Abstract
Objective
During infection, metabolism and immunity react dynamically to promote survival through mechanisms that remain unclear. Pro-opiomelanocortin (POMC) cleavage products are produced and released in the brain and in the pituitary gland. One POMC cleavage product, alpha-melanocyte-stimulating hormone (α-MSH), is known to regulate food intake and energy expenditure and has anti-inflammatory effects. However, it is not known whether α-MSH is required to regulate physiological anti-inflammatory responses. We recently developed a novel mouse model with a targeted mutation in Pomc (Pomctm1/tm1 mice) to block production of all α-MSH forms which are required to regulate metabolism. To test whether endogenous α-MSH is required to regulate immune responses, we compared acute bacterial lipopolysaccharide (LPS)-induced inflammation between Pomctm1/tm1 and wild-type Pomcwt/wt mice.
Methods
We challenged 10- to 14-week-old male Pomctm1/tm1 and Pomcwt/wt mice with single i.p. injections of either saline or low-dose LPS (100 μg/kg) and monitored immune and metabolic responses. We used telemetry to measure core body temperature (Tb), ELISA to measure circulating cytokines, corticosterone and α-MSH, and metabolic chambers to measure body weight, food intake, activity, and respiration. We also developed a mass spectrometry method to measure three forms of α-MSH produced in the mouse hypothalamus and pituitary gland.
Results
LPS induced an exaggerated immune response in Pomctm1/tm1 compared to Pomcwt/wt mice. Both groups of mice were hypoactive and hypothermic following LPS administration, but Pomctm1/tm1 mice were significantly more hypothermic compared to control mice injected with LPS. Pomctm1/tm1 mice also had reduced oxygen consumption and impaired metabolic responses to LPS compared to controls. Pomctm1/tm1 mice had increased levels of key proinflammatory cytokines at 2 h and 4 h post LPS injection compared to Pomcwt/wt mice. Lastly, Pomcwt/wt mice injected with LPS compared to saline had increased total α-MSH in circulation 2 h post injection.
Conclusions
Our data indicate endogenous α-MSH contributes to the inflammatory immune responses triggered by low-dose LPS administration and suggest that targeting the melanocortin system could be a potential therapeutic for the treatment of sepsis or inflammatory disease.
Keywords: LPS, Thermogenesis, POMC, α-MSH, Mouse model
Highlights
-
•
Pomctm1/tm1 mice lacking α-MSH were hyper-responsive to low-dose LPS compared with Pomcwt/wt mice.
-
•
Following LPS injection, Pomctm1/tm1 mice had significantly lower core body temperature compared with Pomcwt/wt mice.
-
•
Following LPS injection, Pomctm1/tm1 mice had a significant decrease in O2 consumption and RER compared with Pomcwt/wt mice.
-
•
Following LPS injection, key inflammatory cytokines including leptin were increased in Pomctm1/tm1 mice vs. Pomcwt/wt mice.
1. Introduction
The melanocortin system has been extensively studied and established as a key regulator of whole-body metabolism (see reviews [[1], [2], [3]]). All mammals express melanocortins, a set of cleavage products from the prohormone pro-opiomelanocortin (POMC) that includes alpha-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropin (ACTH) [[1], [2], [3]]. The diversity of POMC-derived products and their roles in a variety of physiological processes make POMC peptides inherently complex to study in vivo [1]. However, pharmacological studies identified α-MSH as a key regulator of satiety in the hypothalamus by activating the melanocortin 4 receptor (MC4R) [[4], [5], [6], [7]]. POMC or MC4R deletion causes a severe metabolic phenotype characterized by severe obesity and hyperphagia in humans and mice [[8], [9], [10], [11]], and MC4R haploinsufficiency in humans is the most common monogenic cause of obesity accounting for up to 5% of cases [12,13].
Of note, the role of melanocortins in regulating immune responses has been investigated for longer than their roles in metabolism. The melanocortin ACTH is required for the production of glucocorticoids in rodents and humans through the well-characterized hypothalamic-pituitary-adrenal (HPA) axis that regulates stress and immune responses [14,15]. While glucocorticoids have been extensively studied and used in the clinic to reduce inflammation and suppress the immune system [16,17], less is known about the roles of ACTH itself and its cleavage product α-MSH in regulating inflammation. Work by Lipton and colleagues demonstrated that hypothalamic ACTH and α-MSH infusions had antipyretic effects following a challenge with the prominent proinflammatory cytokine interleukin-1β (IL-1β) that causes fever [18]. Furthermore, circulating α-MSH, ACTH, and corticosteroids increased in rabbits following LPS injection as part of a normal immune response [19], which is similar to the immune response in mice and humans [14,20,21]. Subsequent studies confirmed anti-inflammatory and antipyretic roles for α-MSH in rodents [[22], [23], [24], [25]], but it is not clear if α-MSH is required to mount an anti-inflammatory response. Until recently, a major obstacle in understanding the role of α-MSH in coordinating metabolic and immune responses has been the lack of an adequate animal model. We overcome this obstacle by studying a novel mouse line that specifically deletes α-MSH but preserves all other POMC cleavage products (Pomctm1/tm1 mice) [26].
POMC is cleaved into multiple melanocortins, which includes ACTH, α-MSH, and γ-MSH. In humans, β-MSH is also produced from the cleavage of POMC, but β-MSH is not present in rodents [2]. Melanocortins are most highly expressed in the pituitary gland in mice and humans [27], where they are released into circulation and activate one of 5 types of melanocortin receptors (MC1–5R) [2,3,6]. Each melanocortin has a different affinity to each receptor subtype, which allows for melanocortins to have specialized functions. For example, MC2R is only activated by ACTH, and other melanocortin receptors like MC4R have low affinity to γ-MSH but high affinity to α-MSH [2,28]. In mice, most pituitary α-MSH is produced in the pars intermedia (a rudimentary structure in humans) of the anterior lobe [2,27], whereas most pituitary α-MSH in humans is produced in pars intermedia-like cells scattered throughout the pars distalis of the anterior lobe [29,30]. The α-MSH peptides are also produced at much lower levels in the hypothalamus in all mammals, where they function as neurotransmitters and potentially neurohormones to regulate metabolism [3,31]. Most research to date has focused on studying monoacetylated α-MSH (commonly referred to simply as α-MSH), while little is known about the two other forms: desacetyl- and diacetyl-α-MSH. Monoacetylated α-MSH is generally understood to be the most abundant form produced by mouse pituitary melanotrophs while desacetyl-α-MSH is believed to be the most abundant form produced in the brain [32,33]. All three forms of α-MSH are biologically active in vitro and in vivo, but their potencies and functions can differ [[33], [34], [35]]. We recently reported Pomctm1/tm1 mice lacking all forms of α-MSH developed obesity and showed that their obesity was rescued by intracerebroventricular (i.c.v.) infusion of either desacetyl-α-MSH or monoacetyl-α-MSH. This work demonstrated that endogenous α-MSH is required to regulate metabolism and i.c.v. infusion of desacetyl-α-MSH, like α-MSH, regulates body weight [26,36]. Here, we studied Pomctm1/tm1 mice to determine whether endogenous α-MSH is required for regulating immune responses, and the potential role of α-MSH in these responses.
