defect of Sprague-Dawley rats were examined after 4 and 12 weeks. (B1) Micro-CT scans. (B2, 3) Micro-CT assessment-derived BMD and the proportion of newly regenerated bone to tissue volume in the critical-sized area. (C) The ability of hydrogels cultivated with MC3T3-E1 cells to promote osteogenesis. (C1) ALP activity for MC3T3-E1 grown for 7 and 14 days on hydrogels. (C2, 3) Alizarin red staining and amount for MC3T3-E1 grown on hydrogels for 21 days. (D) Section staining of the critical-sized calvarial defect area following 4 and 12 weeks of hydrogel implantation. (D1) Representative pictures of the defect region stained with H&E following hydrogel implantation for 4 and 12 weeks. Data are expressed as mean ± SD (n = 6). *P < 0.05. HB, NB, and RM (hydrogels). (D2) OCN, an osteogenic marker, and CD31, an angiogenic marker, stained with an immunohistochemical reaction. Reprinted with permission from Chen et al.27 Copyright 2021, American Chemical Society. ALP: alkaline phosphatase; BMD: bone mineral density; BV/TV: bone volume fraction; CD31: platelet endothelial cell adhesion molecule-1; CS: chitosan; CSMP: phosphocreatine-functionalized chitosan; CT: computed tomography; H&E: haematoxylin and eosin; HB: host bone; HUVEC: human umbilical vein endothelial cell; IHC: immunohistochemistry; MgO: magnesium oxide; NB: newly regenerated bone; NP: nanoparticle; OCN: osteocalcin; OD: optical density; RM: leftover materials.
Application of Mg-containing bioceramic coatings on bone implants
Bone implants are widely applied in clinical practice due to its strength and bioinertness, which create a favourable microenvironment for osteogenesis and immunoregulation. However, the lack of dynamic interaction with the host hinders their effective osteogenesis. Therefore, coating bone implants with various Mg-containing bioceramic can enhance their osteogenic potential and corrosion resistance to further improve osseointegration of bone implants.
Mg2+, being the second most abundant intracellular cation, play a crucial role in stabilising various intracellular functions and mineralisation processes involved in bone formation and absorption. However, Due to rapid degradation, Mg-based implants can lead to the release of large amounts of hydrogen, compromising the mechanical strength of the implants under physiological conditions. The phenomenon can result in poor bone healing and internal fixation failure.145 These issues highlight the need for careful consideration and further research to address the challenges associated with Mg-based implants in medical applications. To address the challenges, researchers have focused on the Mg-containing bioceramic coatings bone implants to intergrate the advantages of Mg2+ ions and bone implants.
In various pathological microenvironments, the use of Mg-containing bioceramics as a surface modification for implants has recently shown to improve initial healing and shorten healing times. Tao et al.146 suggested that Mg-HA coatings could improve implant osseointegration in osteoporotic rats. Additionally, Varshney et al.147 indicated that nanocomposite coatings of HA/MgO/ZnO could enhance corrosion resistance and antibacterial properties of medical implants to further help reduce infections associated with implant-related diseases. Wang et al.98 used alkali heat treatment to produce Mg(OH)2 nanosheet coating directly on pure Mg, significantly boosting its ability to resist corrosion and combat bacteria. The development of functional composite coatings with superior corrosion resistance, osteogenic activity, and antibacterial properties on Mg alloys surfaces may be achievable through a combination of Mg(OH)2. Yuan et al.97 successfully developed the Mg(OH)2/graphene oxide/HA composite coating. This coating greatly enhances the corrosion resistance of ZQ71 alloy and slow down its degradation rate, owing to its high bond strength and nanoscale topography. Our previous study also indicated that nano-structured Akt-coated Ti-6Al-4V implants promoted the proliferation, osteogenesis, angiogenesis and inhibited osteoclastogenesis of OVX-rat BMSCs in vitro and improved new bone formation and osseointegration in an osteoporosis rabbit model in vivo, which might provide a promising strategy to improve the bone regeneration and osseointegration capability of orthopaedic implants under osteoporosis conditions.148
Overall, Mg-containing bioceramic coating bone implants have been demonstrated to promote osteogenesis and exhibit superior bactericidal effects under both normal and infected conditions.
Conclusion
In conclusion, Mg plays a vital role in intracellular functions, mineralisation, and orthopaedic clinical treatments due to its resemblance to bone. By combining Mg with other elements such as Si, calcium, and phosphorus, as well as organic and biological materials, its performance can be optimised. Mg-containing bioceramics hold a great promise for bone regeneration in various conditions, although their clinical application encounters some challenges. Strategies to tackle the high degradation rate of Mg materials include coatings, alloys, and ion implantation, yet these approaches may induce adverse reactions and side effects. Utilising Mg in compound forms could potentially alleviate these issues. Further study is imperative to explore safe and effective methods for reducing the degradation rate of Mg-containing bioceramics, particularly in case of critical-size defects and age-related diseases like osteoporosis and inflammation. The uniqueness of individual patient and the variability of bone defect sites necessitate precise medical treatments, underscoring the need for advanced materials to meet clinical demands. Comprehensive two-way research on the performance and structure of novel materials is crucial to effectively address these diverse clinical needs.
Footnotes
Author contributions: KL, XW and LZ conceptualised and designed the review; LQ, TZ and JY drafted the manuscript; WG, WJ, JW and MG checked and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Financial support: The work was supported by the National Key R&D Program of China (No. 2023YFC2414106), National Natural Science Foundation of China (Nos. 32271379, 82072396), Science and Technology Commission of Shanghai Municipality (Nos. 21140900102, 21490711700, 21140900103), Disciplinary Characteristic Biobank Project of Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (No. YBKB202110), the Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2021ZD12), Cross disciplinary Research Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYJC202219), Shanghai’s Top Priority Research Center (No. 2022ZZ01017), and CAMS Innovation Fund for Medical Sciences (No. CIFMS, 2019-I2M-5-037).
Acknowledgement: None.
Conflicts of interest statement: The authors declare no conflict of interest.
