Abstract
Our understanding of the spread of yeasts in natural ecosystems remains somewhat limited. The recent momentum of yeast ecology research has unveiled novel habitats and vectors that, alongside human activities, impact yeast communities in their natural environments. Yeasts, as non-airborne microorganisms, rely on animal vectors, predominantly insects. However, the overlooked actor in this interplay is the environmental matrix, a player potentially influencing yeast populations and their vectors. This study aims to delve deeper into the intricate, multi-layered connections between yeast populations and ecosystems, focusing on the interactions between the attributes of the environmental matrix, arthropod diversity, and the mycobiota within a renowned yeast-inhabited framework: the vineyard. To investigate these relationships, we sampled both invertebrate and yeast diversity in six organic and conventional vineyards described in terms of management and landscape composition. We identified 80 different invertebrate taxa and isolated 170 yeast strains belonging to 18 species. Notably, new species-specific yeast-insect associations were observed, including the exclusive association between Candida orthopsilosis and Hymenoptera and between Metschnikowia pulcherrima and Coleoptera. These newly identified potential associations provide valuable insights into insect and yeast physiology, hence holding the promise of enhancing our understanding of yeast and arthropod ecology and their collective impact on overall ecosystem health.
Keywords: Interspecific associations, Vectors, Systematic interactions, Mycobiota, Ecology
Subject terms: Microbial ecology, Ecology
Introduction
Yeasts are globally distributed and inhabit diverse environments, potentially establishing significant relationships with the entire systems they colonized1. Despite their importance, yeast ecology has been neglected for a long time, possibly because of the mistaken belief that these microorganisms play a lesser role in the ecosystem compared to bacteria and filamentous fungi2. On the other side, major efforts in studying yeast populations have been targeted to contexts related to human health (e.g., pathogenic species such as Candida spp.) and biotechnological (e.g., wine fermentation) applications, hence limiting at the same time the range of investigated environments and yeast species. However, a renewed general interest, potentially encouraged by observing previously overlooked environmental roles of yeast, brought yeast ecology to the limelight. In the phyllosphere, several yeasts can compete, mostly through toxin production, with other yeasts (i.e. Pichia, Sporobolomyces, Rhodotorula, Candida, Metschnikowia, Debaryomyces, Aureobasidium) or with filamentous fungi (e.g. Metschnikowia pulcherrima), thus influencing the plant microbiota3. Yeasts can also impact plant health by triggering systemic responses leading to resistance, as demonstrated experimentally for Rhodosporidium paludigenum, Metschnikovia fructicola, Candida oleophila, and Yarrowia lipolytica3. The involvement of yeasts in multi-player relationships has long been recognized, starting from the milestone discovery of yeast-drosophilid-cacti interactions, proving these associations' intricate, yet beneficial, nature4. Still, the interaction between yeasts and invertebrates is only beginning to be understood5,6. Most studies focussed on insect-yeast associations, for instance, highlighting the impact on Drosophilid behavior and development7,8 or the role of social wasps on Saccharomyces cerevisiae ecology and evolution9,10, while other arthropods’ mycobiome has been rarely investigated11. Recently, the composition of the insect-vectored mycobiota has been linked to the presence of forests12, recognized as essential environmental source and niche for yeast species13,14. Fine-tuned regulations have evolved among yeasts, insects, and environmental factors for a reciprocal healthy interaction, such as in the case of Candida and Kuraishia yeast species converting the pheromone verbenol produced by bark beetles from a monoterpene of tree resin into verbenone, an insect repellent15. This exquisite interplay ensures, at the same time, the spread of the yeasts (vectored to other trees by the repelled insects), the survival of the tree (limiting the beetle colonization), and the survival and spread of new beetle generations. Those pieces of evidence provide clear clues on the relevance of studying yeast ecology to gather fundamental insights on inter-kingdom interactions modulating and preserving natural settings. Research on peculiar agroecosystems, such as vineyards, provides some hints on ecological factors affecting yeast diversity and biology. Various studies showed that the microbial composition in vineyards varies depending on their geographical location16–18, with factors like solar radiation, temperature, precipitation, and soil characteristics influencing microbial communities19,20. Furthermore, a comprehensive mapping and phenotypic characterization of yeast species isolated from diverse environments in the USA and Alaska revealed associations between yeast taxa and substrates, with temperature playing a predominant role in yeast ecological distribution1. Despite advancing our understanding of the large-scale distribution and potential ecological selection of yeast species, these findings have yet to identify potential multi-level interactions that could shape the actual natural spreading of yeasts. To address this gap, we conducted an interdisciplinary study examining invertebrate biodiversity, environmental characteristics, including soil and land cover, and the ecology of various yeast species. Our results unveil intricate relationships between environmental factors, arthropod vectors, and yeast species within vineyard ecosystems and the surrounding environmental matrix, providing insights into broader ecological dynamics.
Results
Site selection
To adequately address the potential relationships among yeast populations, insect vectors, and environmental matrix, a pivotal step involves selecting study sites that represent the broadest spectrum of environmental characteristics while also considering agricultural management. To achieve this, we compared 55 vineyards that could be included in our research according to their soil and environmental matrix characteristics (Supplementary Table 1 and Fig. 1). The comparison of the environmental matrices of the available vineyards revealed 7 groups of sites characterized by specific combinations of the monitored attributes (K-means analysis on PCA components, Supplementary Fig. 1a). In our site selection process, we also considered the agricultural management practices of the vineyards to assess potential variations in invertebrate and yeast biodiversity associated with human practices. Consequently, we chose three couples of sites from different identified clusters, each characterized by markedly different environmental matrices and including one organic and one conventional vineyard (Supplementary Fig. 1a). To further standardize the study, we selected vineyards cultivated with the same vine type, i.e. Nebbiolo. Briefly, the resulting selection included the following vineyard couples: RB13 (B: organic) and BC55 (C: conventional); PB1 (organic) and SC4 (conventional); RB2 (organic) and TC49 (conventional) (Fig. 1). The organic vineyard RB13 (cluster 4) was characterized by the predominance of wood, water coverage, slope, altitude, and, to a lesser extent, Northing and river length. Although none of the conventional Nebbiolo vineyards exhibited a comparable level of woodland presence, BC55 closely approximated RB13 in terms of other environmental features. On the other hand, the conventional SC4 and organic PB1 vineyards were associated with high soil organic and stock carbon concentrations and urban environments. The organic RB2 and conventional TC49 vineyards were linked to extensive agricultural areas in the surrounding buffered area and were characterized by Easting exposure (Fig. 1 and Supplementary Fig. 1a).
Figure 1.