We administered lipopolysaccharide (LPS, a gram-negative bacterial cell wall component and a toll-like 4 receptor [TLR4] agonist [37]) to mice lacking α-MSH to study the role of this peptide in regulating immune responses. LPS stimulates immune responses in the host through a well-characterized pathway involving immune cell activation and cytokine production [37,38]. LPS is a commonly used model vs other methods to challenge the immune system in mice due to the ease of administration, controlled dose, and consistency in the responses between mice [39]. We challenged male Pomcwt/wt and Pomctm1/tm1 mice with intraperitoneal (i.p.) injections of saline (control) or low-dose LPS (100 μg/kg) and studied immune and metabolic responses post-injection. We then adapted a previously published mass spectrometry method [40] to measure total peptide levels of the 3 forms of α-MSH in the mediobasal hypothalamus (MBH, which includes the entire arcuate nucleus) and pituitary gland. Overall, we identified a critical role for melanocortins in coordinating metabolic and immune systems in mice after LPS injection.
2. Materials and methods
2.1. Animals
Animal work performed in this study was approved and conducted under the oversight of the UT Southwestern Institutional Animal Care and Use Committee (IACUC). Pomcwt/wt and Pomctm1/tm1 mice were obtained by breeding Pomcwt/tm1 littermates and genotyping offspring as previously described [26]. All mice were housed 2–5 per cage and maintained at UT Southwestern Medical Center at an ambient temperature of 23 ± 1 °C with a 12 h light/dark cycle (lights on 0700–1900). Mice were fed normal mouse chow diet (Harlan, Teklad Global 16% Protein Rodent Diet 2016: 12% kcal from fat, 3 kcal/g). All data were collected from 10- to 14-week-old male mice.
2.2. Peptide quantification
Tissues were collected and immediately frozen on dry ice then stored at −80 °C until further processing. The mediobasal hypothalamus (MBH) was removed using a mouse brain matrix and scalpel. The whole brain was placed on its dorsal side into the brain matrix and razor blades were used to remove a section between the end of the optic tracts and the beginning of the brainstem (from about Bregma −1 to −3 mm). The slice was laid coronally, and a scalpel was used to remove the MBH by cutting from the lateral sides of the hypothalamus adjacent to the cerebral cortex to the third ventricle just under the thalamus. This region includes the entire arcuate nucleus (ARH) and some surrounding nuclei such as the ventromedial hypothalamus, dorsomedial hypothalamus, lateral hypothalamus, and periventricular hypothalamus, which may contain α-MSH in axon terminals. The whole pituitary gland was removed from the sella turcica of the sphenoid bone using fine forceps. Frozen tissues were processed using a modified version of a previously reported method [40]. Two mouse MBH were combined into 1 sample for processing due to the low levels of α-MSH known to be expressed in the ARH, and total peptide concentrations calculated by mass spectrometry were divided by 2 to approximate the total peptide levels per mouse. Pituitary glands were processed individually. Guanidine hydrochloride (50 μL of 6M) was added to each frozen sample consisting of 1 pituitary gland or 2 combined MBH in low protein-binding microcentrifuge tubes. The tissues were then pipette-lysed and snap frozen on dry ice. Homogenates were then thawed and frozen a total of 3 times before 200 μL of 80% acetonitrile was added to each sample and mixed by pipette before centrifugation at 12,000 g × 5 min at 4 °C. The lower aqueous phase (50 μL) was transferred to a new low protein-binding microcentrifuge tube. Stable heavy-isotopic-labeled MSH peptides were synthesized (by 21st Century Biochemicals, Inc.) with isotopically labeled phenylalanine (13C9, 15C1) with purities of >90% for desacetyl- and monoacetyl-α-MSH, and 60% for diacetyl-α-MSH determined by HPLC and used as standards for mass spectrometry. One pmol of each heavy standard (desacetyl-α-MSH: SYSM[+16]EHF[+10]RWGKPV[-1], α-MSH: S[+42]YSM[+16]EHF[+10]RWGKPV[-1], diacetyl-α-MSH: S[+84]YSM[+16]EHF[+10]RWGKPV[-1]) in a total volume of 1 μL of 2% acetonitrile containing 0.1% trifluoroacetic acid was spiked into each 50 uL sample. Next, 4 μL of 0.3% of freshly made hydrogen peroxide in water was added and the samples were incubated for 30 min at room temperature to oxidize methionine residues. Sample volumes were then reduced to less than 10 μL in a SpeedVac. Following this, samples were resuspended in 100 μL of 0.1% formic acid in water and solid-phase extraction was performed using a 96 well Oasis PRIME HLB uElution Plate (Waters). The samples were washed with 200 μL of 0.1% formic acid, followed by 200 μL of 5% methanol containing 1% acetic acid, before the peptides were eluted with 2 × 30 μL of 60% methanol containing 10% acetic acid. Peptide eluates were then completely dried in a SpeedVac and reconstituted in 10 μL of 2% acetonitrile containing 0.1% trifluoroacetic acid for selected reaction monitoring (SRM) mass spectrometry analysis.