References
- 1.Rao S. H., Harini B., Shadamarshan R. P. K., Balagangadharan K., Selvamurugan N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int J Biol Macromol. 2018;110:88–96. doi: 10.1016/j.ijbiomac.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Tan B., Tang Q., Zhong Y., Wei Y., He L., Wu Y., Wu J., Liao J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int J Oral Sci. 2021;13:9. doi: 10.1038/s41368-021-00113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z., Wang Y., Yan J., Zhang K., Lin F., Xiang L., Deng L., Guan Z., Cui W., Zhang H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–534. doi: 10.1016/j.addr.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C., Liu W., Zhu M., Wu C., Zhu Y. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: a review. Bioact Mater. 2022;18:383–398. doi: 10.1016/j.bioactmat.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhi W., Wang X., Sun D., Chen T., Yuan B., Li X., Chen X., Wang J., Xie Z., Zhu X., Zhang K., Zhang X. Optimal regenerative repair of large segmental bone defect in a goat model with osteoinductive calcium phosphate bioceramic implants. Bioact Mater. 2022;11:240–253. doi: 10.1016/j.bioactmat.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai L., Song P., Su J. Bioactive elements manipulate bone regeneration. Biomater Transl. 2023;4:248–269. doi: 10.12336/biomatertransl.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B. Early immunomodulation by magnesium ion: catalyst for superior osteogenesis. Biomater Transl. 2023;4:294–296. doi: 10.12336/biomatertransl.2023.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz-Tocados J. M., Herencia C., Martínez-Moreno J. M., Montes de Oca A., Rodríguez-Ortiz M. E., Vergara N., Blanco A., Steppan S., Almadén Y., Rodríguez M., Muñoz-Castañeda J. R. Magnesium Chloride promotes osteogenesis through Notch signaling activation and expansion of mesenchymal stem cells. Sci Rep. 2017;7:7839. doi: 10.1038/s41598-017-08379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nourisa J., Zeller-Plumhoff B., Helmholz H., Luthringer-Feyerabend B., Ivannikov V., Willumeit-Römer R. Magnesium ions regulate mesenchymal stem cells population and osteogenic differentiation: A fuzzy agent-based modeling approach. Comput Struct Biotechnol J. 2021;19:4110–4122. doi: 10.1016/j.csbj.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao W., Wong K. H. M., Shen J., Wang W., Wu J., Li J., Lin Z., Chen Z., Matinlinna J. P., Zheng Y., Wu S., Liu X., Lai K. P., Chen Z., Lam Y. W., Cheung K. M. C., Yeung K. W. K. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat Commun. 2021;12:2885. doi: 10.1038/s41467-021-23005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castiglioni S., Cazzaniga A., Albisetti W., Maier J. A. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5:3022–3033. doi: 10.3390/nu5083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Dai B., Guo J., Zhu Y., Xu J., Xu S., Yao Z., Chang L., Li Y., He X., Chow D. H. K., Zhang S., Yao H., Tong W., Ngai T., Qin L. Biosynthesized bandages carrying magnesium oxide nanoparticles induce cortical bone formation by modulating endogenous periosteal cells. ACS Nano. 2022;16:18071–18089. doi: 10.1021/acsnano.2c04747. [DOI] [PubMed] [Google Scholar]
- 13.Maier J. A., Castiglioni S., Locatelli L., Zocchi M., Mazur A. Magnesium and inflammation: Advances and perspectives. Semin Cell Dev Biol. 2021;115:37–44. doi: 10.1016/j.semcdb.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Bessa-Gonçalves M., Silva A. M., Brás J. P., Helmholz H., Luthringer-Feyerabend B. J. C., Willumeit-Römer R., Barbosa M. A., Santos S. G. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020;114:471–484. doi: 10.1016/j.actbio.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Tang L., Qi H., Zhao Q., Liu Y., Zhang Y. Dual function of magnesium in bone biomineralization. Adv Healthc Mater. 2019;8:e1901030. doi: 10.1002/adhm.201901030. [DOI] [PubMed] [Google Scholar]
- 16.Danks A. E., Hall S. R., Schnepp Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater Horiz. 2016;3:91–112. [Google Scholar]
- 17.Peng Z. D., Yang J. H., Zhou Z., Liu Y. X., Li Z. Q. Thermodynamic analysis on preparing doped zinc oxide varistor ceramic powders by coprecipitation process. Wuji Cailiao Xuebao. 1999;14:733–738. [Google Scholar]
- 18.Sōmiya S. Hydrothermal preparation and sintering of fine ceramic powders. MRS Online Proc Libr. 1983;24:255–271. [Google Scholar]
- 19.Debuigne F., Jeunieau L., Wiame M. B., Nagy J. Synthesis of organic nanoparticles in different W/O microemulsions. Langmuir. 2000;16:7605–7611. [Google Scholar]
- 20.Eslamian M., Ahmed M., Ashgriz N. Modelling of nanoparticle formation during spray pyrolysis. Nanotechnology. 2006;17:1674–1685. doi: 10.1088/0957-4484/17/6/023. [DOI] [PubMed] [Google Scholar]
- 21.Eslamian M., Shekarriz M. Recent advances in nanoparticle preparation by spray and micro-emulsion methods. Recent Pat Nanotechnol. 2009;3:99–115. doi: 10.2174/187221009788490068. [DOI] [PubMed] [Google Scholar]
- 22.Brewster J. H., Kodas T. T. Generation of unagglomerated, dense, BaTiO3 particles by flame-spray pyrolysis. AIChE J. 1997;43:2665–2669. [Google Scholar]
- 23.Deville S., Saiz E., Nalla R. K., Tomsia A. P. Freezing as a path to build complex composites. Science. 2006;311:515–518. doi: 10.1126/science.1120937. [DOI] [PubMed] [Google Scholar]
