Abstract
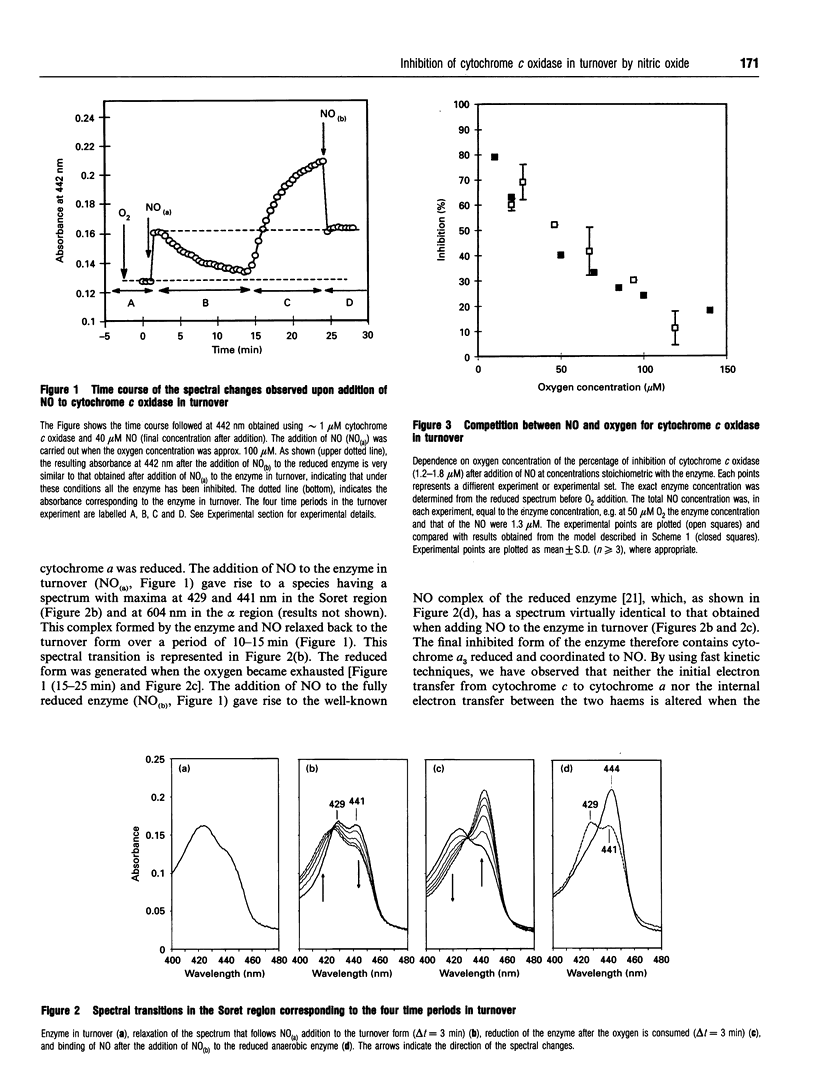
Binding of nitric oxide (NO) to isolated cytochrome c oxidase in turnover was investigated by static and kinetic spectroscopic methods. These studies indicate that cytochrome c oxidase rapidly binds NO when the enzyme enters turnover. Our results show that NO binds to ferrocytochrome a3, competing with oxygen for this binding site. However, the main features of the binding process, in particular the rapid onset of inhibition, cannot be fully explained on this basis. We suggest, therefore, that there is a second binding site for NO, which has lower affinity but nevertheless plays an important role in the inhibitory process. A likely possibility is that CuB+ constitutes this second binding site. The fast onset of inhibition observed in the presence of NO, along with the dependence on the oxygen concentration, suggests that under physiological conditions, where the oxygen concentration is low, nanomolar concentrations of NO can effectively act as a regulator of the mitochondrial respiratory chain.
