Abstract
The relative frequency of primary cutaneous lymphoma (PCL) subtypes shows wide variation across different geographical regions. This retrospective study was conducted in a tertiary referral center located in Korea to describe the relative frequency, demographics, survival outcomes, and temporal trend in PCL. A total of 627 PCL cases diagnosed between January 1994 and December 2022 were included. The majority of PCL cases (87.2%) were of T-/NK-cell lineage (CTCL), while the remaining cases (12.8%) were B-cell lineage lymphomas (CBCL). The prevalence of mycosis fungoides (MF) in CTCL increased significantly over time, while other CTCL subtypes, including primary cutaneous extranodal NK/T-cell lymphoma and subcutaneous panniculitis-like T-cell lymphoma (SPTCL), decreased in frequency. Notably, the prevalence of CD4-positive small/medium T-cell lymphoproliferative disorder showed a substantial increase over time. Primary cutaneous marginal zone lymphoma was consistently the commonest CBCL subtype. Survival analysis demonstrated that CTCL had a more favorable 5-year overall survival (OS) than CBCL. OS rate of MF, SPTCL, and primary cutaneous peripheral T-cell lymphoma, NOS improved significantly over time. This study provides comprehensive insights into the dynamic change in the relative frequency and overall survival of PCL subtypes over time.
Keywords: Primary cutaneous lymphoma, T-/NK-cell lineage, B-cell lineage lymphomas, Mycosis fungoides, Incidence, Survival analysis
Subject terms: Skin cancer, Lymphoma
Introduction
Primary cutaneous lymphomas (PCL) are rare cutaneous malignancies, accounting for 19% of extranodal non-Hodgkin's lymphomas. PCL are heterogeneous lymphoid tumors with varying clinical, histological, cytogenetic, and molecular features. Since the classification of PCL was established by the World Health Organization-European Organisation for Research and Treatment of Cancer (WHO-EORTC) in 2005, several studies have examined the prevalence of PCL1. However, most large-scale studies have been conducted in the USA and Europe, with few from Asia. Previously we observed that PCL shows a temporal change, with a significant increase in the relative incidence of B-cell lineage PCL in recent years2. Additionally, T- and natural killer (NK)-cell lineage PCL were more prevalent in Korea than in Western countries. Since several years have gone by, our previous findings must be reassessed.
This study aimed to assess PCL prevalence, patient characteristics, and survival outcomes from 1994 to 2022 according to subtypes and subgroups of patients diagnosed in different periods.
Methods
Study design
This study was approved by the Institutional Review Board of Asan Medical Center (2022–0832). All research was performed in accordance with relevant guidelines/regulations. Due to its retrospective nature, this study was exempt from obtaining informed consent. A database search was performed for all PCL cases that had been confirmed by skin biopsy between January 1994 and December 2022. PCL was defined as non-Hodgkin’s lymphoma in the skin without evidence of extracutaneous disease at diagnosis.
Mycosis fungoides (MF) and Sézary syndrome (SS) were classified as PCL, even with extracutaneous dissemination unlike other subtypes. In cases when clinical data were insufficient to distinguish primary cutaneous anaplastic large cell lymphoma (pcALCL) from lymphomatoid papulosis (LYP), pcALCL was diagnosed based on histopathological examination findings characterized by cohesive sheets with predominance (> 75%) of large CD30-positive cells showing anaplastic, pleomorphic, or immunoblastic morphology. PCL was classified in accordance with the 5th edition of WHO classification of lymphoid neoplasms3.
Data collection
The following clinical data were collected from the patient’s medical records: age at diagnosis, sex, location, extent, multiplicity, and morphology of the skin lesion(s), follow-up results, and survival status. The degree of skin involvement was evaluated in patients with MF according to the proposed tumor-node-metastases (TNM) classification system for MF4. The extent of skin lesions in PCLs other than MF was evaluated using the International Society for Cutaneous Lymphomas (ISCL)-EORTC TNM classification5. The number of skin lesions was grouped as single or multiple (two or more lesions). Overall survival (OS) was calculated based on the date of initial diagnosis to the date of death from any cause or the last follow-up.
Statistical analysis
The subgroups were compared using a χ2 test for categorical variables and a t-test or Mann–Whitney test for continuous variables. The Kaplan–Meier method was used to analyze survival. Subgroups survival differences were tested for significance using the log-rank test. The endpoint was patient death or last follow-up. The study endpoint was 31 December 2022 for survivors. All analyses were performed with a statistical software package (SPSS, version 22.0; SPSS Inc., Chicago, IL, USA). P < 0.05 were considered to be statistically significant.