- 24.Putra N. E., Borg K. G. N., Diaz-Payno P. J., Leeflang M. A., Klimopoulou M., Taheri P., Mol J. M. C., Fratila-Apachitei L. E., Huan Z., Chang J., Zhou J., Zadpoor A. A. Additive manufacturing of bioactive and biodegradable porous iron-akermanite composites for bone regeneration. Acta Biomater. 2022;148:355–373. doi: 10.1016/j.actbio.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Putra N. E., Leeflang M. A., Klimopoulou M., Dong J., Taheri P., Huan Z., Fratila-Apachitei L. E., Mol J. M. C., Chang J., Zhou J., Zadpoor A. A. Extrusion-based 3D printing of biodegradable, osteogenic, paramagnetic, and porous FeMn-akermanite bone substitutes. Acta Biomater. 2023;162:182–198. doi: 10.1016/j.actbio.2023.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Canales D. A., Reyes F., Saavedra M., Peponi L., Leonés A., Palza H., Boccaccini A. R., Grünewald A., Zapata P. A. Electrospun fibers of poly (lactic acid) containing bioactive glass and magnesium oxide nanoparticles for bone tissue regeneration. Int J Biol Macromol. 2022;210:324–336. doi: 10.1016/j.ijbiomac.2022.05.047. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Sheng W., Lin J., Fang C., Deng J., Zhang P., Zhou M., Liu P., Weng J., Yu F., Wang D., Kang B., Zeng H. Magnesium oxide nanoparticle coordinated phosphate-functionalized chitosan injectable hydrogel for osteogenesis and angiogenesis in bone regeneration. ACS Appl Mater Interfaces. 2022;14:7592–7608. doi: 10.1021/acsami.1c21260. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X., Wei S., Yang Z., Yang P., Liu A. High-strength and tough bioactive Mg-doped hydroxyapatite bioceramics with oriented microchannels. Ceram Int. 2022;48:13494–13507. [Google Scholar]
- 29.Duda G. N., Geissler S., Checa S., Tsitsilonis S., Petersen A., Schmidt-Bleek K. The decisive early phase of bone regeneration. Nat Rev Rheumatol. 2023;19:78–95. doi: 10.1038/s41584-022-00887-0. [DOI] [PubMed] [Google Scholar]
- 30.Jang J. W., Min K. E., Kim C., Shin J., Lee J., Yi S. Review: Scaffold characteristics, fabrication methods, and biomaterials for the bone tissue engineering. Int J Precis Eng Manuf. 2023;24:511–529. [Google Scholar]
- 31.Beattie J. H., Avenell A. Trace element nutrition and bone metabolism. Nutr Res Rev. 1992;5:167–188. doi: 10.1079/NRR19920013. [DOI] [PubMed] [Google Scholar]
- 32.Kanasan N., Adzila S., Koh C. T., Rahman H. A., Panerselvan G. Effects of magnesium doping on the properties of hydroxyapatite/sodium alginate biocomposite. Adv Appl Ceram. 2019;118:381–386. [Google Scholar]
- 33.Tonelli M., Faralli A., Ridi F., Bonini M. 3D printable magnesium-based cements towards the preparation of bioceramics. J Colloid Interface Sci. 2021;598:24–35. doi: 10.1016/j.jcis.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Nabiyouni M., Brückner T., Zhou H., Gbureck U., Bhaduri S. B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018;66:23–43. doi: 10.1016/j.actbio.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y., Wang J., Ong M. T. Y., Yung P. S., Wang J., Qin L. Update on the research and development of magnesium-based biodegradable implants and their clinical translation in orthopaedics. Biomater Transl. 2021;2:188–196. doi: 10.12336/biomatertransl.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trabelsi M., AlShahrani I., Algarni H., Ben Ayed F., Yousef E. S. Mechanical and tribological properties of the tricalcium phosphate - magnesium oxide composites. Mater Sci Eng C Mater Biol Appl. 2019;96:716–729. doi: 10.1016/j.msec.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 37.Sikder P., Ren Y., Bhaduri S. B. Microwave processing of calcium phosphate and magnesium phosphate based orthopedic bioceramics: A state-of-the-art review. Acta Biomater. 2020;111:29–53. doi: 10.1016/j.actbio.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 38.George S. M., Nayak C., Singh I., Balani K. Multifunctional hydroxyapatite composites for orthopedic applications: a review. ACS Biomater Sci Eng. 2022;8:3162–3186. doi: 10.1021/acsbiomaterials.2c00140. [DOI] [PubMed] [Google Scholar]
- 39.Ullah I., Gloria A., Zhang W., Ullah M. W., Wu B., Li W., Domingos M., Zhang X. Synthesis and characterization of sintered Sr/Fe-modified hydroxyapatite bioceramics for bone tissue engineering applications. ACS Biomater Sci Eng. 2020;6:375–388. doi: 10.1021/acsbiomaterials.9b01666. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X., Yang Z., Liu Q., Yang P., Wang P., Wei S., Liu A., Zhao Z. Potential load-bearing bone substitution material: carbon-fiber-reinforced magnesium-doped hydroxyapatite composites with excellent mechanical performance and tailored biological properties. ACS Biomater Sci Eng. 2022;8:921–938. doi: 10.1021/acsbiomaterials.1c01247. [DOI] [PubMed] [Google Scholar]
- 41.Coelho C. C., Padrão T., Costa L., Pinto M. T., Costa P. C., Domingues V. F., Quadros P. A., Monteiro F. J., Sousa S. R. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Sci Rep. 2020;10:19098. doi: 10.1038/s41598-020-76063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coelho C. C., Araújo R., Quadros P. A., Sousa S. R., Monteiro F. J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater Sci Eng C Mater Biol Appl. 2019;97:529–538. doi: 10.1016/j.msec.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 43.Ballouze R., Marahat M. H., Mohamad S., Saidin N. A., Kasim S. R., Ooi J. P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J Biomed Mater Res B Appl Biomater. 2021;109:1426–1435. doi: 10.1002/jbm.b.34802. [DOI] [PubMed] [Google Scholar]