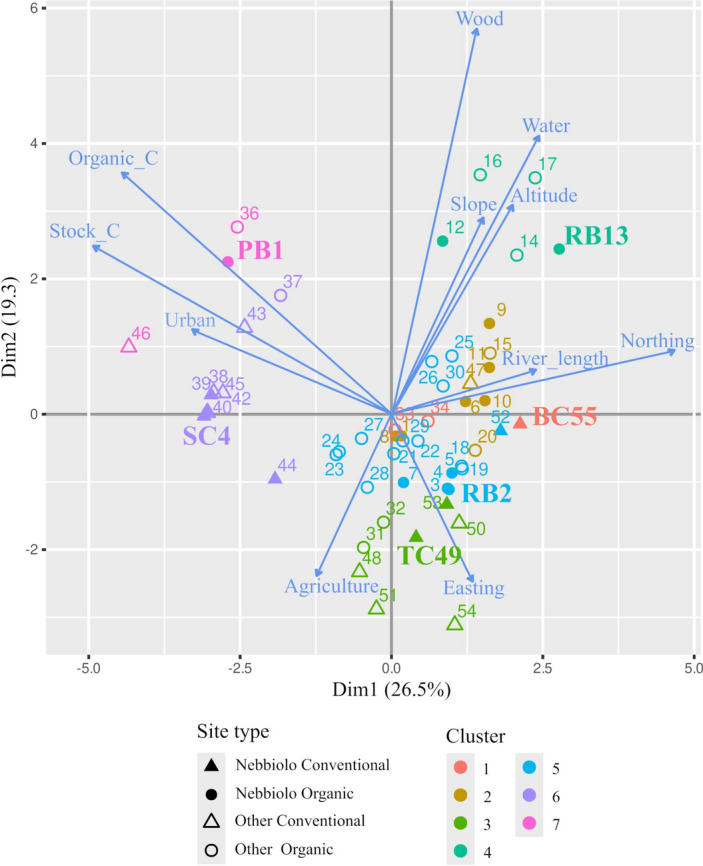
Site selection. Differentiation of vineyards under investigation according to the first two PCA components derived from the environmental matrix and soil features. The contribution of environmental matrix and soil features to explaining sample variance is illustrated by blue arrows and labeled in blue text. Sites are shown with symbols indicating the type of vine cultivated in the corresponding vineyard and the adopted management approach (organic or conventional), as reported in the graphical legend (site types). The color of site points indicates the corresponding clustering determined by K-means analysis. Sites selected for further investigations are identified by their respective IDs mentioned in the text (BC55, SC4, TC49, RB2, RB13, PB1).
After selecting the six vineyards, we delved into analyzing the features of the environmental matrix (Supplementary Fig. 1b and Supplementary Table 1). This analysis involved the examination of a 500-m radius surrounding each of the nine sampling points within every vineyard, corresponding to the locations of nine pitfall traps placed in each vineyard (Supplementary Fig. 1c). Alpha (Shannon index) and beta diversities (Bray–Curtis distances) of the environmental matrix features revealed significant differences among sites and managements (Supplementary Fig. 2, Wilcoxon–Mann–Whitney fdr and permANOVA p.values in Supplementary Table 1), hence further confirming that the selected couples of sites properly represent divergent environmental settings.
Census of Invertebrate Biodiversity
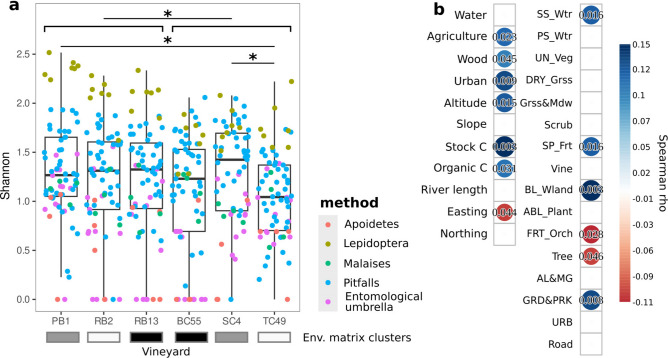
Before addressing potential associations between the features of the environmental matrix, management, and the yeast populations vectored by arthropods, we assessed and compared the selected sites in terms of overall invertebrate biodiversity. To achieve this, we employed multiple sampling approaches, each designed to monitor specific groups of invertebrates, as indicated in the materials and methods section (Supplementary Fig. 3). A total of 25,628 individuals were surveyed through all the sampling techniques, representing 80 different taxa, resulting in a rich overall biodiversity occurring in the studied sites (Fig. 2). We evaluated alpha and beta diversities to compare invertebrate biodiversity among the studied sites and management practices. Overall, organic vineyards showed a higher alpha diversity than conventional vineyards (Wilcoxon-Mann-Withney test, fdr = 0.039, Fig. 2a), confirming that the use of conventional practices may severely impact invertebrate diversity as previously observed21–24. In addition, by comparing the alpha diversities among vineyards, the conventional TC49 exhibited the lowest arthropod biodiversity, significantly differing from the organic PB1 and conventional SC4 vineyards, which share similar environmental features (Fig. 1), and hosted the highest invertebrate richness (Wilcoxon-Mann-Withney test, fdr TC49 vs PB1 = 0.022, fdr TC49 vs SC4 = 0.02, Fig. 2a).
Figure 2.
Overall arthropod alpha diversity at the study sites. (a) Alpha diversities are calculated as Shannon indices for each sample, based on the adopted method for monitoring different groups of invertebrates, as indicated in the legend. * = Wilcoxon-Mann–Whitney test, fdr < 0.05. (b) Spearman correlations among arthropod alpha diversity and environmental features of the sampling sites. Only significant correlations are shown (the numbers superimposed to circles indicating the Spearman rho indicate the corresponding fdr values). Env. Matrix clusters: different colors indicate the clusters identified in the analysis of the environmental matrix reported in Fig. 1. A full description of environmental features is reported in Supplementary Table 1.
Although the management practices affected the invertebrates' biodiversity, our study also highlighted changes in the arthropod assemblages driven by the environmental matrix. In particular, the two vineyards PB1 and SC4 (organic and conventional, respectively, Fig. 2b) characterized by high Carbon soil contents and urban environment (percentage of the area dedicated to urban use, as described in materials and methods) shared the same invertebrate richness, significantly higher compared with the TC49 vineyard (high agriculture and easting features), showing the lowest biodiversity (Fig. 2a). In support of this, we found correlations between invertebrates’ alpha biodiversity and matrix features, with positive associations between soil chemical features (stock and organic C), but also with landscape characteristics, such as surface standing waters (SS_Wtr), shrub plantations (SP_Frt), artificial broadleaved plantations (BL_Wland), and gardens and parks (GRD&PRK) (Fig. 2b). In addition, negative correlations were found between alpha invertebrates’ diversities and landscapes dedicated to cultivated trees (Tree and FRT_Orch, Fruit orchards) (Fig. 2b), where the repeated use of insecticides is likely employed to contain pests25. These results pointed out that some environmental features can have a greater impact on invertebrate diversity compared to management.