Results
Relative frequency of PCL
We identified 627 cases of PCL between January 1994 and December 2022. Of these cases, 87.2% (547 cases) were PCL of T-/NK-cell lineage (CTCL), while the remaining 12.8% (80 cases) were B-cell lineage lymphomas (CBCL). MF (38.1%) was the commonest PCL subtype, followed by LYP (15.6%), pcALCL (11.6%), primary cutaneous marginal zone lymphoma (pcMZL, 8.0%), subcutaneous panniculitis-like T-cell lymphoma (SPTCL, 6.1%), primary cutaneous peripheral T-cell lymphoma, NOS (pcPTCL-NOS, 6.1%), primary cutaneous extranodal natural killer (NK)/T-cell lymphoma (pcENKTL, 5.6%), primary cutaneous diffuse large B-cell lymphoma, leg type (pcDLBCL-LT, 4.1%), primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder (pcCD4+SMTCLD, 3.5%), primary cutaneous follicle center lymphoma (pcFCL, 0.5%), SS (0.6%), and EBV-positive mucocutaneous ulcer (0.2%). No patient with primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma, primary cutaneous gamma/delta T-cell lymphoma, primary cutaneous acral CD8-positive T-cell lymphoproliferative disorder was identified. The summarized data can be found in Table 1, along with a comparison with large-scale reports from other countries and our previous work2,6–8.
Table 1.
Relative frequency of primary cutaneous lymphoma subtypes in this study and in previous reports from other countries.
| This study (n = 627) | Our previous study, 2016 (n = 289)2 | Japan, 2020 (n = 2090)6 | Argentina, 2019 (n = 416)7 | France, 2020 (n = 8593)8 | |
|---|---|---|---|---|---|
| n (%) | n (%) | (%) | (%) | (%) | |
| Mature T-and NK-cell lymphomas | 547 (87.2) | 244 (84.4) | 77.0 | 93.0 | 72.5 |
| Mycosis fungoides | 239 (38.1) | 85 (29.4) | 48.0 | 75.7 | 37.8 |
| Sézary syndrome | 4 (0.6) | No data | 1.1 | 3.1 | 5.1 |
| Primary cutaneous anaplastic large cell lymphoma | 73 (11.6) | 34 (11.8) | 6.0 | 3.6 | 2.1 |
| Lymphomatoid papulosis | 98 (15.6) | 46 (15.9) | 3.2 | 3.6 | 6.1 |
| Primary cutaneous extranodal NK/T-cell lymphoma | 35 (5.6) | 28 (9.7) | 2.1 | 1.7 | 0.6 |
| Subcutaneous panniculitis-like T-cell lymphoma | 38 (6.1) | 21 (7.3) | 2.0 | 1.2 | 1.0 |
| Primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder | 22 (3.5) | 3 (1.0) | 1.8 | 0.2 | 3.7 |
| Primary cutaneous peripheral T-cell lymphoma, NOS | 38 (6.1) | 27 (9.3) | 5.0 | 1.9 | 2.0 |
| Mature B-cell lymphomas | 80 (12.8) | 40 (13.8) | 21.1 | 6.7 | 26.4 |
| Primary cutaneous marginal zone lymphoma | 50 (8.0) | 24 (8.3) | 5.6 | 2.2 | 10.0 |
| Primary cutaneous diffuse large B-cell lymphoma, leg type | 26 (4.1) | 14 (4.8) | 8.5 | 0.5 | 2.3 |
| Primary cutaneous follicle center lymphoma | 3 (0.5) | 1 (0.4) | 3.6 | 2.9 | 10.1 |
| EBV-positive mucocutaneous ulcer | 1 (0.2) | 0 (0) | No data | No data | No data |
Change in the relative frequency of PCL over time
Table 2 illustrates the relative frequency of PCL in different periods. MF was the commonest subtype of PCL between 1994 and 2022. The prevalence of MF increased steadily. In 1994–2003, 26.7% of CTCL was MF; in 2014–2022, 55.3% of CTCL was MF. This increase in the proportion of MF in CTCL was statistically significant (p < 0.001). While the relative frequency of pcENKTL, SPTCL, and pcPTCL-NOS decreased over time, possibly due to the increase in MF cases, the relative frequency of pcCD4+SMTCLD showed a substantial increase, accounting for 6.9% of all CTCL between 2014 and 2022. This temporal change in the relative frequency of pcCD4+SMTCLD was statistically significant (p = 0.012). pcMZL was the commonest CBCL subtype across all periods, accounting for 63.3% of all CBCL cases. Similar to pcCD4+SMTCLD with regard to CTCL, the relative frequency of pcFCL increased gradually over time, from 0 to 5.1%. CBCL frequency relative to CTCL did not change significantly between periods, it was not statistically significant (p = 0.21 from 1994 to 2003 and from 2004 to 2013, p = 0.40 from 2004 to 2013 and from 2014 to 2022).
Table 2.
Comparison of the prevalence of primary cutaneous lymphomas between subgroups according to the year of diagnosis.
