Abstract
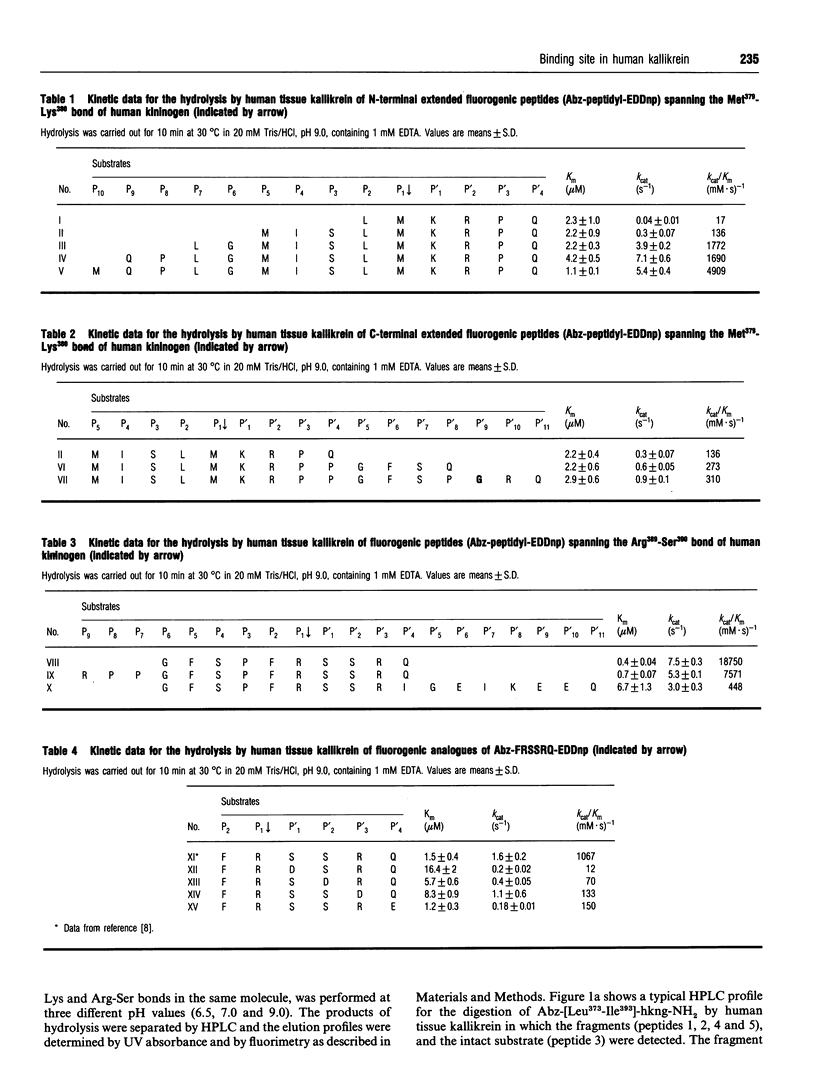
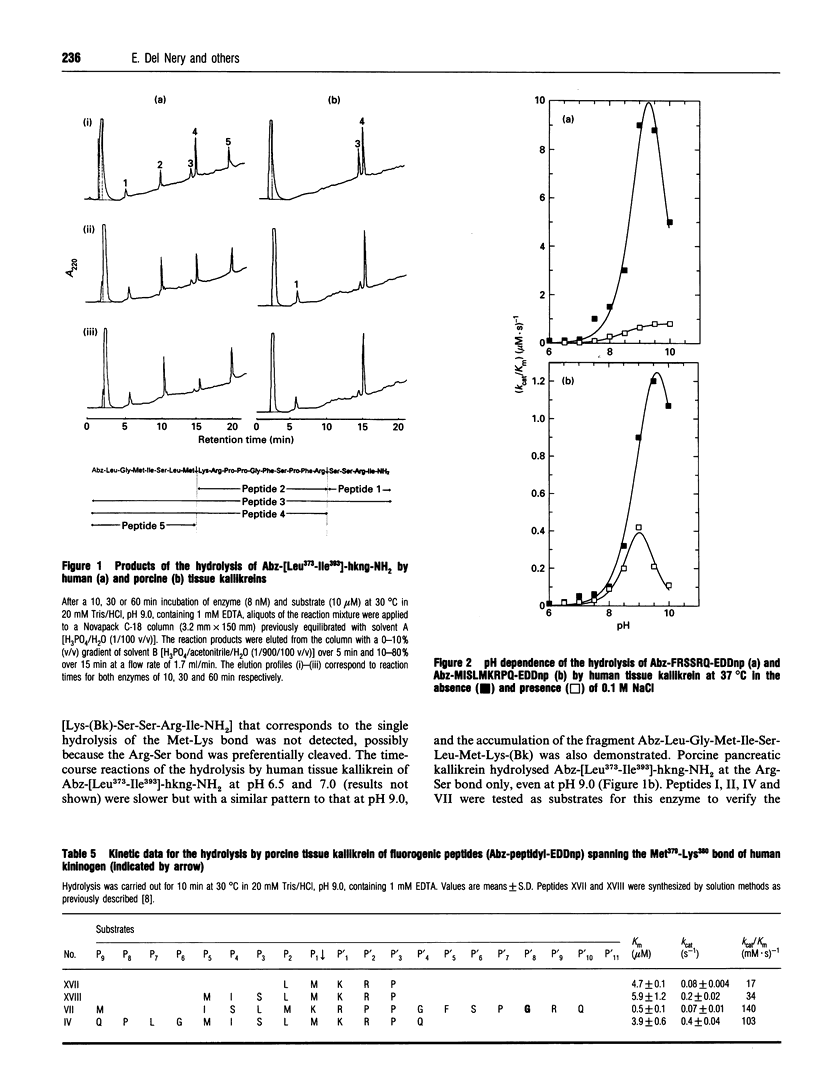
We have synthesized internally quenched peptides spanning the Met379-Lys380 or Arg389-Ser390 bonds of human kininogen (hkng) that flank lysyl-bradykinin and have studied the kinetics of their hydrolysis by human tissue kallikrein. The kinetic data for the hydrolysis of the Met-Lys bond in substrates with an N-terminal extension showed that interactions up to position residue P10 contribute to the efficiency of cleavage. In contrast, there were no significant variations in the kinetic data for the hydrolysis of substrates with C-terminal extensions at sites P'4 to P'11. A similar pattern was observed for the cleavage of substrates containing an Arg-Ser bond because substrates extended up to residue P6 were hydrolysed with the highest kcat/Km values in the series, whereas those extended to P'11 on the C-terminal side had a lower susceptibility to hydrolysis. Time-course studies of hydrolysis by human and porcine tissue kallikreins of a Leu373 to Ile393 human kininogen fragment containing omicron-aminobenzoic acid (Abz) at the N-terminus and an amidated C-terminal carboxyl group Abz-Leu-Gly-Met-Ile-Ser-Leu-Met-Lys-Arg- Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-Ser-Ser-Arg-Ile-NH2 (Abz-[Leu373-Ile393]-hkng-NH2) indicated that the cleavage of Met-Lys and Arg-Ser bonds in the same molecule occurs via the formation of independent enzyme-substrate complexes. The hydrolysis of Abz-F-R-S-S-R-Q-EDDnp [where EDDnp is N-(2,4-dinitrophenyl)ethylenediamine] and Abz-M-I-S-L-M-K-R-P-Q-EDDnp by human tissue kallikrein had maximal kcat/Km values at pH 9-9.5 for both substrates. The pH-dependent variations in this kinetic parameter were almost exclusively due to variations in kcat. A significant decrease in kcat/Km values was observed for the hydrolysis of Arg-Ser and Met-Lys bonds in the presence of 0.1 M NaCl. Because this effect was closely related to an increase in Km, it is likely that sodium competes with the positive charges of the substrate side chains for the same enzyme subsites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo-Viel M. S., Juliano L., Prado E. S. The cleavage of the Met-Lys bond in a bradykinin derivative by glandular kallikreins. Hoppe Seylers Z Physiol Chem. 1981 Mar;362(3):337–345. doi: 10.1515/bchm2.1981.362.1.337. [DOI] [PubMed] [Google Scholar]