- 44.Frasnelli M., Sglavo V. M. Effect of Mg(2+) doping on beta-alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomater. 2016;33:283–289. doi: 10.1016/j.actbio.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 45.He F., Rao J., Zhou J., Fu W., Wang Y., Zhang Y., Zuo F., Shi H. Fabrication of 3D printed Ca(3)Mg(3)(PO(4))(4)-based bioceramic scaffolds with tailorable high mechanical strength and osteostimulation effect. Colloids Surf B Biointerfaces. 2023;229:113472. doi: 10.1016/j.colsurfb.2023.113472. [DOI] [PubMed] [Google Scholar]
- 46.Ge M., Xie D., Yang Y., Tian Z. Sintering densification mechanism and mechanical properties of the 3D-printed high-melting-point-difference magnesium oxide/calcium phosphate composite bio-ceramic scaffold. J Mech Behav Biomed Mater. 2023;144:105978. doi: 10.1016/j.jmbbm.2023.105978. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Lin T., Meng H., Wang X., Peng H., Liu G., Wei S., Lu Q., Wang Y., Wang A., Xu W., Shao H., Peng J. 3D gel-printed porous magnesium scaffold coated with dibasic calcium phosphate dihydrate for bone repair in vivo. J Orthop Translat. 2022;33:13–23. doi: 10.1016/j.jot.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao X., Zhu J., Zhang C., Xian J., Li M., Nath Varma S., Qin Z., Deng Q., Zhang X., Yang W., Liu C. Magnesium-rich calcium phosphate derived from tilapia bone has superior osteogenic potential. J Funct Biomater. 2023;14:390. doi: 10.3390/jfb14070390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu F., Su J., Wei J., Guo H., Liu C. Injectable bioactive calcium-magnesium phosphate cement for bone regeneration. Biomed Mater. 2008;3:044105. doi: 10.1088/1748-6041/3/4/044105. [DOI] [PubMed] [Google Scholar]
- 50.Karfarma M., Esnaashary M. H., Rezaie H. R., Javadpour J., Naimi-Jamal M. R. Poly(propylene fumarate)/magnesium calcium phosphate injectable bone composite: effect of filler size and its weight fraction on mechanical properties. Proc Inst Mech Eng H. 2019;233:1165–1174. doi: 10.1177/0954411919877277. [DOI] [PubMed] [Google Scholar]
- 51.Wu J., Liu F., Wang Z., Liu Y., Zhao X., Fang C., Leung F., Yeung K. W. K., Wong T. M. The development of a magnesium-releasing and long-term mechanically stable calcium phosphate bone cement possessing osteogenic and immunomodulation effects for promoting bone fracture regeneration. Front Bioeng Biotechnol. 2021;9:803723. doi: 10.3389/fbioe.2021.803723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X., Dai H., Yu S., Zhao Y., Long Y., Li W., Tu J. Magnesium calcium phosphate cement incorporating citrate for vascularized bone regeneration. ACS Biomater Sci Eng. 2020;6:6299–6308. doi: 10.1021/acsbiomaterials.0c00929. [DOI] [PubMed] [Google Scholar]
- 53.Kanter B., Vikman A., Brückner T., Schamel M., Gbureck U., Ignatius A. Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater. 2018;69:352–361. doi: 10.1016/j.actbio.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser F., Schröter L., Stein S., Krüger B., Weichhold J., Stahlhut P., Ignatius A., Gbureck U. Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater. 2022;145:358–371. doi: 10.1016/j.actbio.2022.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Brückner T., Meininger M., Groll J., Kübler A. C., Gbureck U. Magnesium phosphate cement as mineral bone adhesive. Materials (Basel) 2019;12:3819. doi: 10.3390/ma12233819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azeena S., Subhapradha N., Selvamurugan N., Narayan S., Srinivasan N., Murugesan R., Chung T. W., Moorthi A. Antibacterial activity of agricultural waste derived wollastonite doped with copper for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;71:1156–1165. doi: 10.1016/j.msec.2016.11.118. [DOI] [PubMed] [Google Scholar]
- 57.Namdar A., Salahinejad E. Advances in ion-doping of Ca-Mg silicate bioceramics for bone tissue engineering. Coord Chem Rev. 2023;478:215001. [Google Scholar]
- 58.Bavya Devi K., Nandi S. K., Roy M. Magnesium silicate bioceramics for bone regeneration: a review. J Indian Inst Sci. 2019;99:261–288. [Google Scholar]
- 59.Huang Y., Jin X., Zhang X., Sun H., Tu J., Tang T., Chang J., Dai K. In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration. Biomaterials. 2009;30:5041–5048. doi: 10.1016/j.biomaterials.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 60.Xia L., Yin Z., Mao L., Wang X., Liu J., Jiang X., Zhang Z., Lin K., Chang J., Fang B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci Rep. 2016;6:22005. doi: 10.1038/srep22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi L., Fang X., Yan J., Pan C., Ge W., Wang J., Shen S. G., Lin K., Zhang L. Magnesium-containing bioceramics stimulate exosomal miR-196a-5p secretion to promote senescent osteogenesis through targeting Hoxa7/MAPK signaling axis. Bioact Mater. 2024;33:14–29. doi: 10.1016/j.bioactmat.2023.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saran U., Gemini Piperni S., Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109–117. doi: 10.1016/j.abb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Zhai W., Lu H., Chen L., Lin X., Huang Y., Dai K., Naoki K., Chen G., Chang J. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012;8:341–349. doi: 10.1016/j.actbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Mayr-Wohlfart U., Waltenberger J., Hausser H., Kessler S., Günther K. P., Dehio C., Puhl W., Brenner R. E. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 65.Diba M., Goudouri O. M., Tapia F., Boccaccini A. R. Magnesium-containing bioactive polycrystalline silicate-based ceramics and glass-ceramics for biomedical applications. Curr Opin Solid State Mater Sci. 2014;18:147–167. [Google Scholar]