| Relative frequency of primary cutaneous lymphomas (n, %) | ||||
|---|---|---|---|---|
| 1994–2003 | 2004–2013 | 2014–2022 | Overall | |
| Cutaneous mature T-and NK-cell lymphomas (CTCL) | n = 86 | n = 186 | n = 275 | n = 547 |
| Mycosis fungoides | 23 (26.7) | 64 (34.4) | 152 (55.3) | 239 (43.7) |
| Sézary syndrome | 1 (1.2) | 3 (1.6) | 0 (0.0) | 4 (0.7) |
| Primary cutaneous anaplastic large cell lymphoma | 12 (14.0) | 36 (19.4) | 25 (9.1) | 73 (13.3) |
| Lymphomatoid papulosis | 22 (25.6) | 37 (19.9) | 39 (14.2) | 98 (17.9) |
| Primary cutaneous extranodal NK/T-cell lymphoma | 8 (9.3) | 16 (8.6) | 11 (4.0) | 35 (6.4) |
| Subcutaneous panniculitis-like T-cell lymphoma | 8 (9.3) | 13 (7.0) | 17 (6.2) | 38 (6.9) |
| Primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder | 1 (1.2) | 2 (1.1) | 19 (6.9) | 22 (4.0) |
| Primary cutaneous peripheral T-cell lymphoma, NOS | 11 (12.8) | 15 (8.1) | 12 (4.4) | 38 (6.9) |
| Cutaneous Mature B-cell lymphomas (CBCL) | n = 9 | n = 32 | n = 39 | n = 80 |
| Primary cutaneous marginal zone lymphoma | 5 (55.6) | 22 (68.8) | 23 (59.0) | 50 (63.3) |
| Primary cutaneous diffuse large B-cell lymphoma, leg typer | 4 (44.4) | 9 (28.1) | 13 (33.3) | 26 (32.9) |
| Primary cutaneous follicle center lymphoma | 0 (0) | 1 (3.1) | 2 (5.1) | 3 (3.8) |
| EBV-positive mucocutaneous ulcer | 0 (0) | 0 (0) | 1 (2.6) | 1 (1.3) |
| CTCL/CBCL ratio | 9.6:1 | 5.8:1 | 7.1:1 | 6.8:1 |
Demographics of patients with PCL
Table 3 shows the patients with PCL demographics according to cell lineage. Analysis of the age and sex ratio in PCL subtypes showed that patients with CBCL were significantly older than those with CTCL (mean age for CTCL = 43.7 years, mean age for CBCL = 51.3 years, p = 0.007). The age at diagnosis ranged from 14 months (LYP) to 98 years (pcDLBCL-LT). Excluding EBV-positive mucocutaneous ulcer and SS, which included only one and four patients, respectively, pcDLBCL-LT (58.6 years) and pcCD4+SMTCLD (46.8 years) had the highest mean age at diagnosis among patients with CBCL and CTCL, respectively. The male to female ratio was highest in MF (1.69:1) and pcDLBCL-LT (1.6:1) in CTCL and CBCL, respectively. Although the proportion of men was higher in patients with CTCL (M: F = 1.32:1) than in those with CBCL (M: F = 1:1), this difference was not statistically significant (p = 0.30).
Table 3.
Demographic data of patients with primary cutaneous lymphoma.
| Age distribution, years | Sex distribution, male/female | |
|---|---|---|
| Mean (range) | Ratio; n | |
| Primary cutaneous lymphoma | ||
| Total | 44.6 (1–98) | 1.28:1; 338/265 |
| Mature T-cell lymphoma | 43.7 (1–91) | 1.32:1; 298/225 |
| Mature B-cell lymphoma | 51.3 (13–98) | 1:1; 40/40 |
| Classification | ||
| Mycosis fungoides | 44.7 (2–91) | 1.69:1; 135/80 |
| Sézary syndrome | 59.3 (40–74) | 1:1; 2/2 |
| Lymphomatoid papulosis | 39.5 (1–73) | 1.18:1; 53/45 |
| Primary cutaneous anaplastic large cell lymphoma | 47.0 (3–83) | 1.35:1; 42/31 |
| Primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder | 46.8 (11–91) | 0.83:1; 10/12 |
| Subcutaneous panniculitis-like T-cell lymphoma | 38.2 (6–74) | 0.81:1; 17/21 |
| Primary cutaneous extranodal NK/T-cell lymphoma | 44.6 (16–82) | 0.94:1; 17/18 |
| Primary cutaneous peripheral T-cell lymphoma, NOS | 56.6 (29–91) | 1.4:1; 22/16 |
| Primary cutaneous diffuse large B-cell lymphoma, leg type | 58.6 (29–98) | 1.6:1; 16/10 |
| Primary cutaneous marginal zone lymphoma | 45.0 (13–77) | 0.79:1; 22/28 |
| Primary cutaneous follicle center lymphoma | 57.7 (52–68) | 0.5:1; 1/2 |
| EBV-positive mucocutaneous ulcer | 76 (one case) | 1:0; 1/0 |
Clinical characteristics of patients with PCL
Supplementary Table 1 summarizes the location and morphology of PCL lesions according to subtype. The clinical features of PCL varied by subtype, with the legs (57.6%) being the commonest site of involvement for CTCL, followed by the arms (57.2%) and the head and neck (46.3%). However, the head and neck (50.0%) was the commonest location for CBCL, followed by the trunk (36.3%) and the legs (25.0%). Generalized skin lesions were seen in 9.1% and 30.3% of patients with MF/SS and CTCL other than MF, respectively. No patient with CBCL had generalized lesions. Of all patients with CTCL, 60.8% had multiple skin lesions at diagnosis. However, 27.5% of patients with CBCL had multiple lesions. This difference in lesion multiplicity between CTCL and CBCL was statistically significant (p < 0.001). The TNM classification showed significant differences in the extent of skin lesions between CTCL and CBCL. Table 4 shows the T stage at initial diagnosis in PCL cases and the frequency of extracutaneous involvement during disease course. The ISCL-EORTC TNM classification revealed that T1 or T2 category was found in 60.2% of CTCL and 77.6% of CBCL. Lymph node involvement was discovered in 59 (11.3%) of 523 CTCL cases and 7 (8.8%) of 80 CBCL cases, a difference that was not statistically significant (p = 0.79). CBCL (10.0%) had more extracutaneous involvement than CTCL (6.9%). However, the difference was not significant (p = 0.41). pcENKTL had the highest lymph node and visceral dissemination occurrence, which was observed in 51.4% of patients. In contrast, no extracutaneous involvement was seen during the clinical course in patients with LYP.