SRM analysis was performed on an AB Sciex 6500 QTRAP mass spectrometer in positive-ion low-mass mode, using a OptiFlow source with an OptiFlow Nano Electrode (100 nl/min – 1 μL/min, P/N: 5070382). The source settings were as follows: curtain gas = 20, ion spray voltage = 3400, ion source gas 1 = 20. Analyst Software v.1.6 was used to run the mass spectrometer. Reconstituted samples (100 μL) were separated on a Dionex Acclaim PepMap100 reverse-phase C18 column (75 μm × 15 cm) using an Ultimate 3000 RSLCnano HPLC system. The HPLC was controlled using Chromeleon Xpress (version 6.8 SR10) and Dionex Chromatography MS Link v. 2.12. Separation of peptides was carried out at 250 nL/min using a gradient from 5% to 35% B for 15 min, 35%–50% B for 4 min, and 50%–80% B for 5 min, where mobile phase A was 2% acetonitrile (ACN), 0.1% formic acid in water and mobile phase B was 80% ACN, 10% trifluoroethanol, 10% water, 0.1% formic acid. The SRM method monitored the top seven transitions for each form of the MSH peptide and its corresponding heavy-isotope-labeled standard. These transitions were determined by monitoring peak areas for all singly and doubly charged b and y ions below m/z = 1,250 and all doubly and triply charged peptide ions, and for a mix of the heavy-labeled peptide standards. These data were analyzed using Skyline v4.2 software (http://skyline.maccosslab.org). Collision energies were optimized by subsequent sample injections. Transitions that had interference from impurities or noise peaks were not included when performing peptide quantification.
2.3. LPS challenge
Mice were group housed for all experiments except metabolic cage and telemetry experiments, which required single housing. All experiments were performed either at room temperature or 23 °C in temperature-controlled metabolic cages and the LPS used was made up from the same stock of Escherichia coli O55:B5 (Sigma Aldrich L2880). The LPS stock solution was reconstituted in saline to a concentration of 1 mg/mL, aliquoted, and stored at −80 °C. LPS aliquots were thawed, diluted further in saline to a working solution, and injected i.p. with a dose of 100 μg/kg for all experiments. Control mice were injected i.p. with saline. Injection volumes for all experiments and treatments were 1% of the animal's body weight (e.g., 0.25 mL for a 25 g mouse).
2.4. Hormone and cytokine measurements
Mice were euthanized by decapitation in a dedicated room to minimize stress to the animals between 2 PM and 5 PM for all experiments. Trunk blood was collected into EDTA coated tubes and centrifuged (2000 g × 15 min at 4 °C) to extract plasma and stored at −80 °C until analysis. Circulating cytokines (Bio-Rad, M60009RDPD), leptin (Crystal Chem, 90030), and total α-MSH peptides (Mybiosource, MBS2563826) were measured using commercial ELISA kits following manufacturer's instructions. Plasma was shipped on dry ice to Vanderbilt University Hormone Assay & Analytical Services Core for corticosterone quantification.
2.5. Telemetry
Pomcwt/wt and Pomctm1/tm1 mice were anesthetized with 3% isoflurane in a chamber before being transferred to a nose cone and maintained on 2% isoflurane for surgeries. Mice were injected subcutaneously with Buprenorphine SR (0.5 mg/mL, 50 μL per 25 g mouse) and Carprofen (1.3 mg/mL, 100 μL per 25 g mouse) into the interscapular region and rested on a heating pad with eye lubricant applied. The abdominal surgical region was shaved, and excess hair was removed with Nair hair removal cream for 30 s. The surgical field was sterilized using 3 alternating applications of Betadine and ethanol wipes. A horizontal incision was made through the skin and muscle layers and the telemeter (model TA-F10, Data Sciences International, Minnesota, USA) was inserted i.p. and slid laterally towards the bladder and away from vital organs. The muscle layer was then sutured with absorbable 4-0 sutures and the skin was closed with wound clips. After surgery, each mouse was placed on a warming pad in a new cage and given 30 min to recover before the cage was moved back to the housing room. Mice were given an additional injection of Carprofen analgesic every 24 h and checked twice per day, up to 72 h post-surgery. All mice were singly housed after surgery and throughout the telemetry experiment.
After the surgery, mice were given 7–10 days to recover before being moved to a dedicated telemetry room for recordings. Mice were habituated in the telemetry room for at least 24 h before commencing baseline recordings. Telemetry plate receivers were placed under each cage, and up to 12 mice were recorded simultaneously. Injections on experimental Days 1 and 2 (after the 24 h habituation period) were performed 1 h before lights off. Telemetry data were recorded for another 24–48 h after Day 2 injections before mice were euthanized. Telemeters recorded 10 s of core body temperature (Tb) and locomotor activity every 10 min during the entire recording period.
2.6. Metabolic cages
A combined indirect calorimetry system (CaloSys Calorimetry System, TSE Systems Inc.) was used for all metabolic studies. Individually housed mice were acclimated for 5 days in a metabolic chamber with food and water before their cages were moved to recording positions. Oxygen consumption (VO2), carbon dioxide production (VCO2), food intake, and water intake were then measured for 10 days. Respiratory exchange ratio (RER) was calculated from VO2 and VCO2 values [41]. On Day 3 of the 10-day testing period, mice were injected i.p. with saline. On Day 6, mice were injected i.p. with 100 μg/kg LPS. All i.p. injections were performed 1 h before lights off.
2.7. Statistical analysis
A detailed list of the statistical analyses performed is found in Table S1. Data are expressed as mean ± SEM. Comparisons between two experimental conditions were analyzed by Student's unpaired t-test. Comparisons between more than two experimental conditions were analyzed by Two-way ANOVA followed by Šidák post hoc test. Outlying data were identified using the ROUT method (Q = 1%) and removed from analysis. All statistical tests were performed using GraphPad Prism (Version 10) and P < 0.05 was considered statistically significant.
3. Results
3.1. Validation of α-MSH depletion using mass spectrometry
In order to determine the efficacy of the depletion of all three forms α-MSH in Pomctm1/tm1 mice, we modified a method [40] to quantify total peptide levels using SRM mass spectrometry analysis in the MBH and pituitary gland. Surprisingly, we found that in control mice, diacetyl-α-MSH was the most highly produced form in both the MBH and pituitary gland (Figs. S1A–B). As expected, we also found a robust depletion of all three forms of α-MSH in both tissues in Pomctm1/tm1 mice (Fig. S1), further validating the functional knockout of all α-MSH forms by the targeted mutation in the Pomc gene in Pomctm1/tm1 mice.