- Bode W., Chen Z., Bartels K., Kutzbach C., Schmidt-Kastner G., Bartunik H. Refined 2 A X-ray crystal structure of porcine pancreatic kallikrein A, a specific trypsin-like serine proteinase. Crystallization, structure determination, crystallographic refinement, structure and its comparison with bovine trypsin. J Mol Biol. 1983 Feb 25;164(2):237–282. doi: 10.1016/0022-2836(83)90077-3. [DOI] [PubMed] [Google Scholar]
- Brillard-Bourdet M., Moreau T., Gauthier F. Substrate specificity of tissue kallikreins: importance of an extended interaction site. Biochim Biophys Acta. 1995 Jan 5;1246(1):47–52. doi: 10.1016/0167-4838(94)00179-k. [DOI] [PubMed] [Google Scholar]
- Chagas J. R., Hirata I. Y., Juliano M. A., Xiong W., Wang C., Chao J., Juliano L., Prado E. S. Substrate specificities of tissue kallikrein and T-kininogenase: their possible role in kininogen processing. Biochemistry. 1992 Jun 2;31(21):4969–4974. doi: 10.1021/bi00136a008. [DOI] [PubMed] [Google Scholar]
- Chagas J. R., Juliano L., Prado E. S. Intramolecularly quenched fluorogenic tetrapeptide substrates for tissue and plasma kallikreins. Anal Biochem. 1991 Feb 1;192(2):419–425. doi: 10.1016/0003-2697(91)90558-b. [DOI] [PubMed] [Google Scholar]
- Chagas J. R., Portaro F. C., Hirata I. Y., Almeida P. C., Juliano M. A., Juliano L., Prado E. S. Determinants of the unusual cleavage specificity of lysyl-bradykinin-releasing kallikreins. Biochem J. 1995 Feb 15;306(Pt 1):63–69. doi: 10.1042/bj3060063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F. Enzymology of porcine tissue kallikrein. Adv Exp Med Biol. 1983;156:263–274. [PubMed] [Google Scholar]
- Fiedler F., Fink E., Tschesche H., Fritz H. Porcine glandular kallikreins. Methods Enzymol. 1981;80(Pt 100):493–532. doi: 10.1016/s0076-6879(81)80042-0. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Hinz H. Kinetics of bond cleavages at kallidin release by tissue kallikrein: cleavage of two peptide bonds in a single enzyme-substrate complex? Agents Actions Suppl. 1992;38(Pt 1):82–88. doi: 10.1007/978-3-0348-7321-5_11. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Hinz H., Lottspeich F. Individual reaction steps in the release of kallidin from kininogen by tissue kallikrein. Adv Exp Med Biol. 1986;198(Pt A):283–289. doi: 10.1007/978-1-4684-5143-6_39. [DOI] [PubMed] [Google Scholar]
