Deng et al.’s study1 provides a detailed protocol for isolating and evaluating mesenchymal stem cells (MSCs) from in vivo osteo-organoids by constructing in vivo organoids using gelatin sponge scaffolds loaded with recombinant human bone morphogenetic protein-2. Stem cells and tissue-derived stromal cells stimulate the repair of degraded and damaged tissues. MSCs have a good self-renewal ability and can differentiate into osteoblasts, chondrocytes, adipocytes, fibroblasts, tendon cells, and nerve cells. In recent years, preclinical and clinical studies have demonstrated that multiple sources of MSCs, such as bone marrow MSCs (BM-MSCs), have therapeutic potential for the regeneration of injured musculoskeletal tissues. However, MSCs derived from these tissues have low primary cell yield, require long in vitro expansion time, and exhibit weakened differentiation ability after passage.
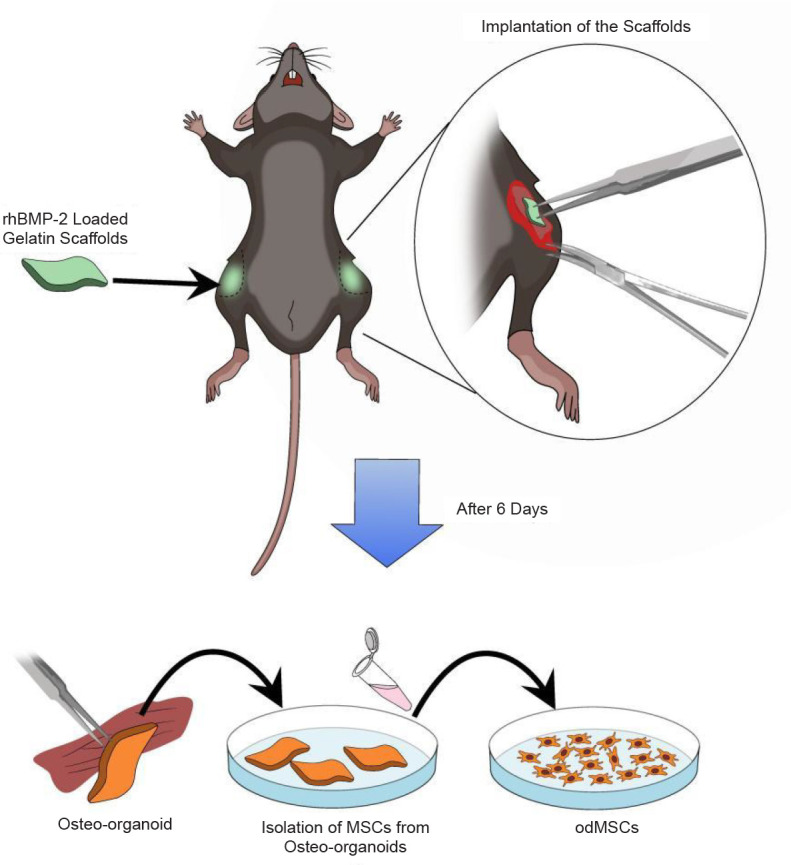
It should be noted that previous studies have found that a gelatin sponge loaded with recombinant human bone morphogenetic protein-2 can form periosteum-like tissues rich in functional stem cells after several days of implantation in mice. From these osteo-organoids, stem cells were isolated and subsequently defined as osteo-organoid-derived MSCs (odMSCs).2 In addition, it takes only 6 days to obtain the first generation of MSCs, which significantly reduces the time cost compared to 12 days to obtain the first generation of BM-MSCs (Figure 1).