After observing significant differences in the overall arthropod biodiversity among the chosen vineyards, we explored these differences in detail by comparing the arthropod diversity monitored using different approaches. A total of 18,722 invertebrates were collected in the pitfall traps, encompassing 21 different taxa identified at either the order or class taxonomic levels (Supplementary Table 2). Temporal disparities were evident, distinguishing a lower alpha diversity in late September (T8) compared to early July (T2), irrespectively of the sampling site. Conversely, no significant differences were observed among alpha diversities of sites subjected to different management practices (Wilcoxon–Mann–Whitney test, fdr = 0.48; Supplementary Fig. 4a). A significantly lower Shannon index was observed in the TC49 site compared with all the other sites, except for PB1 (Supplementary Fig. 4a, Wilcoxon–Mann–Whitney test, fdr < 0.05, Supplementary Table 2). This result indicates that soil arthropod (sampled with pitfalls) alpha diversity is also associated with environmental features rather than management, as confirmed by significant correlations between the alpha diversity and environmental features (Supplementary Fig. 4b). Conversely, the comparison of the environmental matrix and pitfall invertebrates’ alpha diversities were found to be negatively correlated (Spearman rho = − 0.45, p.value = 6*10–04, Supplementary Table 2). The composition of invertebrate communities (Bray–Curtis beta diversity) significantly varied according to the vineyard, management practices, and sampling time (PermANOVA vineyard fdr = 0.001, management fdr = 0.002, sampling time fdr = 0.001; Supplementary Table 2, Supplementary Fig. 4b). Differently from what was observed for alpha diversities, environmental matrix and pitfall invertebrates’ Bray–Curtis distances showed positive correlations (Spearman rho = 0.38, p.value = 4 * 10–09; Mantel test r = 0.481, p.value = 0.001, Supplementary Table 2), suggesting a correlation between the environmental matrix and the structure of the invertebrate community, rather than their diversity. Walking on the transects, we recorded 1078 Lepidoptera (butterflies) and 246 Apoidea (Supplementary Table 2). Overall, we observed 52 species of butterflies belonging to 35 genera (Supplementary Table 2). The alpha diversity analysis revealed notable disparities in butterflies' biodiversity levels across vineyards and management practices (Wilcoxon-Mann–Whitney fdr in Supplementary Table 2, Supplementary Fig. 5a), potentially ascribable to significant correlations with the environmental features (Supplementary Fig. 5b). More precisely, alpha diversities remained consistent among conventional vineyards while varying significantly among organic vineyards (Supplementary Table 2, Supplementary Fig. 5a). However, the RB2 vineyard displayed no significant differences compared to the TC49 vineyard (Supplementary Table 2 and Supplementary Fig. 5a), which indeed was selected as having similar environmental characteristics to RB2 (Fig. 2). The lack of potential associations between the butterflies and environmental matrix diversities was further supported by the absence of significant correlations between the corresponding alpha diversities (Spearman rho = 0.53, p.value = 0.284, Supplementary Table 2). Conversely, butterflies and environmental matrix beta diversities (Bray–Curtis distances) showed significant positive correlations (Spearman rho = 0.38, p.value = 0.001; Mantel r = 0.338, p.value = 0.003, Supplementary Table 2). PermANOVA analysis corroborated these results, indicating significant differences among butterfly community composition associated with vineyard and management practices (Supplementary Table 2); furthermore, beta diversity highlighted differences among Lepidoptera taxa composition over sampling times, not observed according to alpha diversity (Supplementary Table 2, Supplementary Fig. 5c). The observed temporal stability of butterfly abundance over sampling time, considering the susceptibilities of butterflies to environmental variations26, indicates that the systems under analysis were not perturbed during the period of examination, with variation in the monitored species (time-dependent differentiation by beta diversity), ascribable to diverse life cycles. Apoidea alpha diversities did not show significant differences across management practices, vineyards, or sampling times (Wilcoxon-Mann–Whitney fdr in Supplementary Table 2, Supplementary Fig. 6a), also confirmed by lack of significant correlations with the environmental matrix alpha diversity (Spearman rho = 0.24, p.value = 0.652, Supplementary Table 2). In contrast, the coenosis composition (beta diversities) revealed significant differences associated with management practices (Supplementary Fig. 6b), and not-significant correlations with environmental matrix (Spearman rho = 0.62, p.value = 0.17; Mantel r = 0.286, p.value = 0.16; Supplementary Table 2). Using malaise traps, we collected a total of 4,816 insects spanning 4 orders, including Coleoptera, Diptera, Hemiptera (Rhynchota), and Hymenoptera (Supplementary Table 2, Supplementary Fig. 7). No significant differences were observed in alpha or beta diversities of insects captured across sites (Supplementary Table 2, Supplementary Fig. 7).
The entomological umbrella and direct sampling from the vine plants allowed us to collect a total of 714 arthropods for subsequent dissection to investigate the yeast populations they vector (Supplementary Table 2). Samples belonged to 11 taxa, including Acarina, Araneae, Coleoptera, Dermaptera, Diptera, Hymenoptera, Hemiptera, Lepidoptera, Odonata, Opiliones, and Orthoptera. The statistical analysis indicated no significant differences in alpha diversity among vineyards and management, besides the BC55 vineyard showing a lower alpha diversity compared to PB1, SC4, and TC49 (Supplementary Table 2, Supplementary Fig. 8a), indicating that agronomic management and environmental factors have minimal impact on the biodiversity of arthropods getting in direct contact with the vine and that our investigation of the yeast population vectored by this group of insects is not biased by an uneven cohort size among studied locations. Still, there were significant differences among beta diversities according to vineyard and management, while temporal variations were not significant (Supplementary Table 2, Supplementary Fig. 8). Thus, these results suggest that different vineyards (hence the corresponding environmental matrices) and management are characterized by the same levels of biodiversity but different compositions of insect communities potentially vectoring yeasts to grapes. This hypothesis was corroborated by the observation of significant positive correlations of alpha or beta diversities between arthropods and environmental matrix (alpha diversity, Spearman rho = 0.481, p.value = 0.001; beta-diversity Spearman rho = 0.5, p.value = 0.01 and Mantel r = 0.481, p.value = 0.001, Supplementary Table 2).
Vineyard mycobiota is associated with environmental matrix and arthropod biodiversity
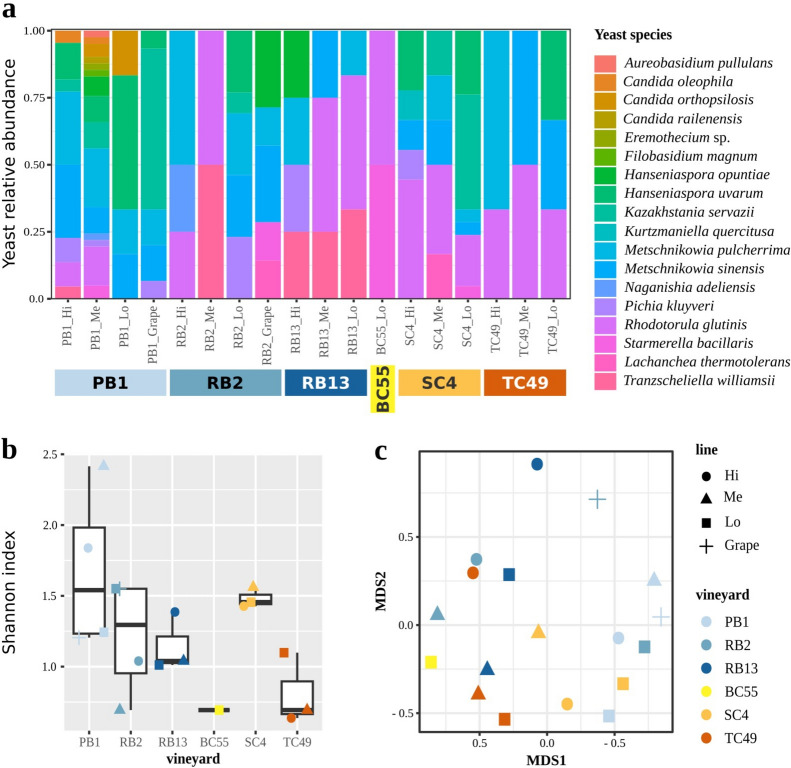
