Abstract
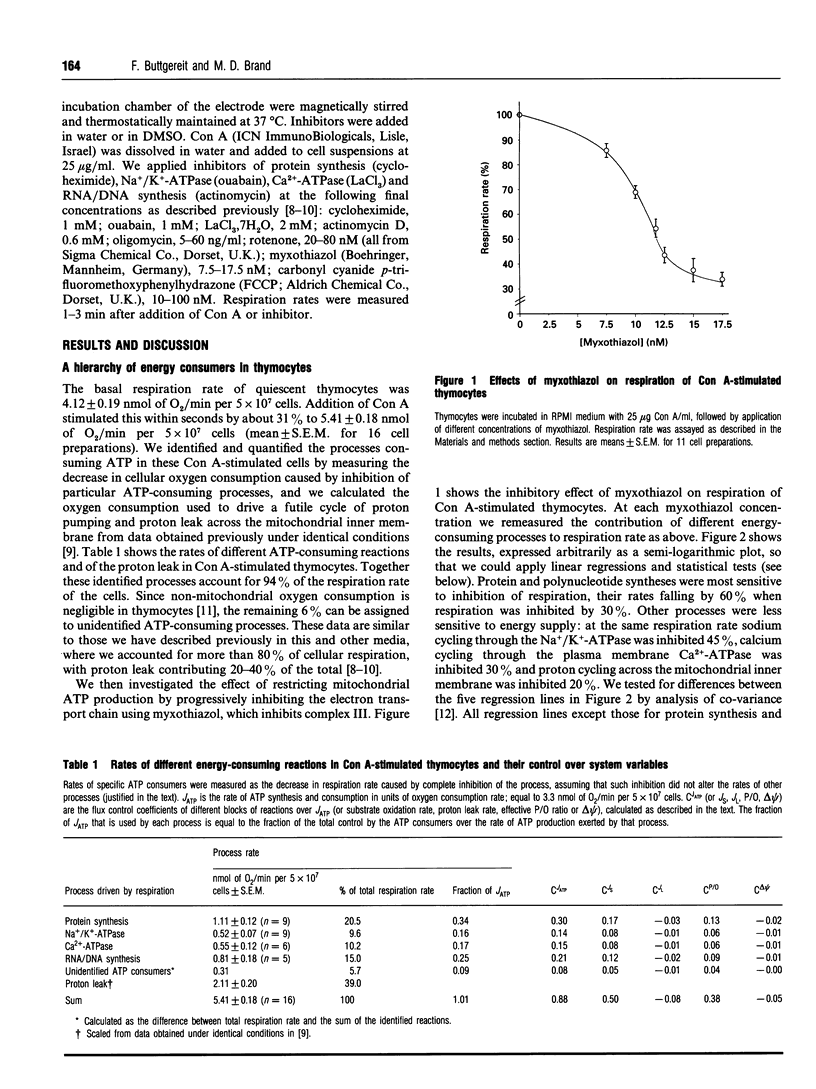
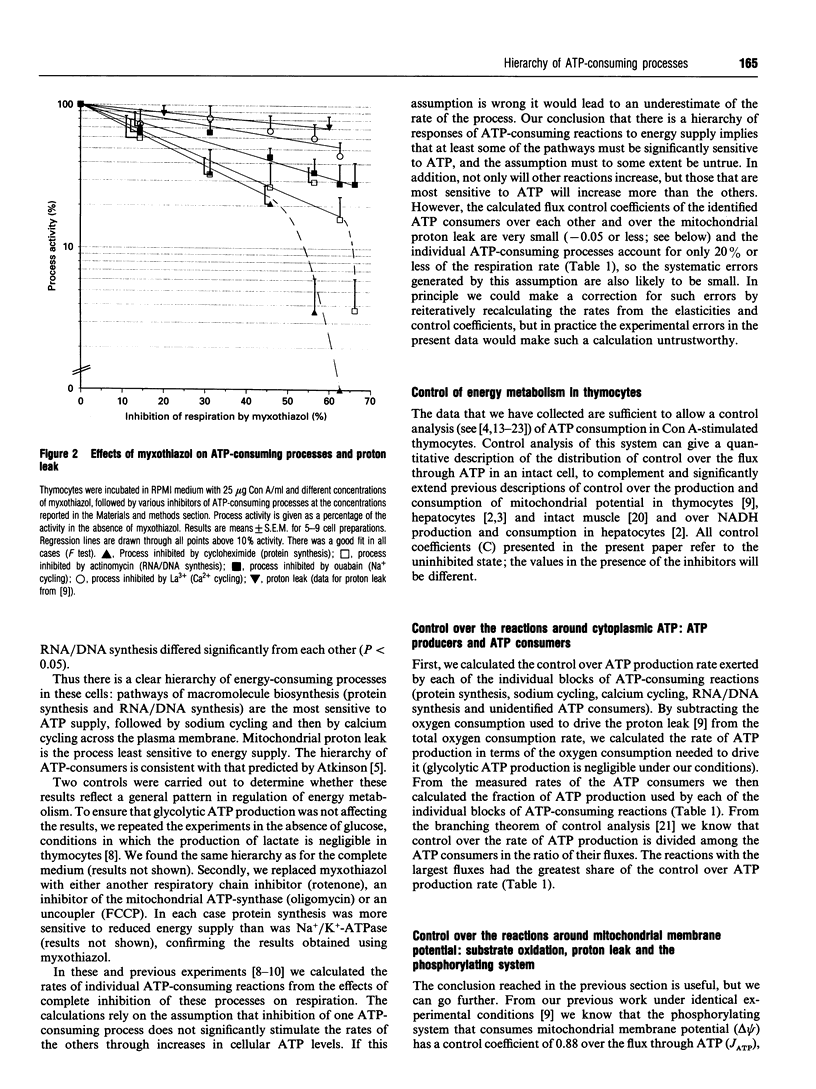
The rates of different ATP-consuming reactions were measured in concanavalin A-stimulated thymocytes, a model system in which more than 80% of the ATP consumption can be accounted for. There was a clear hierarchy of the responses of different energy-consuming reactions to changes in energy supply: pathways of macromolecule biosynthesis (protein synthesis and RNA/DNA synthesis) were most sensitive to energy supply, followed by sodium cycling and then calcium cycling across the plasma membrane. Mitochondrial proton leak was the least sensitive to energy supply. Control analysis was used to quantify the relative control over ATP production exerted by the individual groups of ATP-consuming reactions. Control was widely shared; no block of reactions had more than one-third of the control. A fuller control analysis showed that there appeared to be a hierarchy of control over the flux through ATP: protein synthesis > RNA/DNA synthesis and substrate oxidation > Na+ cycling and Ca2+ cycling > other ATP consumers and mitochondrial proton leak. Control analysis also indicated that there was significant control over the rates of individual ATP consumers by energy supply. Each ATP consumer had strong control over its own rate but very little control over the rates of the other ATP consumers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohnensack R. Theory of steady-state control in complex metabolic networks. Biomed Biochim Acta. 1985;44(11-12):1567–1578. [PubMed] [Google Scholar]
- Brand M. D., Chien L. F., Ainscow E. K., Rolfe D. F., Porter R. K. The causes and functions of mitochondrial proton leak. Biochim Biophys Acta. 1994 Aug 30;1187(2):132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Chien L. F., Rolfe D. F. Control of oxidative phosphorylation in liver mitochondria and hepatocytes. Biochem Soc Trans. 1993 Aug;21(3):757–762. doi: 10.1042/bst0210757. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Harper M. E., Taylor H. C. Control of the effective P/O ratio of oxidative phosphorylation in liver mitochondria and hepatocytes. Biochem J. 1993 May 1;291(Pt 3):739–748. doi: 10.1042/bj2910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992 May 15;284(Pt 1):1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C., Hafner R. P., Brand M. D. A 'top-down' approach to the determination of control coefficients in metabolic control theory. Eur J Biochem. 1990 Mar 10;188(2):321–325. doi: 10.1111/j.1432-1033.1990.tb15406.x. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Lakin-Thomas P. L., Brand M. D. Control of respiration and oxidative phosphorylation in isolated rat liver cells. Eur J Biochem. 