Table 4.
T stage at initial diagnosis and extracutaneous involvement during disease course of primary cutaneous lymphoma.
| T classification at initial work-up | |||||||
|---|---|---|---|---|---|---|---|
| T1, n (%) | T2, n (%) | T3, n (%) | T4, n (%) | LN involvement, n (%) | Visceral involvement, n (%) | Site of visceral involvement | |
| Mature T-and natural killer-cell lymphoma | 215 (41.1) | 109 (19.1) | 163 (31.2) | 12 (2.3) | 59 (11.3) | 36 (6.9) | |
| Mycosis fungoides/Sézary syndrome (n = 219) | 125 (57.1) | 56 (25.6) | 21 (9.6) | 12 (5.5) | 21 (9.6) | 2 (0.9) | Pleura (n = 1), nasopharynx (n = 1) |
| Lymphomatoid papulosis (n = 98) | 10 (10.2) | 25 (25.5) | 59 (60.2) | Not applicable | 0 (0) | 0 (0) | |
| Primary cutaneous anaplastic large cell lymphoma (n = 73) | 21 (28.8) | 7 (9.6) | 29 (39.7) | Not applicable | 2 (2.7) | 10 (13.7) | Bone (n = 4), lung (n = 3), liver (n = 2), breast (n = 1), kidney (n = 1), spleen (n = 1), stomach (n = 1) |
| Subcutaneous panniculitis-like T-cell lymphoma (n = 38) | 6 (15.8) | 5 (13.2) | 27 (71.1) | Not applicable | 4 (10.5) | 2 (5.3) | Breast (n = 1), omentum (n = 1) |
| Primary cutaneous extranodal natural killer/T-cell lymphoma (n = 35) | 12 (34.3) | 0 (0) | 23 (65.7) | Not applicable | 18 (51.4) | 18 (51.4) | Bone marrow (n = 4), lung (n = 4), bone (n = 3), liver (n = 3), spleen (n = 2), muscle (n = 2), testis (n = 2), GI tract (n = 2), adrenal gland (n = 1), pancreas (n = 1), prostate (n = 1), kidney (n = 1) |
| Primary cutaneous peripheral T-cell lymphoma, NOS (n = 38) | 21 (55.3) | 7 (18.4) | 10 (26.3) | Not applicable | 13 (37.1) | 4 (11.4) | Bone marrow (n = 2), lung (n = 1), liver (n = 1), spleen (n = 1) |
| Primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder (n = 22) | 20 (90.1) | 2 (0.9) | Not applicable | Not applicable | 1 (4.5) | 0 (0) | |
| Mature B-cell lymphoma | 51 (63.8) | 11 (13.8) | 14 (17.5) | Not applicable | 7 (8.0) | 8 (10.0) | |
| Primary cutaneous diffuse large B-cell lymphoma, leg type (n = 26) | 16 (61.5) | 4 (15.4) | 3 (11.5) | Not applicable | 6 (23.1) | 7 (27.0) | Adrenal gland (n = 1), bone (n = 1), liver (n = 1), lung (n = 1), testis (n = 1), peritoneum (n = 1), salivary gland (n = 1), spleen (n = 1) |
| Primary cutaneous marginal zone lymphoma (n = 50) | 31 (62) | 7 (14) | 11 (22) | Not applicable | 1 (2) | 1 (2) | Breast (n = 1) |
| Primary cutaneous follicle center lymphoma (n = 3) | 3 (100) | 0 (0) | 0 (0) | Not applicable | 0 (0) | 0 (0) | |
| EBV-positive mucocutaneous ulcer (n = 1) | 1 (100) | 0 (0) | 0 (0) | Not applicable | 0 (0) | 0 (0) | |
Survival outcomes of patients with PCL
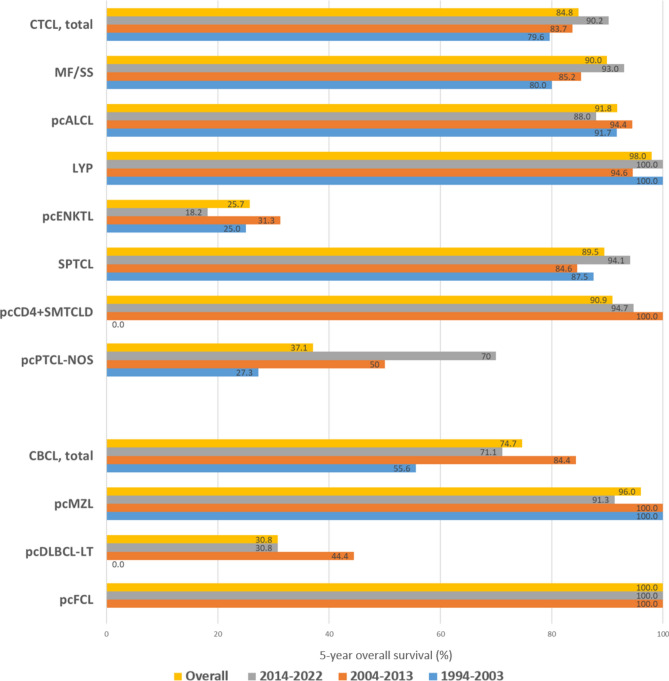
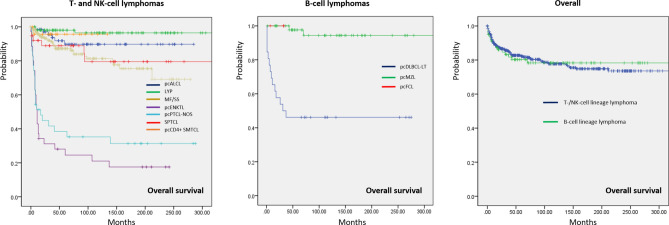