- 66.Winet H. The role of microvasculature in normal and perturbed bone healing as revealed by intravital microscopy. Bone. 1996;19:39S–57S. doi: 10.1016/s8756-3282(96)00133-0. [DOI] [PubMed] [Google Scholar]
- 67.Yi D., Wu C., Ma X., Ji H., Zheng X., Chang J. Preparation and in vitro evaluation of plasma-sprayed bioactive akermanite coatings. Biomed Mater. 2012;7:065004. doi: 10.1088/1748-6041/7/6/065004. [DOI] [PubMed] [Google Scholar]
- 68.Kharaziha M., Fathi M. H. Synthesis and characterization of bioactive forsterite nanopowder. Ceram Int. 2009;35:2449–2454. [Google Scholar]
- 69.Ni S., Chang J., Chou L. In vitro studies of novel CaO-SiO2-MgO system composite bioceramics. J Mater Sci Mater Med. 2008;19:359–367. doi: 10.1007/s10856-007-3186-3. [DOI] [PubMed] [Google Scholar]
- 70.Fathi M. H., Kharaziha M. Two-step sintering of dense, nanostructural forsterite. Mater Lett. 2009;63:1455–1458. [Google Scholar]
- 71.Kharaziha M., Fathi M. H. Improvement of mechanical properties and biocompatibility of forsterite bioceramic addressed to bone tissue engineering materials. J Mech Behav Biomed Mater. 2010;3:530–537. doi: 10.1016/j.jmbbm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Naghiu M. A., Gorea M., Mutch E., Kristaly F., Tomoaia-Cotisel M. Forsterite nanopowder: structural characterization and biocompatibility evaluation. J Mater Sci Technol. 2013;29:628–632. [Google Scholar]
- 73.Devi K. B., Tripathy B., Kumta P. N., Nandi S. K., Roy M. In vivo biocompatibility of zinc-doped magnesium silicate bio-ceramics. ACS Biomater Sci Eng. 2018;4:2126–2133. doi: 10.1021/acsbiomaterials.8b00297. [DOI] [PubMed] [Google Scholar]
- 74.Devi K. B., Tripathy B., Roy A., Lee B., Kumta P. N., Nandi S. K., Roy M. In vitro biodegradation and in vivo biocompatibility of forsterite bio-ceramics: effects of strontium substitution. ACS Biomater Sci Eng. 2019;5:530–543. doi: 10.1021/acsbiomaterials.8b00788. [DOI] [PubMed] [Google Scholar]
- 75.Choudhary R., Chatterjee A., Venkatraman S. K., Koppala S., Abraham J., Swamiappan S. Antibacterial forsterite (Mg(2)SiO(4)) scaffold: A promising bioceramic for load bearing applications. Bioact Mater. 2018;3:218–224. doi: 10.1016/j.bioactmat.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie Y., Zhai W., Chen L., Chang J., Zheng X., Ding C. Preparation and in vitro evaluation of plasma-sprayed Mg(2)SiO(4) coating on titanium alloy. Acta Biomater. 2009;5:2331–2337. doi: 10.1016/j.actbio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Rizwan M., Hamdi M., Basirun W. J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J Biomed Mater Res A. 2017;105:3197–3223. doi: 10.1002/jbm.a.36156. [DOI] [PubMed] [Google Scholar]
- 78.Lalzawmliana V., Anand A., Roy M., Kundu B., Nandi S. K. Mesoporous bioactive glasses for bone healing and biomolecules delivery. Mater Sci Eng C Mater Biol Appl. 2020;106:110180. doi: 10.1016/j.msec.2019.110180. [DOI] [PubMed] [Google Scholar]
- 79.Thanigachalam M., Subramanian A. V. M. Fabrication, microstructure and properties of advanced ceramic-reinforced composites for dental implants: a review. Biomater Transl. 2023;4:151–165. doi: 10.12336/biomatertransl.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sivakumar P. M., Yetisgin A. A., Demir E., Sahin S. B., Cetinel S. Polysaccharide-bioceramic composites for bone tissue engineering: A review. Int J Biol Macromol. 2023;250:126237. doi: 10.1016/j.ijbiomac.2023.126237. [DOI] [PubMed] [Google Scholar]
- 81.Bellucci D., Cannillo V., Anesi A., Salvatori R., Chiarini L., Manfredini T., Zaffe D. Bone regeneration by novel bioactive glasses containing strontium and/or magnesium: a preliminary in-vivo study. Materials (Basel) 2018;11:2223. doi: 10.3390/ma11112223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verné E., Bretcanu O., Balagna C., Bianchi C. L., Cannas M., Gatti S., Vitale-Brovarone C. Early stage reactivity and in vitro behavior of silica-based bioactive glasses and glass-ceramics. J Mater Sci Mater Med. 2009;20:75–87. doi: 10.1007/s10856-008-3537-8. [DOI] [PubMed] [Google Scholar]
- 83.Diba M., Tapia F., Boccaccini A. R., Strobel L. A. Magnesium-containing bioactive glasses for biomedical applications. Int J Appl Glass Sci. 2012;3:221–253. [Google Scholar]
- 84.Oliveira J. M., Correia R. N., Fernandes M. H. Effects of Si speciation on the in vitro bioactivity of glasses. Biomaterials. 2002;23:371–379. doi: 10.1016/s0142-9612(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 85.Magallanes-Perdomo M., De Aza A. H., Sobrados I., Sanz J., Pena P. Structure and properties of bioactive eutectic glasses based on the Ca3(PO4)2-CaSiO3-CaMg(SiO3)2 system. Acta Biomater. 2012;8:820–829. doi: 10.1016/j.actbio.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 86.Hand R. J., Tadjiev D. R. Mechanical properties of silicate glasses as a function of composition. J Non Cryst Solids. 2010;356:2417–2423. [Google Scholar]
- 87.Zheng Q., Potuzak M., Mauro J. C., Smedskjaer M. M., Youngman R. E., Yue Y. Composition–structure–property relationships in boroaluminosilicate glasses. J Non Cryst Solids. 2012;358:993–1002. [Google Scholar]