3.2. Pomctm1/tm1 mice are hyper-responsive to LPS injection
Baseline activity was similar in Pomcwt/wt and Pomctm1/tm1 mice following saline injection on Day 1 (Figure 1A), marked by a normal increase in locomotion during their active phase (lights off) [42]. However, activity dropped markedly (∼50%) in both Pomcwt/wt and Pomctm1/tm1 mice injected i.p. with 100 μg/kg LPS on Day 2 during the acute phase of the immune response (0–10 h post-injection), as part of the normal sickness response induced by LPS (Figure 1B–C) [43]. Tb was also similar in Pomcwt/wt and Pomctm1/tm1 mice on Day 1 after saline injection and increased normally during their active phase (Figure 1D) [42]. On Day 2, LPS injection induced an acute hypothermic response in both Pomcwt/wt and Pomctm1/tm1 mice (Figure 1E–F). Strikingly, Tb in Pomctm1/tm1 mice dropped nearly 2° lower than Pomcwt/wt control mice following low-dose LPS injection, suggesting that Pomctm1/tm1 mice are hyper-responsive to 100 μg/kg LPS. Differences in activity and Tb were not observed following the acute phase for up to 30 h post LPS (Fig. S2). Together, these data show that Pomctm1/tm1 mice exhibit an exaggerated acute LPS-induced thermoregulatory response compared to Pomcwt/wt mice.
Figure 1.
LPS reduced activity and core body temperature in Pomcwt/wtand Pomctm1/tm1mice.Pomcwt/wt and Pomctm1/tm1 mice were injected (i.p.) with saline on Day 1 (A) and 100 μg/kg LPS on Day 2 (B) 1 h before lights off, and relative activity and core body temperature was recorded by telemeter every 10 min. Activity data is binned in 1 h blocks. (C) Summary figure of all 10 min recordings for Days 1 and 2 superimposed. Core body temperature was also recorded after i.p. injections of saline on Day 1 (D) and 100 μg/kg LPS on Day 2 (E). Summary data are shown for Days 1 and 2 superimposed (F). The data are expressed as the mean ± SEM. ∗p < 0.05 compared to littermate controls.
3.3. Pomctm1/tm1 mice have impaired metabolism following LPS injection
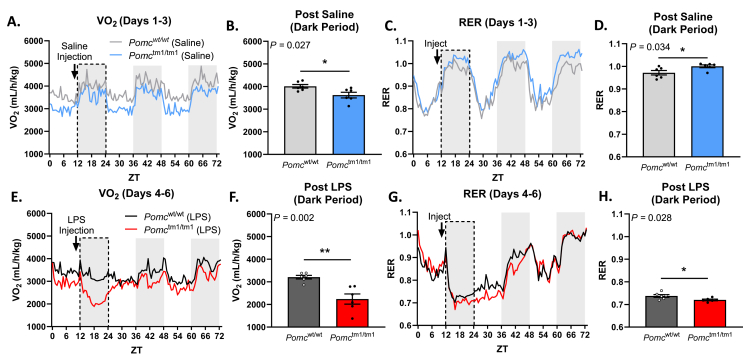
We next challenged mice with LPS in metabolic cages to measure other aspects of sickness behavior (Figure 2). Oxygen consumption and respiratory exchange ratio (RER) are used as indicators of macronutrient preference; higher RER indicates a preference towards carbohydrate energy utilization while a lower RER indicates a preference towards lipids as an energy substrate [41]. These measurements are important in the context of immune responses as many immune cells are sensitive to and modified by macronutrients [[44], [45], [46]]. Furthermore, reduced oxygen consumption is an independent predictor of mortality in critically ill patients and is therefore of clinical significance [47].
Figure 2.
Pomctm1/tm1mice had reduced oxygen consumption and RER after LPS injection. (A–D)Pomcwt/wt and Pomctm1/tm1 mice were injected (i.p.) with saline 1 h before lights off on Day 1. VO2 was measured and RER calculated for 3 days. (E–H)Pomcwt/wt and Pomctm1/tm1 mice were injected i.p. with 100 μg/kg LPS. Gray boxes indicate dark cycle, and bar graphs show average values per mouse for the entire dark period after saline or LPS injection (dotted line region on the line graphs). The data are expressed as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 compared to littermate controls.
After saline injection, Pomctm1/tm1 mice decreased oxygen consumption (Figure 2A–B) which resulted in an elevated respiratory exchange ratio (RER) compared to Pomcwt/wt mice, indicating the preferential use of glucose over lipids as an energy substrate in Pomctm1/tm1 mice [41](Figure 2C–D). However, Pomctm1/tm1 had drastically reduced oxygen consumption following LPS injection (Figure 2E–F), marked by a 38% decrease from post–saline dark period VO2 compared to a 20% decrease in Pomcwt/wt mice. Furthermore, while RER was elevated in Pomctm1/tm1 mice after saline treatment, LPS administration induced a change in energy preference, indicating Pomctm1/tm1 mice may rely more on lipids as an energy substrate compared to Pomcwt/wt littermate controls during immune responses (Figure 2G–H). Increasing fatty acid metabolism is a typical response in mice and humans during infection [[48], [49], [50], [51]], and may indicate an exaggerated immune response in Pomctm1/tm1 mice. This metabolic shift lasted up to 36 h after LPS treatment, and this was not due to a difference in food intake in LPS-treated groups (Fig. S3). Taken together, these data suggest α-MSH is an important regulator of baseline metabolism and metabolic adaptations in response to LPS that may influence the ability of the immune system to properly initiate an immune response.
3.4. Peripheral α-MSH increased in Pomcwt/wt mice while Pomctm1/tm1 mice had normal corticosteroid production but increased leptin following LPS injection
Adrenal-derived glucocorticoids (corticosterone in mice, cortisol in humans) are a central part of the immune and stress responses following an infection [52,53]. Others have shown that LPS administration in rodents causes an increase in pituitary gland Pomc expression and corresponding increases in circulating ACTH, α-MSH, and glucocorticoid production [14,19,54]. There is currently no method to measure individual forms of α-MSH in circulation, but commercial ELISA kits likely detect total (three forms combined) α-MSH. We found that LPS induces an increase in total circulating α-MSH in Pomcwt/wt mice 2 h post-injection (Figure 3A), suggesting this protein may be produced in response to an infection and potentially involved in immune responses.
Figure 3.
Peripheral α-MSH increased in Pomcwt/wtmice and Pomctm1/tm1mice had normal corticosteroid production but increased leptin after LPS injection.Pomcwt/wt and Pomctm1/tm1 mice were injected with 100 μg/kg LPS and plasma was collected 2 h later. (A) Total α-MSH (all 3 forms combined) was measured by ELISA in Pomcwt/wt mice. (B) Corticosterone and (C) leptin were measured in Pomcwt/wt and Pomctm1/tm1 mice by ELISA. The data are expressed as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 compared to littermate controls.