The follow-up period for all patients with PCL ranged from 1 to 381 months (mean ± standard deviation: 85.4 ± 85.0 months, median: 85 months). Supplementary Table 2 summarizes the follow-up period for each PCL subtype. The 5-year survival data according to PCL subtypes and across different periods is presented in Fig. 1. CTCL generally showed a more favorable 5-year OS (84.8%) than CBCL (74.7%). LYP was associated with the best survival outcome, with a 5-year OS of 98%. pcMZL and pcALCL also showed a good prognosis, with a 5-year OS of 96.0% and 91.8%, respectively. In contrast, patients diagnosed with pcENKTL demonstrated the lowest 5-year OS (25.7%), followed by patients with pcDLBCL-LT (30.8%). A significant increase in the 5-year OS of patients with MF/SS was observed over time (80.0% between 1997 and 2003 and 93.0% between 2014 and 2022, p < 0.001). The most remarkable increase in the 5-year OS was seen in pcPTCL-NOS (27.3% in 2004–2013 vs. 70.0% in 2014–2022, p < 0.001). Similarly, the 5-year OS of SPTCL increased in recent years (84.6% in 2004–2013 vs. 94.1% in 2014–2022, p < 0.001). However, the 5-year OS of pcENKTL declined from 31.3% (2004–2013) to 18.2% (2014–2022), but this change was not statistically significant (p = 0.06). No documented death was observed for all three patients diagnosed with pcFCL within 5 years after diagnosis. Figure 2 shows the Kaplan–Meier survival analysis of PCL subtypes. pcPTCL-NOS and pcENKTL had significantly worse OS than all other CTCL subtypes (p < 0.001). The difference in OS of these two subtypes was not statistically significant (p = 0.322). LYP showed a significantly better OS than MF/SS (p < 0.001), pcCD4+SMTCLD (p = 0.013), and SPTCL (p = 0.004). Survival comparisons between other subtypes did not yield statistically significant differences. The survival analysis performed specifically for patients with MF/SS demonstrated a significant (p < 0.001) difference between those with early MF (stage IA–IIA) and those with advanced MF/SS (stage IIB–IVB) (Fig. 3). Among CBCL subtypes, the OS of pcDLBCL-LT was significantly worse than that of pcMZL (p < 0.001). Survival comparison between pcFCL and other CBCL subtypes was limited due to the small number of pcFCL cases. The difference in OS between CTCL (mean = 52.8 months, 95% confidence interval (CI) 51.5–54.1 months) and CBCL (mean = 51.0 months, 95% CI 46.58–55.40 months) was not statistically significant (p = 0.732).
Fig. 1.
Five-year overall survival (OS) rate of patients with primary cutaneous lymphoma. Primary cutaneous extranodal natural killer/T-cell lymphoma and diffuse large B-cell lymphoma, leg type have the lowest OS rate among primary cutaneous mature T-and NK-cell lymphomas (CTCL) and primary cutaneous mature B-cell lymphomas (CBCL), respectively. The 5-year OS rate of mycosis fungoides and primary cutaneous peripheral T-cell lymphoma, NOS showed significant improvement over time. EBV-positive mucocutaneous ulcer is not shown because only one patient was identified in our cohort. LYP: lymphomatoid papulosis; MF: mycosis fungoides; pcALCL: primary cutaneous anaplastic large cell lymphoma; pcCD4+SMTCLD: primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder; pcDLBCL-LT: primary cutaneous diffuse large B-cell lymphoma, leg type; pcENKTL: primary cutaneous extranodal natural killer/T-cell lymphoma; pcFCL: primary cutaneous follicle center lymphoma; pcMZL: primary cutaneous marginal zone lymphoma; pcPTCL-NOS, primary cutaneous peripheral T-cell lymphoma, NOS; SPTCL: subcutaneous panniculitis-like T-cell lymphoma; SS: Sézary syndrome.