- Maier M., Austen K. F., Spragg J. Kinetic analysis of the interaction of human tissue kallikrein with single-chain human high and low molecular weight kininogens. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3928–3932. doi: 10.1073/pnas.80.13.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L., Araujo-Viel M. S., Juliano L., Prado E. S. Substrate activation of porcine pancreatic kallikrein by N alpha derivatives of arginine 4-nitroanilides. Biochemistry. 1987 Aug 11;26(16):5032–5035. doi: 10.1021/bi00390a022. [DOI] [PubMed] [Google Scholar]
- Prado E. S., Prado de Carvalho L., Araujo-Viel M. S., Ling N., Rossier J. A Met-enkephalin-containing-peptide, BAM 22P, as a novel substrate for glandular kallikreins. Biochem Biophys Res Commun. 1983 Apr 29;112(2):366–371. doi: 10.1016/0006-291x(83)91472-9. [DOI] [PubMed] [Google Scholar]
- Sampaio C. A., Sampaio M. U., Prado E. S. Active-site titration of horse urinary kallikrein. Hoppe Seylers Z Physiol Chem. 1984 Mar;365(3):297–302. doi: 10.1515/bchm2.1984.365.1.297. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shimamoto K., Chao J., Margolius H. S. The radioimmunoassay of human urinary kallikrein and comparisons with kallikrein activity measurements. J Clin Endocrinol Metab. 1980 Oct;51(4):840–848. doi: 10.1210/jcem-51-4-840. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Kitamura N., Nakanishi S. Cloning and sequence analysis of cDNAs for human high molecular weight and low molecular weight prekininogens. Primary structures of two human prekininogens. J Biol Chem. 1985 Jul 15;260(14):8601–8609. [PubMed] [Google Scholar]
- Taylor J. M., Cohen S., Mitchell W. M. Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci U S A. 1970 Sep;67(1):164–171. doi: 10.1073/pnas.67.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Baglan N. C., Bradshaw R. A. The amino acid sequence of the gamma-subunit of mouse submaxillary gland 7 S nerve growth factor. J Biol Chem. 1981 Sep 10;256(17):9156–9166. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