- 88.Chen X., Ou J., Wei Y., Huang Z., Kang Y., Yin G. Effect of MgO contents on the mechanical properties and biological performances of bioceramics in the MgO-CaO-SiO2 system. J Mater Sci Mater Med. 2010;21:1463–1471. doi: 10.1007/s10856-010-4025-5. [DOI] [PubMed] [Google Scholar]
- 89.Yang S., Liang L., Liu L., Yin Y., Liu Y., Lei G., Zhou K., Huang Q., Wu H. Using MgO nanoparticles as a potential platform to precisely load and steadily release Ag ions for enhanced osteogenesis and bacterial killing. Mater Sci Eng C Mater Biol Appl. 2021;119:111399. doi: 10.1016/j.msec.2020.111399. [DOI] [PubMed] [Google Scholar]
- 90.Alinda Shaly A., Hannah Priya G., Mahendiran M., Mary Linet J. A behavioural study of hydrothermally derived novel alumina/magnesia/hydroxyapatite (Al(2)O(3)/MgO/HA) bioceramic nanocomposite. J Mech Behav Biomed Mater. 2022;133:105313. doi: 10.1016/j.jmbbm.2022.105313. [DOI] [PubMed] [Google Scholar]
- 91.Hao L., Lawrence J., Chian K. S. Effects of CO2 laser irradiation on the surface properties of magnesia-partially stabilised zirconia (MgO-PSZ) bioceramic and the subsequent improvements in human osteoblast cell adhesion. J Biomater Appl. 2004;19:81–105. doi: 10.1177/0885328204043546. [DOI] [PubMed] [Google Scholar]
- 92.Lengyel J. S., Milne J. L., Subramaniam S. Electron tomography in nanoparticle imaging and analysis. Nanomedicine (Lond) 2008;3:125–131. doi: 10.2217/17435889.3.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nandi S. K., Roy M., Bandyopadhyay A., Bose S. In vivo biocompatibility of SrO and MgO doped brushite cements. J Biomed Mater Res B Appl Biomater. 2023;111:599–609. doi: 10.1002/jbm.b.35177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li C., Sun J., Shi K., Long J., Li L., Lai Y., Qin L. Preparation and evaluation of osteogenic nano-MgO/PMMA bone cement for bone healing in a rat critical size calvarial defect. J Mater Chem B. 2020;8:4575–4586. doi: 10.1039/d0tb00074d. [DOI] [PubMed] [Google Scholar]
- 95.Ke D., Tarafder S., Vahabzadeh S., Bose S. Effects of MgO, ZnO, SrO, and SiO(2) in tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis. Mater Sci Eng C Mater Biol Appl. 2019;96:10–19. doi: 10.1016/j.msec.2018.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen R., Chen H. B., Xue P. P., Yang W. G., Luo L. Z., Tong M. Q., Zhong B., Xu H. L., Zhao Y. Z., Yuan J. D. HA/MgO nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. J Mater Chem B. 2021;9:1107–1122. doi: 10.1039/d0tb02553d. [DOI] [PubMed] [Google Scholar]
- 97.Yuan B., Chen H., Zhao R., Deng X., Chen G., Yang X., Xiao Z., Aurora A., Iulia B. A., Zhang K., Zhu X., Iulian A. V., Hai S., Zhang X. Construction of a magnesium hydroxide/graphene oxide/hydroxyapatite composite coating on Mg-Ca-Zn-Ag alloy to inhibit bacterial infection and promote bone regeneration. Bioact Mater. 2022;18:354–367. doi: 10.1016/j.bioactmat.2022.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang G., Jiang W., Mo S., Xie L., Liao Q., Hu L., Ruan Q., Tang K., Mehrjou B., Liu M., Tong L., Wang H., Zhuang J., Wu G., Chu P. K. Nonleaching antibacterial concept demonstrated by in situ construction of 2D nanoflakes on magnesium. Adv Sci (Weinh) 2020;7:1902089. doi: 10.1002/advs.201902089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janning C., Willbold E., Vogt C., Nellesen J., Meyer-Lindenberg A., Windhagen H., Thorey F., Witte F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010;6:1861–1868. doi: 10.1016/j.actbio.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 100.Pinho L. C., Alves M. M., Colaço B., Fernandes M. H., Santos C. Green-synthesized magnesium hydroxide nanoparticles induced osteoblastic differentiation in bone co-cultured cells. Pharmaceuticals (Basel) 2021;14:1281. doi: 10.3390/ph14121281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shive M. S., Anderson J. M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 102.Niiranen H., Pyhältö T., Rokkanen P., Kellomäki M., Törmälä P. In vitro and in vivo behavior of self-reinforced bioabsorbable polymer and self-reinforced bioabsorbable polymer/bioactive glass composites. J Biomed Mater Res A. 2004;69:699–708. doi: 10.1002/jbm.a.30043. [DOI] [PubMed] [Google Scholar]
- 103.Wang X., Liu M., Li H., Yin A., Xia C., Lou X., Wang H., Mo X., Wu J. MgO-incorporated porous nanofibrous scaffold promotes osteogenic differentiation of pre-osteoblasts. Mater Lett. 2021;299:130098. [Google Scholar]
- 104.Ji J., Dong X., Ma X., Tang S., Wu Z., Xia J., Wang Q., Wang Y., Wei J. Preparation and characterization of bioactive and degradable composites containing ordered mesoporous calcium-magnesium silicate and poly(l-lactide) Appl Surf Sci. 2014;317:1090–1099. [Google Scholar]
- 105.Wang C., Meng C., Zhang Z., Zhu Q. 3D printing of polycaprolactone/bioactive glass composite scaffolds for in situ bone repair. Ceram Int. 2022;48:7491–7499. [Google Scholar]
- 106.Niknam Z., Golchin A., Rezaei-Tavirani M., Ranjbarvan P., Zali H., Omidi M., Mansouri V. Osteogenic differentiation potential of adipose-derived mesenchymal stem cells cultured on magnesium oxide/polycaprolactone nanofibrous scaffolds for improving bone tissue reconstruction. Adv Pharm Bull. 2022;12:142–154. doi: 10.34172/apb.2022.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salaris V., Leonés A., López D., Kenny J. M., Peponi L. A comparative study on the addition of MgO and Mg(OH)2 nanoparticles into PCL electrospun fibers. Macromol Chem Phys. 2023;224:2200215. [Google Scholar]