We next sought to determine whether Pomctm1/tm1 mice may have impairments in other aspects of their immune responses related to the mutation in Pomc in our mouse model. ACTH is a key POMC-derived hormone involved in the synthesis of corticosterone, a key hormone in regulating the immune system, and peaks 2 h after LPS injection in rodents and functions to prevent excess inflammation [55,56]. We have shown previously that male Pomctm1/tm1 mice have normal baseline corticosterone levels similar to Pomcwt/wt controls, and that the mutated ACTH in these mice still binds and activates MC2R in vitro [26]. However, antibody-based techniques that target ACTH are ineffective with our mouse model due to the location of the targeted mutation in Pomctm1/tm1 mice. In our model, we mutated the cleavage site on the ACTH protein which is also the epitope-binding site targeted by antibody-based techniques; we therefore cannot measure ACTH in Pomctm1/tm1 mice. Instead, we measured the downstream hormone, corticosterone, to assess a component of the early HPA axis response. We found that Pomctm1/tm1 and Pomcwt/wt mice have similar concentrations of corticosterone 2 h post-LPS (Figure 3B). These data suggest that both Pomcwt/wt and Pomctm1/tm1 mice have intact acute glucocorticoid responses to low-dose LPS.
We have previously shown that Pomctm1/tm1 mice have elevated leptin due to increased fat mass [36]. Leptin is a critical metabolic hormone produced and released by white adipose tissue and exerts its effects by binding to receptors in the hypothalamus [57,58]. As fat mass increases, circulating levels of leptin also increase, and this hyperleptinemia is predicted to result in the chronic low-grade inflammation and susceptibility to inflammatory disease observed in obese patients [59]. However, this low-grade inflammation is also due to leptin's role as a proinflammatory cytokine which is induced after LPS administration [60,61]. Leptin regulates the immune system [62] and is expressed in immune cells [[63], [64], [65]]. Activation of the leptin receptor increases phagocytic action of macrophages [66] and promotes proinflammatory cytokine secretion [67,68](for review, see [69]). Given the increased body mass (Fig. S4), which we have previously shown is due to increased fat mass and corresponding leptin concentrations in Pomctm1/tm1 mice [36], we measured leptin 2 h post LPS injection and found that leptin levels were significantly increased compared to Pomcwt/wt mice (Figure 3C). These data suggest mice lacking α-MSH may have impaired leptin regulation following an LPS injection, which could contribute to the exaggerated immune responses seen here.
3.5. Key proinflammatory cytokines are increased in Pomctm1/tm1 mice following LPS injection
Cytokines signal the brain to shift metabolism and behavior to promote survival after infection [70,71]. In gram-negative infections, cytokine production is stimulated via activation of TLR4, primarily through monocytes and macrophages [[72], [73], [74]]. POMC cells in the hypothalamus and pituitary express numerous cytokine receptors and increase Pomc mRNA expression after LPS or IL-1β injection [22,[75], [76], [77]]. However, cytokine production must be tightly regulated since excessive cytokine production can be life-threatening, as seen in septic shock [78,79]. Therefore, we determined whether endogenous α-MSH regulates cytokine expression at 0.5 h, 2 h, and 4 h after saline or low-dose LPS injection (100 μg/kg). LPS injection robustly increased cytokine expression in both Pomcwt/wt and Pomctm1/tm1 mice (Figure 4 and Table S2), consistent with an LPS immune challenge [80]. However, we found that Pomctm1/tm1 mice had significantly greater expression of multiple proinflammatory cytokines and chemokines at 2 h and 4 h post-LPS injection (Figure 4). Pomctm1/tm1 mice had significantly higher concentrations of IL-1β at 2 h and 4 h post-LPS, and significant increases in IL-6, IL-10, monocyte chemoattractant peptide 1 (MCP-1), and macrophage inflammatory peptides 1α and 1β (MIP-1α and MIP-1β, respectively) at 4 h compared to LPS-injected Pomcwt/wt controls (Figure 4). There were no significant differences between Pomctm1/tm1 and Pomcwt/wt controls 30 min after either saline or LPS injection (Table S2). Together, these data indicate α-MSH is an important regulator of cytokine production in response to LPS, and that mice lacking α-MSH are susceptible to excessive inflammation.
Figure 4.
Pomctm1/tm1mice had increased cytokine levels compared to Pomcwt/wtmice 2 h and 4 h after LPS injection. Plasma from Pomcwt/wt and Pomctm1/tm1 mice were collected 2 h (A) and 4 h (B) after 100 μg/kg i.p. LPS injection and cytokine levels were quantified using a cytokine multiplex assay. The data are expressed as the mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01 compared to littermate controls.
4. Discussion
Collectively, our results support the hypothesis that α-MSH regulates inflammatory immune responses triggered by LPS administration [26]. Compared to Pomcwt/wt littermates, Pomctm1/tm1 mice exhibited an exaggerated immune response to low-dose i.p. LPS, characterized by acute hypothermia, an impaired metabolic response, and increased proinflammatory and inflammatory-related cytokines including leptin. Using ELISA, we show that LPS treatment significantly increased total plasma α-MSH in Pomcwt/wt mice 2 h post LPS. These data suggest that α-MSH release, in concert with other immune responses, reduces systemic inflammation to LPS by regulating cytokine production. Without α-MSH, some proinflammatory cytokines are produced in excess following treatment with LPS, which are associated with the exaggerated sickness behaviors observed here in Pomctm1/tm1 mice. Furthermore, the lack of hypothalamic α-MSH may contribute to the impaired metabolic responses in Pomctm1/tm1 mice characterized by reduced O2 consumption and lower RER, and attenuated thermogenesis (resulting in hypothermia and a failure to regulate Tb) compared to Pomcwt/wt mice after LPS injection.