Fig. 2.
Overall survival (OS) rate for primary cutaneous lymphoma. Among T and NK cell lymphoma subtypes, primary cutaneous extranodal NK/T-cell lymphoma was associated with the worst OS. Among primary cutaneous B-cell lymphomas, the OS of marginal zone lymphoma was significantly superior to that of primary cutaneous diffuse large B-cell lymphoma, leg type. The survival curve for EBV-positive mucocutaneous ulcer (n = 1, alive 20 months after diagnosis) is not shown. The cell lineage of primary cutaneous lymphoma (T-/NK-cell vs. B-cell) did not influence OS significantly (p = 0.732). LYP: lymphomatoid papulosis; MF: mycosis fungoides; pcALCL: primary cutaneous anaplastic large cell lymphoma; pcCD4+SMTCLD: primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder; pcDLBCL-LT: primary cutaneous diffuse large B-cell lymphoma, leg type; pcENKTL: primary cutaneous extranodal natural killer/T-cell lymphoma; pcFCL: primary cutaneous follicle center lymphoma; pcMZL: primary cutaneous marginal zone lymphoma; pcPTCL-NOS, primary cutaneous peripheral T-cell lymphoma, NOS; SPTCL: subcutaneous panniculitis-like T-cell lymphoma; SS: Sézary syndrome.
Fig. 3.
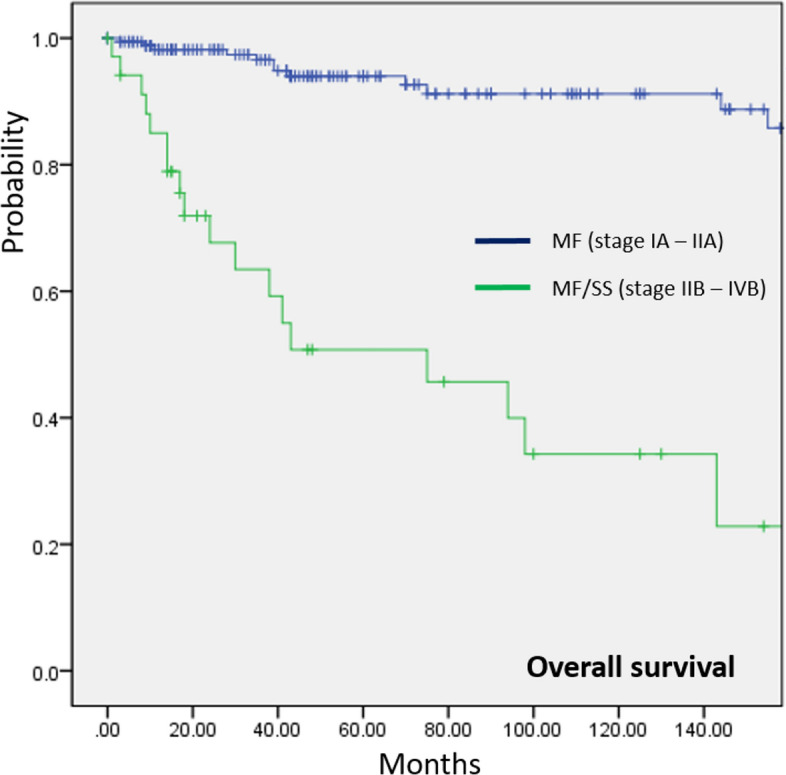
Comparison of overall survival (OS) rate between early (stage IA–IIA) and advanced (stage IIB–IVB) mycosis fungoides (MF)/Sézary syndrome (SS). Patients with advanced MF/SS had a significantly lower OS than those with early MF (p < 0.001).
Discussion
This study outlines the prevalence, demographics, clinical presentation, and survival outcomes of different PCL subtypes, and the temporal change in the relative incidence. In addition to the overall increase in the incidence of PCL over time, we have found that the prevalence of MF and pcCD4+SMTCLD increased steadily and significantly over time.
Asians are more likely than Westerners to have T-/NK-cell lineage PCL8–10. Herein, the overall CTCL/CBCL ratio was 6.5:1. However, it varied overtime. Although not statistically significant, CBCL increased relative to CTCL over time. Previous Korean studies reported CTCL predominance and CBCL increase2,11,12. CBCL diagnosis may have increased due to advances in immunohistological and genetic testing, as well as dermatologists’ and pathologists’ increased awareness of this rare disease entity. Environmental factors, including a more westernized lifestyle in Asia, could have contributed to this trend.
Regarding the relative frequency of CTCL subtypes, the incidences of both pcENKTL and SPTCL are known to be higher in Asians than in Western populations13. This can be, at least partly, attributed to the higher prevalence of lymphoma-associated viruses in the Far East in addition to the higher frequency of the germline mutation of the HAVCR2 gene, which is associated with SPTCL in Asians14,15. However, our results indicate that the relative incidence of pcENKTL and SPTCL gradually decreased over time. This study shows additional dynamic change in the relative frequency of PCL, especially for MF and pcCD4+SMTCLD, whose incidence and relative frequency have increased significantly over time. MF, the commonest type of CTCL, accounts for 40–90% of all CTCL16,17. Notable difference was observed in the relative incidence of MF between geographical regions. MF is more frequent in Europe than in North America9. The proportion of MF in Asia is heterogeneous, ranging from 40% (South Korea) to 92% (Singapore)18,19. Dobos et al. reported a gradual decrease in MF incidence in France9. Nonetheless, this has not been validated in Asian populations. Moreover, pcCD4+SMTCLD, a rare PCL subtype, is uncommon. We cannot explain this difference in relative incidence because we do not know the pathomechanism or risk factors for MF and pcCD4+SMTCLD.