Full text
PDF

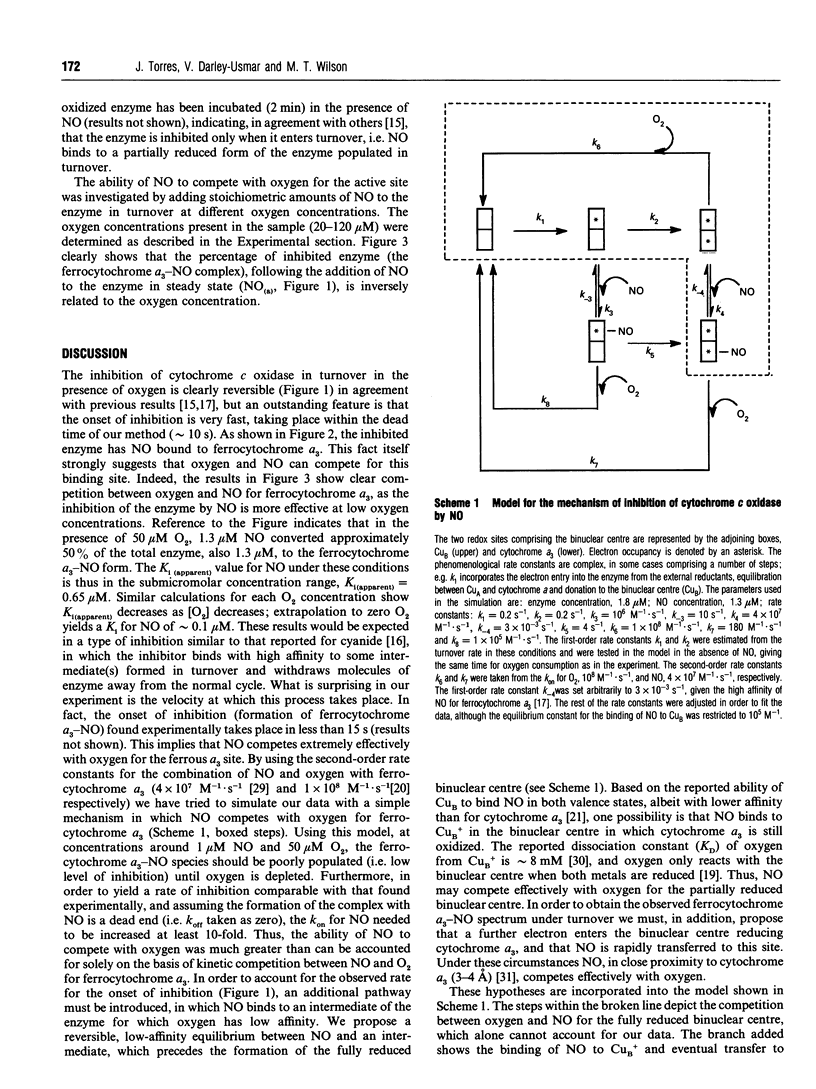

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C., Cooper C. E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994 Dec 19;356(2-3):295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- Brudvig G. W., Stevens T. H., Chan S. I. Reactions of nitric oxide with cytochrome c oxidase. Biochemistry. 1980 Nov 11;19(23):5275–5285. doi: 10.1021/bi00564a020. [DOI] [PubMed] [Google Scholar]
- Brunori M., Antonini G., Malatesta F., Sarti P., Wilson M. T. Structure and function of cytochrome oxidase: a second look. Adv Inorg Biochem. 1988;7:93–153. [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994 May 23;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Cooper C. E. The steady-state kinetics of cytochrome c oxidation by cytochrome oxidase. Biochim Biophys Acta. 1990 Jun 26;1017(3):187–203. doi: 10.1016/0005-2728(90)90184-6. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Jones M. G., Bickar D., Wilson M. T., Brunori M., Colosimo A., Sarti P. A re-examination of the reactions of cyanide with cytochrome c oxidase. Biochem J. 1984 May 15;220(1):57–66. doi: 10.1042/bj2200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Powers L., Chance B., Ching Y., Angiolillo P. Structural features and the reaction mechanism of cytochrome oxidase: iron and copper X-ray absorption fine structure. Biophys J. 1981 Jun;34(3):465–498. doi: 10.1016/S0006-3495(81)84863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. H., Brudvig G. W., Bocian D. F., Chan S. I. Structure of cytochrome a3-Cua3 couple in cytochrome c oxidase as revealed by nitric oxide binding studies. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3320–3324. doi: 10.1073/pnas.76.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A. How does NO activate hemeproteins? FEBS Lett. 1994 Mar 21;341(2-3):141–145. doi: 10.1016/0014-5793(94)80445-1. [DOI] [PubMed] [Google Scholar]
- Verkhovsky M. I., Morgan J. E., Wikström M. Oxygen binding and activation: early steps in the reaction of oxygen with cytochrome c oxidase. Biochemistry. 1994 Mar 15;33(10):3079–3086. doi: 10.1021/bi00176a042. [DOI] [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]
- Wilson M. T., Peterson J., Antonini E., Brunori M., Colosimo A., Wyman J. A plausible two-state model for cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7115–7118. doi: 10.1073/pnas.78.11.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Darbyshire J. F., Nims R. W., Saavedra J. E., Ford P. C. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993 Jan-Feb;6(1):23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. III. Improved preparation and some properties. J Biol Chem. 1961 Jun;236:1680–1688. [PubMed] [Google Scholar]