Macrophages and other immune cells express TLR4, LEPR, and melanocortin receptors in mice and humans [63,64,73,81,82]. In vitro studies have shown that activation of melanocortin receptors on macrophages inhibits cytokine release via suppression of NF-kB and nitric oxide [[83], [84], [85]], and MC1R [[86], [87], [88], [89]] and MC3R [90] agonists have been shown to reduce inflammation through this same pathway. Indeed, murine macrophages express MC1R [84,89,91], MC3R [92], and MC5R [93], which all bind α-MSH and reduce inflammation. Furthermore, central melanocortin signaling plays a critical role in regulating fever [19,94] as well as central [[95], [96], [97]] and peripheral [81,98,99] immune responses. These responses involve microglia, neurons, and astrocytes, all of which express the melanocortin receptors [97,[100], [101], [102]]. We found that macrophage-derived cytokines, IL-1β, IL-6, IL-10, MCP-1, MIP-1α, and MIP-1β were significantly increased in Pomctm1/tm1 mice following low-dose LPS injection compared to LPS-injected Pomcwt/wt control mice. Leptin, another important cytokine and adipokine known to regulate inflammation [60,61], was also significantly increased after LPS injection in our study. The primary targets for leptin are the LEPR-expressing hypothalamic neurons (including POMC-expressing ARH neurons) which also express cytokine receptors and are activated after LPS treatment [103,104]. Thus, the hypothalamus is poised to orchestrate metabolic and behavioral responses to immune challenges through highly interconnected networks linked to every metabolic function associated with the sickness behaviors observed in this study, including activity, food intake, energy expenditure, and thermoregulation [105]. α-MSH release in the periphery, along with feedback from peripheral inflammatory signals such as cytokines and leptin to hypothalamic neurons, may be critical in regulating thermogenesis and sickness behaviors following LPS administration.
While these findings agree with and build upon previous literature, there are important limitations to consider. First, while we have modified a method enabling measurement of three forms of α-MSH in eluate from tissue lysate, further work is required to enable quantitative measurement for these forms of α-MSH in animal tissue and to determine their respective roles in response to inflammation. Future matrix-assisted laser desorption/ionization (MALDI)-imaging mass spectrometry to map cell expression of these α-MSH forms in the pituitary gland and hypothalamus will also help to resolve the regulation of α-MSH forms following LPS treatment. Second, our study was limited to acute immune responses specifically to LPS, mediated via the TLR4-dependent pathway, which is commonly used as a model of Gram-negative sepsis [37]. Our studies used low-dose LPS due to the risk of severe sickness and mortality from higher doses of LPS in a mouse model that was hypothesized to have an impaired immune response [46,106]. Our model relied on modeling bacterial inflammation using LPS, but α-MSH may have other effects (or not be involved) in bacterial clearance or other types of responses, such as adaptive or T-cell-mediated immune responses [44,45]. Future studies using alternative methods such as cecal ligation and puncture to model live bacterial infections, as well as injection of different inflammatory pathogens (e.g., viral proteins) and/or ambient temperatures will further elucidate the role of α-MSH in host defense. Lastly, it is unclear from these data if the dysregulated metabolism in Pomctm1/tm1 mice contribute to, or cause, the exaggerated immune responses observed here: more studies will be needed to untangle the interactions between metabolic and immune responses in Pomctm1/tm1 mice.
Our data support the model that melanocortin agonists could be useful in the context of sepsis and obesity, where leptin and melanocortin signaling may already be deficient. In fact, the side effects of hypertension encountered for treatment of obesity with MC4R agonist [107,108] could also be beneficial in treating refractory septic shock. Furthermore, α-MSH agonists may avoid the undesired side effects of alternative immunosuppressive therapies for sepsis, such as glucocorticoids [109]. Lastly, preclinical studies have highlighted the striking effects of metabolic state on mortality to bacterial and viral infections [46]. Together, these data highlight the interaction between metabolism and inflammation and incentivize further studies that investigate targeting the melanocortin system to treat inflammation.
5. Conclusion
In conclusion, we demonstrate a role of the POMC derivative, α-MSH, in mediating immune responses to LPS. Deletion of three forms of α-MSH in male C57BL6/J mice results in exaggerated immune responses to low-dose LPS, characterized by hypothermia, altered metabolism, and increased circulating proinflammatory and inflammatory-related cytokines including leptin. We developed a mass spectrometry method to distinguish between three forms of endogenous α-MSH to better understand α-MSH regulation in health and disease. Our model predicts that α-MSH is released in response to an immune challenge and prevents excess cytokine production to properly tune the physiological response to pathogens. We believe these data warrant further translational studies exploring the therapeutic potential of α-MSH mimetics that target both metabolic and immune responses.
CRediT authorship contribution statement
R.P. Reynolds: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. R.R. Fan: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. A. Tinajero: Methodology. X. Luo: Writing – original draft, Methodology, Formal analysis. S.C. Huen: Writing – review & editing, Methodology. T. Fujikawa: Writing – review & editing, Writing – original draft. S. Lee: Supervision, Conceptualization. A. Lemoff: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis. K.G. Mountjoy: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization. J.K. Elmquist: Writing – review & editing, Writing – original draft, Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank NIH for its support: P01DK119130, R01DK127274, R01DK118725, and 1P30DK127984-01A1-7989 awarded to JKE; 1T32HL139438-01A1 awarded to UT Southwestern Medical Center; R35GM137984 awarded to SCH. Catalyst: Seeding funding (awarded to KGM and JKE) was provided by the New Zealand Ministry of Business, Innovation and Employment and administered by the Royal Society Te Apārangi.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2024.101986.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Mountjoy K.G. Pro-opiomelanocortin (POMC) neurones, POMC-derived peptides, melanocortin receptors and obesity: how understanding of this system has changed over the last decade. J Neuroendocrinol. 2015;27(6):406–418. doi: 10.1111/jne.12285. [DOI] [PubMed] [Google Scholar]