Among all the monitored groups of insects, those observed employing the entomological umbrella or directly from the vine plants were the most likely to transfer the vectored yeast population to the grapes. The observed variation in the composition of the insect monitored through this approach could imply a corresponding variation in the vectored yeast populations. Hence, we focused on and analyzed their yeast communities through culturomics, which allowed the isolation of 170 yeast isolates belonging to 18 yeast species (Fig. 3a). The most frequently isolated yeast species was Metschnikowia pulcherrima (30 occurrences), followed by Rhodotorula glutinis (29) (Fig. 3a). On the contrary, 5 of the 18 yeast species isolated from the captured insects were observed at a lower frequency, with only one occurrence each: Aureobasidium pullulans, Eremothecium sp., Filobasidium magnum, Kurtzmaniella quercitusa, and Candida railenensis. Alpha diversities of yeast communities did not differ according to source sites (vineyard) and management (Wilcoxon-Mann–Whitney test fdr values in Supplementary Table 3, Fig. 3b). However, analysis of beta diversity (Bray Curtis dissimilarity) revealed significant differences based on the vineyard, but not on management (permANOVA by vineyard fdr = 0.017, by management fdr = 0.107, Supplementary Table 3, Fig. 3c). These findings highlight that vectored yeast populations' composition, but not the diversity, is influenced more by the sampling site, rather than by agricultural techniques. This observation is particularly interesting as compositional variations in yeast communities were associated only with the site of collection, differently from what was observed for vectoring insects (collected through direct or entomological umbrella sampling) that were also associated with management (Supplementary Fig. 8). Overall, contrary to previous studies evidencing the impact of human interventions on natural microbiota27,28, our findings support new perspectives previously suggested29,30, proposing the relevance of environmental settings on the definition of yeast populations. To delve further into the potential species-specific yeast-insects associations, we searched for correlations between yeast (measured as Shannon indices) and vectoring arthropods (Shannon indices) diversities. A significant positive correlation between the two variables was observed (Spearman rho = 0.580, statistics = 559.26, p.value = 0.007) and confirmed by a Generalized Linear Model (GLM) analysis, which revealed a significant coefficient estimate of 0.532 (standard error = 0.244; t-value = 2.180; p.value = 0.043), indicating that a greater diversity of yeast species is associated with an increased diversity of vectors (Supplementary Fig. 9). The distribution of samples suggests a relevant impact of samples from the BC55 vineyard. Indeed, by removing the BC55 samples, the correlation between vector insects and yeasts alpha diversities was not statistically significant (GLM coefficient estimate of 0.404, standard error = 0.831, t-value = 0.486, p.value = 0.635); Spearman rho = 0.13, p.value = 0.635; Supplementary Table 3).Conversely, the evaluation of potential associations between yeasts and vectoring arthropods’ beta diversities (Bray–Curtis distances), revealed significant correlations both including every vineyard (Spearman rho = 0.52, p.val = 0; Mantel test r = 0.392, p.value = 0.001) and excluding the BC55 vineyard (Spearman rho = 0.52, p.val = 0; Mantel test r = 0.39, p.value = 0.001) (Supplementary Table 3). This result suggests a specific yeast-insect association: rather than the number of different taxa (yeast species or arthropod orders), is the presence of a given organism (e.g. a yeast species) to be associated with the presence of a given vector (e.g. arthropod order) or vice-versa.
Figure 3.
Yeasts isolated in the study. (a) Mycobiota composition of yeast species according to vineyard rows (Hi = high, Me = middle, Lo = low, Grape). (b) Alpha yeast diversity, evaluated as Shannon index of yeast isolates grouped by source vineyard row. (c) MDS representation of yeast beta diversity, calculated as Bray–Curtis distances among yeast communities found in different source vineyard lines (Hi = hight, Me = middle, Lo = low).
Multilevel relationships are present among yeast species, arthropod taxa, and environmental matrix characteristics
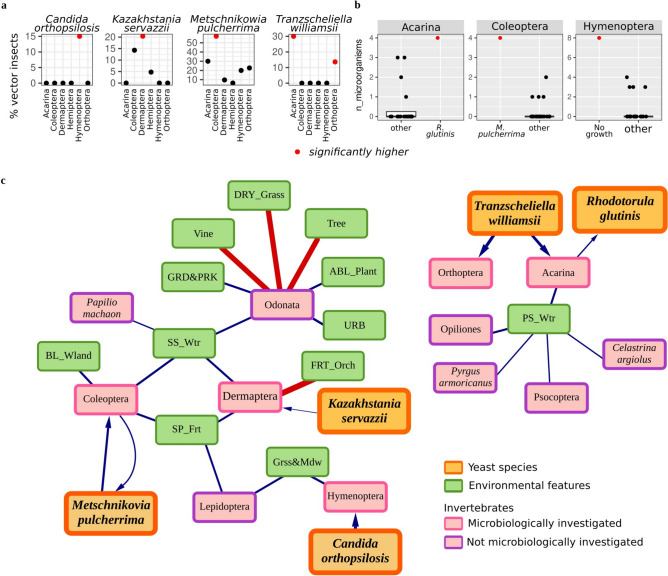
The association of both yeast and compositional features of arthropod populations (beta diversity) with sampling sites supported the hypothesis of the impact of the environmental matrix on the yeast and vector ecology and consequently on potential yeast-arthropod associations. To further delve into this multilevel relationship (yeasts, invertebrates, and matrix), we first assessed whether species-specific associations between vectors (various arthropod taxa) and yeast species could have a role in defining the composition of the vineyard mycobiota. A few yeast species were more frequently vectored by specific taxa: Candida orthopsilosis by Hymenoptera (the only insect order bearing this yeast species), Kazakhstania servazzii was found in 20% of Dermaptera, Metschnikowia pulcherrima in 57% of Coleoptera, and Tranzscheliella williamsii in 30% Acarina and 14% Orthoptera (Wilcoxon-Mann–Whitney test, fdr < 0.05; Fig. 4a and Supplementary Fig. 10). To note, Hymenoptera showed a significantly high number of cases with no yeast isolation, eventually because of an overgrowth of filamentous fungi (Supplementary Fig. 11), suggesting a poor yeast-ant association, a selective ant-yeast association, or the presence of uncultivable yeasts. This observation could support the identification of the association between C. orthopsilosis and Hymenoptera. In fact, previous studies revealed that strains of C. orthopsilosis inhibit the growth of Aspergillus sp.31, hence potentially indicating that this yeast was preferentially isolated from ants as being one of the few yeast species capable of competing against molds or because ants take advantage of the antifungal activity of C. orthopsilosis to control potential fungal pathogens32. On the other hand, it has to be considered that C. orthopsilosis is an opportunistic human pathogen, whose ecology and evolution have only recently been explored33, and the strict association with Hymenoptera could provide an additional insight into its natural distribution.
Figure 4.
Stratified correlations. (a) Percentage of vectoring insects, determined by the proportion of individual insects harboring strains of each of the 18 isolated yeast species. (b) Count of microorganisms isolated for each insect microbiologically examined. (c) Multilevel network linking the correlation between characteristics of the environmental matrix, insect taxa, and yeast species. Blue links indicate positive correlations, while red links denote negative ones. Arrows signify one-way correlations. Within the environmental matrix, green rectangles symbolize its characteristics, grouped by frame color. Pink rectangles represent insects, distinguished by the one collected by visual technique with a darker frame, and the one microbiologically analyzed with a brighter frame. Larger, orange rectangles highlight yeast species.
Hanseniaspora uvarum, Metschnikowia sinensis, and Rhodotorula glutinis were isolated from multiple taxa, each being isolated from 4 out of the 6 taxa microbiologically inspected in this study, indicating a less strict association. In particular, H. uvarum, and R. glutinis were isolated from more than 10% of Acarina, Dermaptera, Hemiptera, and Orthoptera, whereas M. sinensis from Coleoptera, Dermaptera, Hemiptera, Hymenoptera (Supplementary Fig. 10). Despite being isolated from multiple arthropods, these yeast species were associated with different vectors. All three species were isolated from Dermaptera and Hemiptera, but H. uvarum and R. glutinis were also isolated from Acarina and Orthoptera, and M. siniensis from Coleoptera and Hymenoptera. These differences could be ascribed to different ecological distributions of the yeast species: H. uvarum and R. glutinis have been found in broader ranges of environments (air, soil, insects, fruits, fermenting musts, milk, and cheese, and even marine and freshwater ecosystems)34,35 whereas the identification of M. sinensis in natural environments was limited to fruit34,36,37.