Similar to a large-scale study conducted in the USA, pcMZL was found to be the commonest in our study20. However, pcDLBCL-LT was reported as the most frequent CBCL subtype in Japan6,21. Conflicting results have been reported regarding the relative incidence of CBCL subtypes in Korea2,12,13. Previously reported pcMZL: pcDLBCL-LT incidence ratio in Korea varied substantially from 9.4:1 to 1:1.27. Our observations that the incidence of pcMZL is nearly twice as high as that of pcDLBCL-LT and that pcMZL was consistently the commonest CBCL subtype across different time periods further support a higher incidence of pcMZL in Korea than in other regions. While pcFCL is the predominant CBCL subtype in Europe, it is considered extremely rare in Asia. Although the limited sample size prevents a detailed analysis, our study shows an increase in pcFCL cases over time. However, future studies need to confirm the above dynamic variations in the relative frequency of PCL subtypes.
Survival analysis in this study indicated an overall indolent course of PCL except for pcENKTCL, pcPTCL-NOS, and pcDLBCL-LT. The 5-year OS rate was 84.6% and 74.7% for CTCL and CBCL, respectively. This was similar to that of previous reports2,14,22,23. The increase in the 5-year OS for CTCL from 79.6 to 89.9% over 20 years is worthy of attention. In addition to the recent advances in both the diagnosis and treatment for CTCL, the increase in the proportion of MF, which typically shows an indolent clinical course, may have resulted in such improvement in OS.
Our study has some limitations. First, our findings may be difficult to generalize because they were conducted in a single tertiary referral center. Moreover, the few patients with rarer PCL subtypes may have restricted a thorough assessment of clinical characteristics and survival outcomes.
In conclusion, PCL subtype incidence in Korea differed from that observed in other geographical regions and varied over time.
Supplementary Information
Author contributions
Conceptualization: M.L. and W.L.; Methodology: S.C., C.W., C.P., D.Y., and S.S.; Data acquisition: I.M.; Formal analysis and investigation: I.M., S.C., C.W., C.P., D.Y., and S.S.; Writing—original draft preparation: I.M. and W.L.; Writing—review and editing: M.L., W.L.; Funding acquisition: W.L.; Supervision: M.L., W.L.. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Asan Institute for Life Sciences, Asan Medical Center (Grant Number: 2023IL0003–1) and the National Research Foundation of Korea (NRF; grant number: NRF–2023R1A2C100730311), which is funded by the Ministry of Science and Information Technology (MSIT) of the Korean government.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval
Reviewed and approved by Institutional Review Board of Asan Medical Center (2022–0832).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mi Woo Lee and Woo Jin Lee.
Contributor Information
Mi Woo Lee, Email: miumiu@amc.seoul.kr.
Woo Jin Lee, Email: uucm79@hanmail.net.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71210-y.
References
- 1.Willemze, R. et al. WHO-EORTC classification for cutaneous lymphomas. Blood105, 3768–3785. 10.1182/blood-2004-09-3502 (2005). 10.1182/blood-2004-09-3502 [DOI] [PubMed] [Google Scholar]
- 2.Lee, W. J. et al. Relative frequency, clinical features, and survival outcomes of 395 patients with cutaneous lymphoma in Korea: A subgroup analysis per 10-year period. Acta Derm Venereol.96, 888–893. 10.2340/00015555-2404 (2016). 10.2340/00015555-2404 [DOI] [PubMed] [Google Scholar]
- 3.Alaggio, R. et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Lymphoid Neoplasms. Leukemia36, 1720–1748. 10.1038/s41375-022-01620-2 (2022). 10.1038/s41375-022-01620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen, E. et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European organization of research and treatment of cancer (EORTC). Blood110, 1713–1722. 10.1182/blood-2007-03-055749 (2007). 10.1182/blood-2007-03-055749 [DOI] [PubMed] [Google Scholar]
- 5.Kim, Y. H. et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European organization of research and treatment of cancer (EORTC). Blood110, 479–484. 10.1182/blood-2006-10-054601 (2007). 10.1182/blood-2006-10-054601 [DOI] [PubMed] [Google Scholar]
- 6.Fujii, K. et al. Cutaneous lymphoma in Japan, 2012–2017: A nationwide study. J. Dermatol. Sci.97, 187–193. 10.1016/j.jdermsci.2020.01.010 (2020). 10.1016/j.jdermsci.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 7.Abeldaño, A. et al. Primary cutaneous lymphoma in Argentina: A report of a nationwide study of 416 patients. Int. J. Dermatol.58, 449–455. 10.1111/ijd.14262 (2019). 10.1111/ijd.14262 [DOI] [PubMed] [Google Scholar]