- 2.Harno E., Ramamoorthy T.G., Coll A.P., White A. POMC: the physiological power of hormone processing. Physiol Rev. 2018:2381–2430. doi: 10.1152/physrev.00024.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cone R.D. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 4.Poggioli R., Vergoni A.V., Bertolini A. ACTH-(1–24) and α-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986:843–848. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- 5.Abbott C.R., Rossi M., Kim M., AlAhmed S.H., Taylor G.M., Ghatei M.A., et al. Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869(1–2):203–210. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 6.Mountjoy K.G., Robbins L.S., Mortrud M.T., Cone R.D. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 7.Fan W., Boston B.A., Kesterson R.A., Hruby V.J., Cone R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 8.Yeo G.S., Farooqi I.S., Aminian S., Halsall D.J., Stanhope R.G., O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 9.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 10.Vaisse C., Clement K., Guy-Grand B., Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20(2):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 11.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R., et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 12.Farooqi I.S., Yeo G.S., Keogh J.M., Aminian S., Jebb S.A., Butler G., et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Investig. 2000;106(2):271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Investig. 2000;106(2):253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beishuizen A., Thijs L.G. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9(1):3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 15.Keller-Wood M.E., Dallman M.F. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 16.Cain D.W., Cidlowski J.A. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 18.Glyn J.R., Lipton J.M. Hypothermic and antipyretic effects of centrally administered ACTH (1–24) and α-melanotropin. Peptides. 1981:177–187. doi: 10.1016/s0196-9781(81)80032-0. [DOI] [PubMed] [Google Scholar]
- 19.Martin L.W., Lipton J.M. Acute phase response to endotoxin: rise in plasma alpha-MSH and effects of alpha-MSH injection. Am J Physiol. 1990;259(4 Pt 2):R768–R772. doi: 10.1152/ajpregu.1990.259.4.R768. [DOI] [PubMed] [Google Scholar]
- 20.Rivier C., Chizzonite R., Vale W. In the mouse, the activation of the hypothalamic pituitary-adrenal Axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1∗. Endocrinology. 1989:2800–2805. doi: 10.1210/endo-125-6-2800. [DOI] [PubMed] [Google Scholar]
- 21.Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Q.-H., Hruby V.J., Tatro J.B. Systemic α-MSH suppresses LPS fever via central melanocortin receptors independently of its suppression of corticosterone and IL-6 release. Am J Physiol Regul Integr Comp Physiol. 1998:R524–R530. doi: 10.1152/ajpregu.1998.275.2.r524. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso A., McCoy D., Ceriani G., Watanabe T., Biltz J., Catania A., et al. Antiinflammatory influences of alpha-MSH molecules: central neurogenic and peripheral actions. J Neurosci: Off J Soc Neurosci. 1994;14(4):2377–2382. doi: 10.1523/JNEUROSCI.14-04-02377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Q.H., Entwistle M.L., Alvaro J.D., Duman R.S., Hruby V.J., Tatro J.B. Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever. J Neurosci: Off J Soc Neurosci. 1997;17(9):3343–3351. doi: 10.1523/JNEUROSCI.17-09-03343.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catania A., Lonati C., Sordi A., Carlin A., Leonardi P., Gatti S. The melanocortin system in control of inflammation. TheScientificWorldJOURNAL. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mountjoy K.G., Caron A., Hubbard K., Shome A., Grey A.C., Sun B., et al. Desacetyl-α-melanocyte stimulating hormone and α-melanocyte stimulating hormone are required to regulate energy balance. Mol Metabol. 2018;9:207–216. doi: 10.1016/j.molmet.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro S., Soletto L., Puchol S., Rotllant J., Soengas J.L., Cerdá-Reverter J.M. 60 years of POMC: POMC: an evolutionary perspective. J Mol Endocrinol. 2016;56(4):T113–T118. doi: 10.1530/JME-15-0288. [DOI] [PubMed] [Google Scholar]
- 28.Cone R.D. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 29.McNicol A.M. A study of intermediate lobe differentiation in the human pituitary gland. J Pathol. 1986;150(3):169–173. doi: 10.1002/path.1711500304. [DOI] [PubMed] [Google Scholar]
- 30.Coates P.J., Doniach I., Hale A.C., Rees L.H. The distribution of immunoreactive alpha-melanocyte-stimulating hormone cells in the adult human pituitary gland. J Endocrinol. 1986;111(2):335–342. doi: 10.1677/joe.0.1110335. [DOI] [PubMed] [Google Scholar]
- 31.Mountjoy K.G., Mortrud M.T., Low M.J., Simerly R.B., Cone R.D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8(10):1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 32.Mountjoy K.G. Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes. Biochem J. 2010;428(3):305–324. doi: 10.1042/BJ20091957. [DOI] [PubMed] [Google Scholar]
- 33.Rudman D., Hollins B.M., Kutner M.H., Moffitt S.D., Lynn M.J. Three types of alpha-melanocyte-stimulating hormone: bioactivities and half-lives. Am J Physiol. 1983;245(1):E47–E54. doi: 10.1152/ajpendo.1983.245.1.E47. [DOI] [PubMed] [Google Scholar]
- 34.Lamers A.E., Flik G., Atsma W., Wendelaar Bonga S.E. A role for di-acetyl alpha-melanocyte-stimulating hormone in the control of cortisol release in the teleost Oreochromis mossambicus. J Endocrinol. 1992;135(2):285–292. doi: 10.1677/joe.0.1350285. [DOI] [PubMed] [Google Scholar]
- 35.Ellerkmann E., Kineman R.D., Porter T.E., Frawley L.S. Des-acetylated variants of alpha-melanocyte-stimulating hormone and beta-endorphin can antagonize the mammotrope-recruiting activity of their acetylated forms. J Endocrinol. 1993;139(2):295–300. doi: 10.1677/joe.0.1390295. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard K., Shome A., Sun B., Pontré B., McGregor A., Mountjoy K.G. Chronic high-fat diet exacerbates sexually dimorphic pomctm1/tm1 mouse obesity. Endocrinology. 2019;160(5):1081–1096. doi: 10.1210/en.2018-00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y.-C., Yeh W.-C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Buras J.A., Holzmann B., Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 40.Kirwan P., Kay R.G., Brouwers B., Herranz-Pérez V., Jura M., Larraufie P., et al. Quantitative mass spectrometry for human melanocortin peptides in vitro and in vivo suggests prominent roles for β-MSH and desacetyl α-MSH in energy homeostasis. Mol Metabol. 2018;17:82–97. doi: 10.1016/j.molmet.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonson D.C., DeFronzo R.A. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990;258(3 Pt 1):E399–E412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 42.Tokizawa K., Yoda T., Uchida Y., Kanosue K., Nagashima K. Estimation of the core temperature control during ambient temperature changes and the influence of circadian rhythm and metabolic conditions in mice. J Therm Biol. 2015;51:47–54. doi: 10.1016/j.jtherbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Kozak W., Conn C.A., Kluger M.J. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;266(1 Pt 2):R125–R135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 44.Galván-Peña S., O'Neill L.A.J. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang A., Huen S.C., Luan H.H., Yu S., Zhang C., Gallezot J.-D., et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166(6):1512–1525.e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Alvarez M., Marik P., Bellomo R. Sepsis-associated hyperlactatemia. Crit Care/the Society of Critical Care Medicine. 