From the vector viewpoint, the mycobiota of some arthropods were enriched in a few yeast species: Acarina and Coleoptera preferentially bore R. glutinis and M. pulcherrima, respectively (Wilcoxon-Mann–Whitney test, fdr < 0.05; Fig. 4b and Supplementary Fig. 11). Upon identifying these new potential yeast-arthropod associations, we also explored possible relationships between the presence of vectors and the environmental matrix to gain insights into the role of the environment in selecting or promoting the presence of specific arthropods, potentially by providing suitable niches. We evaluated correlations between components of the environmental matrix and dissected specimens for microbiological surveys (Fig. 4c). Dermaptera and Coleoptera, despite occurring in areas sharing two environmental features (surface standing waters -SS_Wtr- and shrub plantations for ornamental or non-vineyard fruit purposes -SP_Fr-; Fig. 4c), harbor at a higher frequency two distinct yeast species (K. servazzii and M. pulcherrima). The peculiar association of these two yeast species with the two insect orders could be ascribed to the individual association of the latter to additional environmental matrix features: Coleoptera with woods (BL_Wland) and Dermaptera negatively correlated with Fruit orchards (FRT_Orch). In light of this, it is relevant to consider that M. pulcherrima is commonly found in nutrient-rich plant materials, also including tree sap fluxes and fruit34. The positive correlation found between Coleoptera, the proposed vector for M. pulcherrima, and woods and fruit trees (Fig. 4c) suggests that this yeast could be picked in the identified environmental sources by Coleoptera. Furthermore, the reciprocity observed in the correlation between Coleoptera and M. pulcherrima species suggests a mutualistic relationship. In this optic, it is worth considering that M. pulcherrima has gained attention as a biocontrol agent for apple rot38 thanks to its capability of controlling Botrytis cinerea and other molds, yeasts, and bacteria through direct competition39 or by producing pulcherrimin, a broad-spectrum antimicrobial compound40. Coleoptera could take advantage of the association with M. pulcherrima to prevent infections from pathogenic fungal species. Unfortunately, the limited information available for K. servazzii does not allow hypothesizing possible explanations for the observed associations with Dermaptera and relatively linked environmental features.
Another relevant association between invertebrates and environmental features provides fundamental hints on the newly identified yeast-insect association. The abundance of Hymenoptera, the proposed vectors for C. orthopsilosis (Fig. 4a), was positively correlated with the extent of gardens and parks (Grss&Mdw) (Fig. 4c and Supplementary Fig. 12a). Strikingly, from the available literature, we know that C. orthopsilosis has been found in various plant sources34, hence providing a potential explanation for the isolation of this yeast species from Hymenoptera. The same environmental feature potentially explaining the relationship between C. orthopsilosis and Hymenoptera, the extension of gardens and parks, is also positively associated with the abundance of Lepidoptera (Fig. 4c). The microbiological analysis of yeast populations bore by Lepidoptera, not addressed in this study, holds the promise of providing further information on yeast-insect interactions e.g., by evaluating the association between one of the three yeast species associated with the co-ecologically linked insect orders. Similarly, Odonata, whose abundance is positively associated with urban-related environmental features (gardens and parks, tree plantations—ABL_Plant-, and buildings -URB-), but not microbiologically investigated in this study, could provide relevant information on the impact of human activities on the composition of the yeast mycobiota transported to the vineyards by insects. It is worth noting that the abundance of Acarina was found to be significantly associated with permanent non-tidal, smooth-flowing watercourses (PS_Wtr) (Fig. 4c), further supporting the previously highlighted association of these arthropods with R. glutinis (Fig. 4b), a yeast species also isolated from even marine and freshwater ecosystems34,35. On the other hand, Acarina were also the principal vectors for T. williamsii, as previously highlighted, together with Orthoptera (Fig. 4a). Unfortunately, the information available on T. williamsii ecology remains poorly explored, hence avoiding the possibility of proposing an explanation for the unspecific association of this yeast with two different invertebrate taxa. Finally, variance partitioning showed that invertebrates variables explain 12.2% of the variation in yeast species composition, the environmental variables explain 13.2% of the variation in yeast species composition, and these two variable groups jointly explain 10.7% of the variation in yeast species composition. This result confirms a joint interaction of environmental matrix and invertebrate community composition in determining yeast species community composition. Nonetheless our results show that 63.8% of the variation in yeast species composition is not explained by our variable sets, this may be caused by missing factors still neglected as their potential influence is not yet recognized or by ubiquitous commensal yeast species that are vectored by most arthropods independently from the features of the environmental matrix (such as Rhodotorula glutinis).
Discussion
Overall, the correlations identified in this study hint at the complex interplay between environmental factors, insect vectors, and yeast species within the vineyard ecosystem, also providing fundamental insights into broader ecological implications of yeast-arthropod-environment interactions. Besides proposing new potential associations between yeasts and invertebrate vectors, our work also provided indirect, although essential, insights into the Saccharomyces cerevisiae-social wasps association, which is known to occur in vine agroecosystems9,10,12. Indeed, the lack of identification of S. cerevisiae in 226 microbiologically inspected insects further supports the specificity of this yeast with Vespa crabro and Polistes wasps. Conversely, several of the observed associations hold the promise to represent a key instrument for the proper understanding of yeast and arthropod ecology, as well as the implications of these interactions from a one-health perspective. The exclusive association of C. orthopsilosis and Hymenoptera could provide a natural and multifaceted tool to safeguard plants and soil, considering the beneficial impact of both partners. In addition, all the identified yeast-vector associations provide resourceful information that will help improve our knowledge of both arthropod and yeast ecology and physiology, such as the one highlighted by the mutual interaction between M. pulcherrima and Coleoptera.
Material and methods
Selection and characteristics of sampling areas
A preliminary survey was conducted to engage local winemakers and identify potential study sites. Vineyard owners willing to take part in the research were asked to complete a questionnaire, resulting in the inclusion of 55 vineyards in the initial screening process. The questionnaire aimed to gather information on various aspects, including vineyard geographical coordinates, grape variety, management (organic or conventional), year of the official application of the management, land use practices in neighboring areas, botanical characteristics (e.g., vine origin: seed or grafted cutting), type of grafting (e.g., whip-and-tongue grafting, bark grafting), vineyard and vine age, use of phytosanitary products (if conventional regulations apply), and other treatments (e.g., fungicides) including frequency and application methods. Additional information on agronomic practices such as ground cover methods (seeding or natural growth) and cultivation systems (e.g., Guyot, spurred cordon, Casarsa, canopy, bush, Sylvoz, Geneva double curtain, and pergola) was documented. Each vineyard was described at a landscape scale, creating a buffer of 500 m from the center of the vineyard and computing woodland areas, impervious areas, water bodies, exposition, and soil carbon content obtained from Geoportale Piemonte as described in Valentini et al.12. Following data collection, Principal Component Analysis (PCA) and K-means clustering analyses were conducted on the vineyard features. Six vineyards were selected as belonging to clusters associated with distinct environmental matrix compositions and their management (organic or conventional practices). For these six selected vineyards, we analyzed further environmental variables available on the Geoportale Piemonte database, which was again integrated using QGIS (v. 3.24.1)41. These characteristics are categorized and listed in Supplementary Table 1, to facilitate the subsequent analyses.