- 8.Dobos, G. et al. Epidemiological changes in cutaneous lymphomas: an analysis of 8593 patients from the French Cutaneous Lymphoma Registry*. Br. J. Dermatol.184, 1059–1067. 10.1111/bjd.19644 (2021). 10.1111/bjd.19644 [DOI] [PubMed] [Google Scholar]
- 9.Dobos, G. et al. Recent advances on cutaneous lymphoma epidemiology. Presse Med.51, 104108. 10.1016/j.lpm.2022.104108 (2022). 10.1016/j.lpm.2022.104108 [DOI] [PubMed] [Google Scholar]
- 10.Liu, J. et al. Relative frequency and survival of primary cutaneous lymphomas: a retrospective analysis of 98 patients. Chin. Med. J.127, 645–650 (2014). 10.3760/cma.j.issn.0366-6999.20132424 [DOI] [PubMed] [Google Scholar]
- 11.Lee, M. W. Characteristics of cutaneous lymphomas in Korea. Clin. Exp. Dermatol.28, 639–646. 10.1046/j.1365-2230.2003.01374.x (2003). 10.1046/j.1365-2230.2003.01374.x [DOI] [PubMed] [Google Scholar]
- 12.Park, J. H. et al. World Health Organization-European organization for research and treatment of cancer classification of cutaneous lymphoma in Korea: A retrospective study at a single tertiary institution. J. Am. Acad. Dermatol.67, 1200–1209. 10.1016/j.jaad.2012.02.033 (2012). 10.1016/j.jaad.2012.02.033 [DOI] [PubMed] [Google Scholar]
- 13.Lee, H. S. et al. Cutaneous lymphoma in Korea: A nationwide retrospective study. Acta Derm. Venereol.96, 535–539. 10.2340/00015555-2283 (2016). 10.2340/00015555-2283 [DOI] [PubMed] [Google Scholar]
- 14.Fujita, A., Hamada, T. & Iwatsuki, K. Retrospective analysis of 133 patients with cutaneous lymphomas from a single Japanese medical center between 1995 and 2008. J. Dermatol.38, 524–530. 10.1111/j.1346-8138.2010.01049.x (2011). 10.1111/j.1346-8138.2010.01049.x [DOI] [PubMed] [Google Scholar]
- 15.Sonigo, G. et al. HAVCR2 mutations are associated with severe hemophagocytic syndrome in subcutaneous panniculitis-like T-cell lymphoma. Blood135, 1058–1061. 10.1182/blood.2019003811 (2020). 10.1182/blood.2019003811 [DOI] [PubMed] [Google Scholar]
- 16.Willemze, R. et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood133, 1703–1714. 10.1182/blood-2018-11-881268 (2019). 10.1182/blood-2018-11-881268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobos, G. et al. Epidemiology of cutaneous T-Cell Lymphomas: A systematic review and meta-analysis of 16,953 patients. Cancers10.3390/cancers12102921 (2020). 10.3390/cancers12102921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han, J. H. et al. Characteristics of cutaneous lymphomas in korea according to the new WHO-EORTC Classification: Report of a nationwide study. Korean J. Pathol.48, 126–132. 10.4132/KoreanJPathol.2014.48.2.126 (2014). 10.4132/KoreanJPathol.2014.48.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan, S. H., Sim, C. S. & Ong, B. H. Cutaneous lymphomas other than mycosis fungoides in Singapore: A clinicopathological analysis using recent classification systems. Br. J. Dermatol.149, 542–553. 10.1046/j.1365-2133.2003.05476.x (2003). 10.1046/j.1365-2133.2003.05476.x [DOI] [PubMed] [Google Scholar]
- 20.Bradford, P. T., Devesa, S. S., Anderson, W. F. & Toro, J. R. Cutaneous lymphoma incidence patterns in the United States: A population-based study of 3884 cases. Blood113, 5064–5073. 10.1182/blood-2008-10-184168 (2009). 10.1182/blood-2008-10-184168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasukawa, K., Kato, N., Kodama, K., Hamasaka, A. & Hata, H. The spectrum of cutaneous lymphomas in Japan: A study of 62 cases based on the World Health Organization classification. J. Cutan. Pathol.33, 487–491. 10.1111/j.1600-0560.2006.00460.x (2006). 10.1111/j.1600-0560.2006.00460.x [DOI] [PubMed] [Google Scholar]
- 22.Hallermann, C., Niermann, C., Fischer, R. J. & Schulze, H. J. Survival data for 299 patients with primary cutaneous lymphomas: A monocentre study. Acta Derm. Venereol.91, 521–525. 10.2340/00015555-1112 (2011). 10.2340/00015555-1112 [DOI] [PubMed] [Google Scholar]
- 23.Senff, N. J. & Willemze, R. The applicability and prognostic value of the new TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: Results on a large cohort of primary cutaneous B-cell lymphomas and comparison with the system used by the Dutch Cutaneous Lymphoma Group. Br. J. Dermatol.157, 1205–1211. 10.1111/j.1365-2133.2007.08239.x (2007). 10.1111/j.1365-2133.2007.08239.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.