1990 Sep 11;192(2):355–362. doi: 10.1111/j.1432-1033.1990.tb19234.x. [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Brand M. D., Müller M. ConA induced changes in energy metabolism of rat thymocytes. Biosci Rep. 1992 Oct;12(5):381–386. doi: 10.1007/BF01121501. [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Brand M. D., Müller M. Effects of methylprednisolone on the energy metabolism of quiescent and ConA-stimulated thymocytes of the rat. Biosci Rep. 1993 Feb;13(1):41–52. doi: 10.1007/BF01138177. [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Grant A., Müller M., Brand M. D. The effects of methylprednisolone on oxidative phosphorylation in Concanavalin-A-stimulated thymocytes. Top-down elasticity analysis and control analysis. Eur J Biochem. 1994 Jul 15;223(2):513–519. doi: 10.1111/j.1432-1033.1994.tb19020.x. [DOI] [PubMed] [Google Scholar]
- Fell D. A. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem J. 1992 Sep 1;286(Pt 2):313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D. A., Sauro H. M. Metabolic control and its analysis. Additional relationships between elasticities and control coefficients. Eur J Biochem. 1985 May 2;148(3):555–561. doi: 10.1111/j.1432-1033.1985.tb08876.x. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Pardee A. B., Goldberg A. L. The ATP dependence of the degradation of short- and long-lived proteins in growing fibroblasts. J Biol Chem. 1985 Mar 25;260(6):3344–3349. [PubMed] [Google Scholar]
- Hafner R. P., Brown G. C., Brand M. D. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the 'top-down' approach of metabolic control theory. Eur J Biochem. 1990 Mar 10;188(2):313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Brand M. D. The quantitative contributions of mitochondrial proton leak and ATP turnover reactions to the changed respiration rates of hepatocytes from rats of different thyroid status. J Biol Chem. 1993 Jul 15;268(20):14850–14860. [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kacser H. The control of enzyme systems in vivo: elasticity analysis of the steady state. Biochem Soc Trans. 1983 Jan;11(1):35–40. doi: 10.1042/bst0110035. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas P. L., Brand M. D. Stimulation of respiration by mitogens in rat thymocytes is independent of mitochondrial calcium. Biochem J. 1988 Nov 15;256(1):167–173. doi: 10.1042/bj2560167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn S. L., Nordeen S. K., Young D. A. Rapid changes in initiation-limited rates of protein synthesis in rat thymic lymphocytes correlate with energy charge. Biochem Biophys Res Commun. 1977 Nov 7;79(1):53–60. doi: 10.1016/0006-291x(77)90059-6. [DOI] [PubMed] [Google Scholar]