Invertebrate monitoring, collection, and dissection
Various methods were employed to monitor the occurrence of invertebrates on vine, soil, or flying in the vineyards. Butterflies were identified down to the species level, while other arthropods were classified by their class or order, depending on the sampling technique used, as explained below. The pitfall trapping was employed to investigate the abundance and diversity of soil invertebrates42. In each vineyard, nine pitfall traps were positioned (Supplementary Fig. 1), three traps for each external vineyard row (High or “Hi”, Low or “Lo”), and three traps in the middle row (Middle or “Me”). Twenty ml of 70% ethylene glycol (v/v) were added to each pitfall (plastic container of 120 ml volume). Sampling occurred at 15-day intervals from mid-June 2023 to the end of September 2023. Invertebrates captured in each pitfall were identified at the order or class level using dichotomous keys43. Neuroptera was excluded from the analysis because only one insect was captured. Lepidoptera and Hymenoptera Apoidea were counted by walking for 45 min along linear transects of 400 m and 150 m, respectively44. These surveys were conducted once every 2 weeks from late June to mid-September for butterflies and once a month for bees on sunny days with scarce wind, between 10 a.m. and 3 p.m. All butterflies were caught by a 40 cm diameter net, and a few individuals (i.e., Pyrgus spp.) were collected and identified in the laboratory. Butterflies were identified at the species level according to the key tables reported in the “Collins Butterfly Guide”45. Bees were identified as Apis mellifera, Bombus spp., or “other Apoidea”. Eight Lepidoptera species (i.e., Everes alcetas, Everes alcetas/decoloratus, Inachis io, Lampides boeticus/pirithous, Leptotes pirithous, Lysandra bellargus, Spialia sertorius, and Thymelicus sylvestris) were excluded from the analysis because they were represented by only a single individual. To capture other flying insects, malaise traps (Omnes Artes s. a. s.), each measuring 1.8 m3, were placed in each vineyard46. Each malaise trap was equipped with a bottle containing a 70% (v/v) ethylene glycol solution to preserve captured samples. All traps were installed between the 4th and 6th of July, and their contents were analyzed monthly from July to October. Direct collection from grape vines, combined with samplings performed by an entomological umbrella47, was employed to gather arthropods for investigating yeast populations. Briefly, a white umbrella was positioned beneath the grape clusters, aligned with each pitfall; a wooden stick was used to beat and shake the plants for two series of 10 strokes to extract arthropods from the vines; the dislodged arthropods accumulated in the entomological umbrella were individually preserved alive in sterile 50 ml tubes. Additionally, arthropods were collected after destemming 5 kg of grapes on-site to survey their yeast community (hereafter identified as “grape” arthropods and relative yeasts). Organisms sampled using these two techniques were picked using tweezers sterilized with 70% ethanol. These samples were sorted at the laboratory, identified according to the same key used for pitfall-collected arthropods, and stored at 4–8 °C until the dissection. The orders Lepidoptera, Odonata, and Opiliones were excluded from the analysis because only a few insects were captured. Culturomics of Araneae and Diptera resulted in the predominance of molds, hindering the isolation of yeasts, and were then excluded from the analysis. The dissection procedure involved subjecting each individual to a temperature of − 20 °C for 20 min. Subsequently, arthropods were washed once in sterile water and dissected using sterile tweezers; the content of the intestine was mechanically dissolved in the same sterile water used for the initial wash to sample gut and surface microbiota simultaneously. Smaller items, such as Hymenoptera and Araneae, were initially subjected to abdomen opening using microscissors and a microscope and were then crushed in 100 µl of sterile water. The intestine content of bigger arthropods was dissolved in 300–600 μl of sterile water according to the size. A 100 μl aliquot from the solutions obtained was spread onto a nutrient-rich solid YPD medium (1% Yeast Extract, 2% Peptone, 2% d-glucose, 2% Agar) supplemented with penicillin (10,000 U/ml) and streptomycin (10 mg/l) to prevent bacterial growth. The plates were then incubated at 28 °C for 48 h.
Yeasts’ isolation, identification, and storage
Yeast colonies cultivated on YPD medium were visually inspected, and colonies displaying distinct morphology were re-isolated on YPD before identification through PCR–RFLP and following confirmation through Sanger sequencing. This identification was based on the interspecific variability of the ITS marker region, encompassing the ITS1 and ITS2 regions and the 5.8S rRNA gene, amplified using the primers ITS1 (FW) 5′-GTTTCCGTAGGTGAACTTGC-3′ and Primer ITS4 (RV) 5′-TCCTCCGCTTATTGATATGC-3′48. After an initial denaturation step at 95 °C for 1 min to activate the GoTaq DNA polymerase (PROMEGA), DNAs were subjected to 35 cycles of amplification (95 °C for 30 s, 53.6 °C for 30 s, and 72 °C for 1 min 30 s), followed by a final extension at 72 °C for 10 min. The PCR products were subjected to digestion with the HaeIII endonuclease for 1 h at 37 °C. The size of digested PCR products was quantified on a 2.5% agarose gel and compared with those in the Esteve-Zarzoso protocol48. Sanger sequences of the ITS1-5.8S-ITS2 region for strains representative of the identified ITS1-5.8S-ITS2 PCR-RFLP profiles were compared with the rRNA_typestrains/ITS_RefSeq_Fungi GenBank database using the standard nucleotide BLAST on the NCBI website (threshold for identification: 98% identity percentage) and deposited on NCBI (ID PQ050703-PQ050731, information in Supplementary Table 3). Yeast cells were preserved in a sterile 15% (w/v) glycerol solution at − 80 °C.
Statistical analysis
All statistical analyses were performed using R version 4.3.249. Principal Component Analysis (PCA) and K-means clustering analyses were conducted by using the prcomp and the eclust functions49,50. Alpha diversity indexes (Shannon and observed) were computed for both invertebrates and yeasts using the vegan package51, followed by visualization employing the ggplot2 package52. Pairwise comparisons among alpha diversities were conducted with Wilcoxon–Mann–Whitney tests implemented with the pairwise.wilcox.test function, followed by multiple testing p.values correction (false discovery rate, fdr). Bray–Curtis dissimilarities were calculated with the vegdist function of the vegan package and visualized through Non-metric Multidimensional Scaling (NMDS), performed with the metaMDS function of the vegan R package51. Permutational Analysis of Variance (permANOVA) was performed using the adonis2 function from the vegan package51 to determine the significance of differences between groups. The various monitoring methods provided information at different levels (vineyard, row, or pitfall). To ensure the highest possible precision of the comparisons, statistical analyses were performed by grouping data according to the deepest level possible, meaning: vineyards for Lepidoptera, Apoidea, and malaise analysis; vineyard rows for direct sampling, entomological umbrella, and environmental analysis; pitfall positions for the pitfall method. The effect of alpha diversity (Shannon Index) of arthropods sampled via direct sampling and entomological umbrella methods on the yeast alpha diversity (Shannon index) was tested through a stepwise Generalized Linear Model (GLM). Spearman correlations were performed between the number of arthropods monitored with various methods and the environmental matrix features (expressed as a percentage of surface in the study area) with the rcorr function of the Hmisc R package53 and significant correlations were plotted with the corrplot function54. Yeast-arthropod associations were evaluated by comparing the distribution of yeast species in the analyzed insects. The percentage of individuals providing strains of the isolated yeast species was calculated for each isolated yeast species (% vectoring insects) to quantify the preferential isolation of yeast species from a specific arthropod taxon. Wilcoxon–Mann–Whitney test was performed with the wilcox.test R function49 by comparing the percentage of isolation of a given yeast species from a given arthropod taxon and the percentage of isolation of the same yeast species from other arthropods. The number of arthropods providing a particular yeast species was calculated to quantify the enrichment of insect mycobiota in specific yeast species. Wilcoxon–Mann–Whitney test was performed with the wilcox.test R function49 to compare the number of arthropods (eventually grouped according to the taxon) providing a given yeast species with the number of arthropods carrying other yeast species. A network plot was implemented with Cytoscape55 on a matrix generated by combining significant results of correlation and enrichment analyses described earlier. Variance partinioning was performed using the function varpart in the vegan package51 to determine the specific contribution of invertebrates and environmental factors to yeast presence.