- 108.Vidhya E., Vijayakumar S., Nilavukkarasi M., Punitha V. N., Snega S., Praseetha P. K. Green fabricated MgO nanoparticles as antimicrobial agent: characterization and evaluation. Mater Today Proc. 2021;45:5579–5583. [Google Scholar]
- 109.Dong Y., Yao L., Cai L., Jin M., Forouzanfar T., Wu L., Liu J., Wu G. Antimicrobial and pro-osteogenic coaxially electrospun magnesium oxide nanoparticles-polycaprolactone/parathyroid hormone-polycaprolactone composite barrier membrane for guided bone regeneration. Int J Nanomedicine. 2023;18:369–383. doi: 10.2147/IJN.S395026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan L., Chen S., Yang M., Liu Y., Liu J. Metallic materials for bone repair. Adv Healthc Mater. 2024;13:e2302132. doi: 10.1002/adhm.202302132. [DOI] [PubMed] [Google Scholar]
- 111.Alipour S., Nour S., Attari S. M., Mohajeri M., Kianersi S., Taromian F., Khalkhali M., Aninwene G. E., 2nd, Tayebi L. A review on in vitro/in vivo response of additively manufactured Ti-6Al-4V alloy. J Mater Chem B. 2022;10:9479–9534. doi: 10.1039/d2tb01616h. [DOI] [PubMed] [Google Scholar]
- 112.Rondanelli M., Faliva M. A., Tartara A., Gasparri C., Perna S., Infantino V., Riva A., Petrangolini G., Peroni G. An update on magnesium and bone health. Biometals. 2021;34:715–736. doi: 10.1007/s10534-021-00305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dorozhkin S. V. Calcium orthophosphate bioceramics. Ceram Int. 2015;41:13913–13966. [Google Scholar]
- 114.Wu C., Chang J. A review of bioactive silicate ceramics. Biomed Mater. 2013;8:032001. doi: 10.1088/1748-6041/8/3/032001. [DOI] [PubMed] [Google Scholar]
- 115.Zhang M., Yang N., Dehghan-Manshadi A., Venezuela J., Bermingham M. J., Dargusch M. S. Fabrication and properties of biodegradable akermanite-reinforced Fe35Mn alloys for temporary orthopedic implant applications. ACS Biomater Sci Eng. 2023;9:1261–1273. doi: 10.1021/acsbiomaterials.2c01228. [DOI] [PubMed] [Google Scholar]
- 116.Siahmard P., Amini Najafabadi R., Meysami A., Meysami M., Isfahani T. Investigation of structural properties of forsterite coating on AZ91 magnesium alloy by sol-gel method. Results Eng. 2023;18:101138. [Google Scholar]
- 117.Bose S., Roy M., Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–554. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewns F. K., Tsigkou O., Cox L. R., Wildman R. D., Grover L. M. Poologasundarampillai, g. hydrogels and bioprinting in bone tissue engineering: creating artificial stem-cell niches for in vitro models. Adv Mater. 2023;35:e2301670. doi: 10.1002/adma.202301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sarian M. N., Iqbal N., Sotoudehbagha P., Razavi M., Ahmed Q. U., Sukotjo C., Hermawan H. Potential bioactive coating system for high-performance absorbable magnesium bone implants. Bioact Mater. 2022;12:42–63. doi: 10.1016/j.bioactmat.2021.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.El-Rashidy A. A., Roether J. A., Harhaus L., Kneser U., Boccaccini A. R. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. doi: 10.1016/j.actbio.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 121.Liu X., Liu Y., Qiang L., Ren Y., Lin Y., Li H., Chen Q., Gao S., Yang X., Zhang C., Fan M., Zheng P., Li S., Wang J. Multifunctional 3D-printed bioceramic scaffolds: recent strategies for osteosarcoma treatment. J Tissue Eng. 2023;14:20417314231170371. doi: 10.1177/20417314231170371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma H., Feng C., Chang J., Wu C. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater. 2018;79:37–59. doi: 10.1016/j.actbio.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 123.McWilliam R. H., Chang W., Liu Z., Wang J., Han F., Black R. A., Wu J., Luo X., Li B., Shu W. Three-dimensional biofabrication of nanosecond laser micromachined nanofibre meshes for tissue engineered scaffolds. Biomater Transl. 2023;4:104–114. doi: 10.12336/biomatertransl.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He F., Yuan X., Lu T., Wang Y., Feng S., Shi X., Wang L., Ye J., Yang H. Preparation and characterization of novel lithium magnesium phosphate bioceramic scaffolds facilitating bone generation. J Mater Chem B. 2022;10:4040–4047. doi: 10.1039/d2tb00471b. [DOI] [PubMed] [Google Scholar]
- 125.Mouriño V., Boccaccini A. R. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010;7:209–227. doi: 10.1098/rsif.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alegrete N., Sousa S. R., Peleteiro B., Monteiro F. J., Gutierres M. Local antibiotic delivery ceramic bone substitutes for the treatment of infected bone cavities and bone regeneration: a systematic review on what we have learned from animal models. Materials (Basel) 2023;16:2387. doi: 10.3390/ma16062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Radwan N. H., Nasr M., Ishak R. A. H., Awad G. A. S. Moxifloxacin-loaded in situ synthesized bioceramic/poly(L-lactide-co-ε-caprolactone) composite scaffolds for treatment of osteomyelitis and orthopedic regeneration. Int J Pharm. 2021;602:120662. doi: 10.1016/j.ijpharm.2021.120662. [DOI] [PubMed] [Google Scholar]
- 128.Soundrapandian C., Datta S., Kundu B., Basu D., Sa B. Porous bioactive glass scaffolds for local drug delivery in osteomyelitis: development and in vitro characterization. AAPS PharmSciTech. 2010;11:1675–1683. doi: 10.1208/s12249-010-9550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kundu B., Soundrapandian C., Nandi S. K., Mukherjee P., Dandapat N., Roy S., Datta B. K., Mandal T. K., Basu D., Bhattacharya R. N. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: a systematic approach for in vitro and in vivo animal trial. Pharm Res. 2010;27:1659–1676. doi: 10.1007/s11095-010-0166-y. [DOI] [PubMed] [Google Scholar]