Figure 1. Illustrations of harvesting functional mesenchymal stem cells from osteo-organoids in vivo. The gelatin scaffolds loaded with rhBMP-2 are lyophilised and frozen for later use. In the experiment, the scaffolds were transplanted into the muscles of both hind limbs of mice. After 6 days, the osteo-organoids were removed in situ, followed by digestion with digestive enzyme mixture to obtain odMSCs. Created with Vectornator 5.4.0. MSCs: mesenchymal stem cells; odMSCs: osteo-organoid-derived MSCs; rhBMP-2: recombinant human bone morphogenetic protein-2.
In the experiments, the researchers established osteo-organoids in the muscle of the hind limbs of mice, using the femur and tibia as controls. Compared with BM-MSCs, osteo-organoids produced a higher number of MSCs. Encouragingly, the experimental results showed that in vivo odMSCs exhibited more vital anti-replication senescence ability during passage and expansion than BM-MSCs. Moreover, odMSCs have more vital osteogenic and lipogenic differentiation ability than BM-MSCs.
In conclusion, this study provides a scheme for constructing osteo-organoids through in vivo tissue engineering technology, providing a solution for generating a large scale, high purity, and high quality of MSCs. odMSCs obtained by this method can maintain a young state during short-term expansion in vitro, and avoid the interference of factors such as cryopreservation and preservation solution, which will be conducive to the clinical transformation and application of autologous stem cells. Deng et al.’s study1 presents an alternative method for obtaining a large number of high-quality MSCs, which could serve as a new source of stem cells.
However, some concerns remain. Firstly, the origin of odMSCs is unclear, and therefore, their subtype composition and specificity are unknown. Secondly, odMSCs have not been compared with other sources of MSCs, limiting the ways in which they can be used. In addition, when affected by the surrounding microenvironment, MSCs can secrete a wide range of cell active factors, exosomes, and various extracellular vesicles to regulate the surrounding internal environment. Therefore, this study lacks validation in animal experiments and was only conducted at the cellular level, which needs further verification. Finally, different diseases may have different therapeutic needs and mechanisms, so it is necessary to further determine its feasibility and efficacy in clinical applications.
Since the 21st century, cell transplantation therapy and tissue bioengineering have been used for tissue regeneration and become potential methods to treat various diseases. The researchers obtained reliable sources of MSCs by constructing osteo-organoids in mouse hind limb muscles. However, the skeleton is a structurally complex and functionally active organ, and tissue-resident stem cells are a group of cells known for tissue-specific self-renewal and multi-lineage differentiation. Therefore, the prospect of odMSCs in clinical application can only be determined after pedigree tracing and adequate experimental verification. As for how to obtain MSCs more efficiently in the future, it can be explored not only from the way of enzymatic hydrolysis of tissues, but also from the aspects of materials that can induce MSC migration and proliferation.
In conclusion, this study proposes a novel treatment for diseases by constructing osteo-organoids to obtain MSCs. This method broadens the range of MSC sources, providing an alternative to cell transplantation strategies.
Footnotes
Author contributions: YX: Investigation, and writing-review & editing; FW: conceptualization, project administration, resources, supervision. Both authors approved the final version of the manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China (No. 82272534), The Guangdong Basic and Applied Basic Research Foundation (No. 2021B1515140056), the Chinese Universities Industry-Academia-Research Innovation Fund (No. 2023HT024), and Shenzhen Key Laboratory of Bone Tissue Repair and Translational Research (No. ZDSYS20230626091402006).
Acknowledgement: None.
Conflicts of interest statement: None.
References
- 1.Deng S., Zhu F., Dai K., Wang J., Liu C. Harvest of functional mesenchymal stem cells derived from in vivo osteo-organoids. Biomater Transl. 2023;4:270–279. doi: 10.12336/biomatertransl.2023.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai K., Zhang Q., Deng S., Yu Y., Zhu F., Zhang S., Pan Y., Long D., Wang J., Liu C. A BMP-2-triggered in vivo osteo-organoid for cell therapy. Sci Adv. 2023;9:eadd1541. doi: 10.1126/sciadv.add1541. [DOI] [PMC free article] [PubMed] [Google Scholar]