Supplementary Information
Acknowledgements
The work was supported by the Human Frontiers Science Program (RGP0060/2021) and Fondazione Cassa di Risparmio di Cuneo. The work was only possible thanks to the contribution, interest, and engagement of the participating winemakers; we would like to express our gratitude to Enrico Rivetto (Azienda Agricola Rivetto, https://rivetto.it/en/), Paolo Ghislandi (I Carpini, https://icarpini.it/en/), Pastoris wines (https://pastorisvini.com/), Tartuflanghe (https://tartuflanghe.com/), Terre del Barolo (https://terredelbarolo.com/en/), and Mauro Canale (Cantine della Serra, https://www.cantinadellaserra.com/en/nomi cantina/azienda). The authors would like to thank Arthur Hais for the support in insect identification, Silvia Abbà, and Davide Bongiovanni for support in fieldwork.
Author contributions
Conceptualization: IS, FB; Data curation: BV; Formal analysis: BV, IS; Investigation: BV, MP, MV, EC, FB; Methodology: FB, LPC, EC, IS, BV; Visualization: BV, IS; Writing—original draft: BV, IS; Writing -review and editing: FB, EC, LPC.
Data availability
All the data produced in this study are provided as supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70327-4.
References
- 1.Spurley, W. J. et al. Substrate, temperature, and geographical patterns among nearly 2000 natural yeast isolates. Yeast.39, 55–68. 10.1002/yea.3679 (2022). 10.1002/yea.3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boynton, P., Patil, K. R., Stefanini, I., Stelkens, R. & Cubillos, F. A. Yeast ecology and communities. Yeast.39, 3. 10.1002/yea.3698 (2022). 10.1002/yea.3698 [DOI] [PubMed] [Google Scholar]
- 3.Gouka, L., Raaijmakers, J. M. & Cordovez, V. Ecology and functional potential of phyllosphere yeasts. Trends Plant Sci.27, 1109–1123. 10.1016/j.tplants.2022.07.008 (2022). 10.1016/j.tplants.2022.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Starmer, W. T., Heed, W. B., Miranda, M., Miller, M. W. & Phaff, H. J. The ecology of yeast flora associated with cactiphilic Drosophila and their host plants in the Sonoran desert. Microb. Ecol.3, 11–30. 10.1007/BF02011450 (1976). 10.1007/BF02011450 [DOI] [PubMed] [Google Scholar]
- 5.Madden, A. A. et al. The ecology of insect-yeast relationships and its relevance to human industry. Proc. Biol. Sci.285, 20172733. 10.1098/rspb.2017.2733 (2018). 10.1098/rspb.2017.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega, F. E. & Dowd, P. F. The role of yeasts as insect endosymbionts. In Insect-Fungal Associations: Ecology and Evolution. 211–243, (2005).
- 7.Lewis, M. T. & Hamby, K. A. Differential impacts of yeasts on feeding behavior and development in larval Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep.9, 13370. 10.1038/s41598-019-49928-9 (2019). 10.1038/s41598-019-49928-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilhot, R. et al. Influence of bacteria on the maintenance of a yeast during Drosophila melanogaster metamorphosis. Anim. Microbiome.3, 68. 10.1186/s42523-021-00138-x (2021). 10.1186/s42523-021-00138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefanini, I. et al. Role of social wasps in Saccharomyces cerevisiae ecology and evolution. Proc. Natl. Acad. Sci. USA109, 13398–13403. 10.1073/pnas.1208362109 (2012). 10.1073/pnas.1208362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanini, I. et al. Social wasps are a Saccharomyces mating nest. Proc. Natl. Acad. Sci. USA113, 2247–2251. 10.1073/pnas.1516453113 (2016). 10.1073/pnas.1516453113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackwell, M. Made for each other: Ascomycete yeasts and insects. Microbiol. Spectr.10.1128/microbiolspec.FUNK-0023-2016 (2017). 10.1128/microbiolspec.FUNK-0023-2016 [DOI] [PubMed] [Google Scholar]
- 12.Valentini, B. et al. Forests influence yeast populations vectored by insects into vineyards. Front. Microbiol.13, 1039939. 10.3389/fmicb.2022.1039939 (2022). 10.3389/fmicb.2022.1039939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozzachiodi, S. et al. Yeasts from temperate forests. Yeast39, 4–24. 10.1002/yea.3699 (2022). 10.1002/yea.3699 [DOI] [PubMed] [Google Scholar]
- 14.Rosa, C. A. et al. Yeasts from tropical forests: Biodiversity, ecological interactions, and as sources of bioinnovation. Yeast.40, 511–539. 10.1002/yea.3903 (2023). 10.1002/yea.3903 [DOI] [PubMed] [Google Scholar]
- 15.Leufvén, A., Bergström, G. & Falsen, E. Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J. Chem. Ecol.10, 1349–1361. 10.1007/BF00988116 (1984). 10.1007/BF00988116 [DOI] [PubMed] [Google Scholar]
- 16.Bokulich, N. A., Thorngate, J. H., Richardson, P. M. & Mills, D. A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA111, E139–E148. 10.1073/pnas.1317377110 (2014). 10.1073/pnas.1317377110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert, J. A., van der Lelie, D. & Zarraonaindia, I. Microbial terroir for wine grapes. Proc. Natl. Acad. Sci. USA111, 5–6. 10.1073/pnas.1320471110 (2014). 10.1073/pnas.1320471110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kioroglou, D., Kraeva-Deloire, E., Schmidtke, L. M., Mas, A. & Portillo, M. C. Geographical origin has a greater impact on grape berry fungal community than grape variety and maturation state. Microorganisms.7, 669. 10.3390/microorganisms7120669 (2019). 10.3390/microorganisms7120669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, R. et al. The biogeography of fungal communities across different Chinese wine-producing regions associated with environmental factors and spontaneous fermentation performance. Front. Microbiol.12, 636639. 10.3389/fmicb.2021.636639 (2022). 10.3389/fmicb.2021.636639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalvantzi, I., Banilas, G., Tassou, C. & Nisiotou, A. Biogeographical regionalization of wine yeast communities in Greece and environmental drivers of species distribution at a local scale. Front. Microbiol.12, 705001. 10.3389/fmicb.2021.705001 (2021). 10.3389/fmicb.2021.705001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wersebeckmann, V., Biegerl, C., Leyer, I. & Mody, K. Orthopteran diversity in steep slope vineyards: The role of vineyard type and vegetation management. Insects.14, 83. 10.3390/insects14010083 (2023). 10.3390/insects14010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kratschmer, S. et al. Response of wild bee diversity, abundance, and functional traits to vineyard inter-row management intensity and landscape diversity across Europe. Ecol. Evol.9, 4103–4115. 10.1002/ece3.5039 (2019). 10.1002/ece3.5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiera, C. et al. Effects of vineyard inter-row management on the diversity and abundance of plants and surface-dwelling invertebrates in Central Romania. J. Insect Conserv.24, 175–185. 10.1007/s10841-020-00227-4 (2020). 10.1007/s10841-020-00227-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sáenz-Romo, M. G. et al. Effects of ground cover management on insect predators and pests in a Mediterranean vineyard. Insects.10, 421. 10.3390/insects10120421 (2019). 10.3390/insects10120421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AliNiazee, M. T. Ecology and management of hazelnut pests. Annu. Rev. Entomol.43, 395–419. 10.1146/annurev.ento.43.1.395 (1998). 10.1146/annurev.ento.43.1.395 [DOI] [PubMed] [Google Scholar]