- 130.Soundrapandian C., Basu D., Sa B., Datta S. Local drug delivery system for the treatment of osteomyelitis: In vitro evaluation. Drug Dev Ind Pharm. 2011;37:538–546. doi: 10.3109/03639045.2010.528427. [DOI] [PubMed] [Google Scholar]
- 131.Thanyaphoo S., Kaewsrichan J. Synthesis and evaluation of novel glass ceramics as drug delivery systems in osteomyelitis. J Pharm Sci. 2012;101:2870–2882. doi: 10.1002/jps.23230. [DOI] [PubMed] [Google Scholar]
- 132.Deng C., Zhou Q., Zhang M., Li T., Chen H., Xu C., Feng Q., Wang X., Yin F., Cheng Y., Wu C. Bioceramic scaffolds with antioxidative functions for ROS scavenging and osteochondral regeneration. Adv Sci (Weinh) 2022;9:e2105727. doi: 10.1002/advs.202105727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Z., Li G., Ruan H., Chen K., Cai Z., Lu G., Li R., Deng L., Cai M., Cui W. Capturing magnesium ions via microfluidic hydrogel microspheres for promoting cancellous bone regeneration. ACS Nano. 2021;15:13041–13054. doi: 10.1021/acsnano.1c02147. [DOI] [PubMed] [Google Scholar]
- 134.Zhang X., Zhu Y., Cao L., Wang X., Zheng A., Chang J., Wu J., Wen J., Jiang X., Li H., Zhang Z. Alginate-aker injectable composite hydrogels promoted irregular bone regeneration through stem cell recruitment and osteogenic differentiation. J Mater Chem B. 2018;6:1951–1964. doi: 10.1039/c7tb03315j. [DOI] [PubMed] [Google Scholar]
- 135.Ostrowski N., Roy A., Kumta P. N. Magnesium phosphate cement systems for hard tissue applications: a review. ACS Biomater Sci Eng. 2016;2:1067–1083. doi: 10.1021/acsbiomaterials.6b00056. [DOI] [PubMed] [Google Scholar]
- 136.Hu J., Shao J., Huang G., Zhang J., Pan S. In vitro and in vivo applications of magnesium-enriched biomaterials for vascularized osteogenesis in bone tissue engineering: a review of literature. J Funct Biomater. 2023;14:326. doi: 10.3390/jfb14060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu Y., Wang J., Liu C., Zhang B., Chen H., Guo H., Zhong G., Qu W., Jiang S., Huang H. Evaluation of inherent toxicology and biocompatibility of magnesium phosphate bone cement. Colloids Surf B Biointerfaces. 2010;76:496–504. doi: 10.1016/j.colsurfb.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 138.Wu F., Wei J., Guo H., Chen F., Hong H., Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4:1873–1884. doi: 10.1016/j.actbio.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 139.Cabrejos-Azama J., Alkhraisat M. H., Rueda C., Torres J., Pintado C., Blanco L., López-Cabarcos E. Magnesium substitution in brushite cements: Efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. Mater Sci Eng C Mater Biol Appl. 2016;61:72–78. doi: 10.1016/j.msec.2015.10.092. [DOI] [PubMed] [Google Scholar]
- 140.Mestres G., Ginebra M. P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011;7:1853–1861. doi: 10.1016/j.actbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 141.Mestres G., Abdolhosseini M., Bowles W., Huang S. H., Aparicio C., Gorr S. U., Ginebra M. P. Antimicrobial properties and dentin bonding strength of magnesium phosphate cements. Acta Biomater. 2013;9:8384–8393. doi: 10.1016/j.actbio.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 142.Lin Z., Wu J., Qiao W., Zhao Y., Wong K. H. M., Chu P. K., Bian L., Wu S., Zheng Y., Cheung K. M. C., Leung F., Yeung K. W. K. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials. 2018;174:1–16. doi: 10.1016/j.biomaterials.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 143.Liu C., Yang G., Zhou M., Zhang X., Wu X., Wu P., Gu X., Jiang X. Magnesium ammonium phosphate composite cell-laden hydrogel promotes osteogenesis and angiogenesis in vitro. ACS Omega. 2021;6:9449–9459. doi: 10.1021/acsomega.0c06083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu Y., Deng S., Ma Z., Kong L., Li H., Chan H. F. Macrophages activated by akermanite/alginate composite hydrogel stimulate migration of bone marrow-derived mesenchymal stem cells. Biomed Mater. 2021;16:045004. doi: 10.1088/1748-605X/abe80a. [DOI] [PubMed] [Google Scholar]
- 145.Wang J. L., Xu J. K., Hopkins C., Chow D. H., Qin L. Biodegradable magnesium-based implants in orthopedics-a general review and perspectives. Adv Sci (Weinh) 2020;7:1902443. doi: 10.1002/advs.201902443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tao Z. S., Zhou W. S., He X. W., Liu W., Bai B. L., Zhou Q., Huang Z. L., Tu K. K., Li H., Sun T., Lv Y. X., Cui W., Yang L. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater Sci Eng C Mater Biol Appl. 2016;62:226–232. doi: 10.1016/j.msec.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 147.Varshney S., Nigam A., Singh A., Samanta S. K., Mishra N., Tewari R. P. Antibacterial, structural, and mechanical properties of MgO/ZnO nanocomposites and its ha-based bio-ceramics; synthesized via physio-chemical route for biomedical applications. Mater Technol. 2022;37:2503–2516. [Google Scholar]
- 148.Cui J., Xia L., Lin K., Wang X. In situ construction of a nano-structured akermanite coating for promoting bone formation and osseointegration of Ti-6Al-4V implants in a rabbit osteoporosis model. J Mater Chem B. 2021;9:9505–9513. doi: 10.1039/d1tb01917a. [DOI] [PubMed] [Google Scholar]