- 26.Warren, M. S. et al. The decline of butterflies in Europe: Problems, significance, and possible solutions. Proc. Natl. Acad. Sci. USA10.1073/pnas.2002551117 (2021). 10.1073/pnas.2002551117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colautti, A., Civilini, M., Contin, M., Celotti, E. & Iacumin, L. Organic vs. conventional: Impact of cultivation treatments on the soil microbiota in the vineyard. Front. Microbiol.14, 1242267. 10.3389/fmicb.2023.1242267 (2023). 10.3389/fmicb.2023.1242267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendgen, M. et al. Effects of different management regimes on microbial biodiversity in vineyard soils. Sci. Rep.8, 9393. 10.1038/s41598-018-27743-0 (2018). 10.1038/s41598-018-27743-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gobbi, A. et al. A global microbiome survey of vineyard soils highlights the microbial dimension of viticultural terroirs. Commun. Biol.5, 241. 10.1038/s42003-022-03214-6 (2022). 10.1038/s42003-022-03214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longa, C. M. O. et al. Soil microbiota respond to green manure in organic vineyards. J. Appl. Microbiol.123, 1547–1560. 10.1111/jam.13606 (2017). 10.1111/jam.13606 [DOI] [PubMed] [Google Scholar]
- 31.Sukmawati, D. et al. Biocontrol activity of Aureobasidium pullulans and Candida orthopsilosis isolated from Tectona grandis L. Phylloplane against Aspergillus sp. in post-harvested citrus fruit. Sustainability.13, 7479. 10.3390/su13137479 (2021). 10.3390/su13137479 [DOI] [Google Scholar]
- 32.Lin, W. J., Chiu, M. C., Lin, C. C., Chung, Y. K. & Chou, J. Y. Efficacy of entomopathogenic fungus Aspergillus nomius against Dolichoderus thoracicus. BioControl.66, 463–473. 10.1007/s10526-021-10103-0 (2021). 10.1007/s10526-021-10103-0 [DOI] [Google Scholar]
- 33.Del Olmo, V. et al. Origin of fungal hybrids with pathogenic potential from warm seawater environments. Nat. Commun.14, 6919. 10.1038/s41467-023-42679-4 (2023). 10.1038/s41467-023-42679-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtzman, C. P., Fell, J. W. & Boekhout, T. The Yeasts, a Taxonomic Study 5th edn. (Elsevier B.V, 2011). 10.1016/C2010-0-67052-X. [Google Scholar]
- 35.Hernandez-Almanza, A. et al.Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci.5, 64–72. 10.1016/j.fbio.2013.11.007 (2014). 10.1016/j.fbio.2013.11.007 [DOI] [Google Scholar]
- 36.Lorenzini, M., Simonato, B. & Zapparoli, G. Yeast species diversity in apple juice for cider production evidenced by culture-based method. Folia Microbiol.63, 677–684. 10.1007/s12223-018-0609-0 (2018). 10.1007/s12223-018-0609-0 [DOI] [PubMed] [Google Scholar]
- 37.Pawlikowska, E., James, S. A., Breierova, E., Antolak, H. & Kregiel, D. Biocontrol capability of local Metschnikowia sp isolates. Antonie van Leeuwenhoek.112, 1425–1445. 10.1007/s10482-019-01272-w (2019). 10.1007/s10482-019-01272-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janisiewicz, W. J., Tworkoski, T. J. & Kurtzman, C. P. Biocontrol potential of Metchnikowia pulcherrima strains against blue mold of apple. Phytopathology.91, 1098–1108. 10.1094/PHYTO.2001.91.11.1098 (2001). 10.1094/PHYTO.2001.91.11.1098 [DOI] [PubMed] [Google Scholar]
- 39.Kregiel, D., Nowacka, M., Rygala, A. & Vadkertiová, R. Biological activity of Pulcherrimin from the Meschnikowia pulcherrima clade. Molecules27, 1855. 10.3390/molecules27061855 (2022). 10.3390/molecules27061855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipiczki, M. Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol.72, 6716–6724. 10.1128/AEM.01275-06 (2006). 10.1128/AEM.01275-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.QGIS Development Team. QGIS geographic information system. Open Source Geospatial Foundation Project. http://qgis.osgeo.org (2022).
- 42.Geldenhuys, M., Gaigher, R., Pryke, J. S. & Samways, M. J. Diverse herbaceous cover crops promote vineyard arthropod diversity across different management regimes. Agric. Ecosyst. Environ.307, 107222. 10.1016/j.agee.2020.107222 (2021). 10.1016/j.agee.2020.107222 [DOI] [Google Scholar]
- 43.Capinera, J. L. Insect keys. Encycl. Entomol.10.1007/978-1-4020-6359-6_1834 (2008). 10.1007/978-1-4020-6359-6_1834 [DOI] [Google Scholar]
- 44.Pollard, E. & Yates, T. J. Monitoring Butterflies for Ecology and Conservation: The British Butterfly Monitoring Scheme (Springer Science & Business Media, 1993). [Google Scholar]
- 45.Tolman, T. Collins Butterfly Guide (HarperCollins UK, 2008). [Google Scholar]
- 46.Uhler, J. et al. A comparison of different Malaise trap types. Insect Conserv. Divers.15, 666–672. 10.1111/icad.12604 (2022). 10.1111/icad.12604 [DOI] [Google Scholar]
- 47.Campos, R. I., Vasconcelos, H. L., Ribeiro, S. P., Neves, F. S. & Soares, J. P. Relationship between tree size and insect assemblages associated with Anadenanthera macrocarpa. Ecography.29, 442–450. 10.1111/j.2006.0906-7590.04586.x (2006). 10.1111/j.2006.0906-7590.04586.x [DOI] [Google Scholar]
- 48.Esteve-Zarzoso, B., Belloch, C., Uruburu, F. & Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol.49, 329–337. 10.1099/00207713-49-1-329 (1999). 10.1099/00207713-49-1-329 [DOI] [PubMed] [Google Scholar]
- 49.R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org (R Foundation for Statistical Computing, 2023)
- 50.Kassambara, A. & Mundt, F. factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.7. http://www.sthda.com/english/rpkgs/factoextra (2020).
- 51.Oksanen, J. et al. Vegan: community ecology package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan (2020).
- 52.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016). 10.1007/978-3-319-24277-4. [Google Scholar]
- 53.Harrell Jr, F. Hmisc: Harrell Miscellaneous. R package version 5.1-1. https://CRAN.R-project.org/package=Hmisc (2023).
- 54.Wei, T. & Simko, V. R package 'corrplot': Visualization of a Correlation Matrix (Version 0.92). https://github.com/taiyun/corrplot (2021).
- 55.Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res.13, 2498–2504. 10.1101/gr.1239303 (2003). 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data produced in this study are provided as supplementary material.