2014;18(5):503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park B.S., Kim Y.J., Jeong D.Y., Kim Y.T., Kim J.K., Lee B.J., et al. Enhanced lipid utilization is coupled to the sickness responses triggered by lipopolysaccharide. Biochem Biophys Res Commun. 2021:44–50. doi: 10.1016/j.bbrc.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 49.Patkova A., Joskova V., Havel E., Kovarik M., Kucharova M., Zadak Z., et al. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic review. Adv Nutr. 2017;8(4):624–634. doi: 10.3945/an.117.015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma K., Mogensen K.M., Robinson M.K. Pathophysiology of critical illness and role of nutrition. Nutr Clin Pract: Off Publ Am Soc Parenteral Enteral Nutr. 2019;34(1):12–22. doi: 10.1002/ncp.10232. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y., Zhong W., Zhang Y., Cheng Y., Lai H., Yu H., et al. Sustained inflammation induced by LPS leads to tolerable anorexia and fat loss via Tlr4 in mice. J Inflamm Res. 2022;15:5635–5648. doi: 10.2147/JIR.S358518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 53.Bornstein S.R., Ziegler C.G., Krug A.W., Kanczkowski W., Rettori V., McCann S.M., et al. The role of toll-like receptors in the immune-adrenal crosstalk. Ann N Y Acad Sci. 2006;1088:307–318. doi: 10.1196/annals.1366.027. [DOI] [PubMed] [Google Scholar]
- 54.Schnydrig S., Korner L., Landweer S., Ernst B., Walker G., Otten U., et al. Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci Lett. 2007;429(1):69–73. doi: 10.1016/j.neulet.2007.09.067. [DOI] [PubMed] [Google Scholar]
- 55.Nakano K., Suzuki S., Oh C. Significance of increased secretion of glucocorticoids in mice and rats injected with bacterial endotoxin. Brain Behav Immun. 1987;1(2):159–172. doi: 10.1016/0889-1591(87)90018-3. [DOI] [PubMed] [Google Scholar]
- 56.Mathias S., Schiffelholz T., Linthorst A.C., Pollmächer T., Lancel M. Diurnal variations in lipopolysaccharide-induced sleep, sickness behavior and changes in corticosterone levels in the rat. Neuroendocrinology. 2000;71(6):375–385. doi: 10.1159/000054558. [DOI] [PubMed] [Google Scholar]
- 57.Ahima R.S., Saper C.B., Flier J.S., Elmquist J.K. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 59.Cava A.L., La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017:51–58. doi: 10.1016/j.cyto.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faggioni R., Moser A., Feingold K.R., Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156(5):1781–1787. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imajo K., Fujita K., Yoneda M., Nozaki Y., Ogawa Y., Shinohara Y., et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metabol. 2012;16(1):44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Zhang F., Basinski M.B., Beals J.M., Briggs S.L., Churgay L.M., Clawson D.K., et al. Crystal structure of the obese protein Ieptin-E100. Nature. 1997;206–9 doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 63.Zarkesh-Esfahani H., Pockley G., Metcalfe R.A., Bidlingmaier M., Wu Z., Ajami A., et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001:4593–4599. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 64.Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R., Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Sun R., You L., Gao C., Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003:247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 66.Mancuso P., Gottschalk A., Phare S.M., Peters-Golden M., Lukacs N.W., Huffnagle G.B. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J Immunol. 2002:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 67.Loffreda S., Yang S.Q., Lin H.Z., Karp C.L., Brengman M.L., Wang D.J., et al. Leptin regulates proinflammatory immune responses. Faseb J. 1998:57–65. doi: 10.1096/fasebj.12.1.57. [DOI] [PubMed] [Google Scholar]
- 68.Gainsford T., Willson T.A., Metcalf D., Handman E., McFarlane C., Ng A., et al. Proceedings of the national academy of Sciences. 1996. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells; pp. 14564–14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matarese G., Moschos S., Mantzoros C.S. Leptin in immunology. J Immunol. 2005:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 70.Borish L.C., Steinke J.W. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 71.Alexander C., Rietschel E.T. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001:167–202. doi: 10.1179/096805101101532675. [DOI] [PubMed] [Google Scholar]
- 72.Erickson M.A., Banks W.A. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun. 2011;25(8):1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12(1):20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Castejon G., Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kariagina A., Romanenko D., Ren S.-G., Chesnokova V. Hypothalamic-pituitary cytokine network. Endocrinology. 2004:104–112. doi: 10.1210/en.2003-0669. [DOI] [PubMed] [Google Scholar]
- 76.Ray D.W., Ren S.G., Melmed S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J Clin Invest. 1996:1852–1859. doi: 10.1172/jci118615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woloski B.M.R.J., Woloski B.M.R. CORTICOTROPIN-RELEASING activity of monokines. Pediatr Infect Dis J. 1986;501 doi: 10.1097/00006454-198607000-00044. [DOI] [Google Scholar]
- 78.Bone R.C. The pathogenesis of sepsis. Ann Intern Med. 1991;457 doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 79.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020:2255–2273. doi: 10.1056/nejmra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skelly D.T., Hennessy E., Dansereau M.-A., Cunningham C. Correction: a systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in C57bl/6 mice. PLoS One. 2013;8(12) doi: 10.1371/annotation/90c76048–2edd–4315–8404–4d9d8cbd411e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catania A., Gatti S., Colombo G., Lipton J.M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56(1):1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 82.Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81(2):383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- 83.Rajora N., Ceriani G., Catania A., Star R.A., Murphy M.T., Lipton J.M. α-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol. 1996:248–253. doi: 10.1002/jlb.59.2.248. [DOI] [PubMed] [Google Scholar]
- 84.Star R.A., Rajora N., Huang J., Stock R.C., Catania A., Lipton J.M. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci USA. 1995:8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brzoska T., Kalden D.-H., Scholzen T., Luger T.A. Molecular basis of the α-MSH/IL-1 antagonism. Ann N Y Acad Sci. 2006:230–238. doi: 10.1111/j.1749-6632.1999.tb08680.x. [DOI] [PubMed] [Google Scholar]
- 86.Taylor A.W., Li D. Through melanocortin 1 receptor (MC1r), alpha-melanocyte stimulating hormone (α-MSH) regulates macrophage function (100.2) J Immunol. 2007;178(1_Supplement) S197–S197. [Google Scholar]
- 87.Salazar-Onfray F., López M., Lundqvist A., Aguirre A., Escobar A., Serrano A., et al. Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker. Br J Cancer. 2002;87(4):414–422. doi: 10.1038/sj.bjc.6600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mun Y., Kim W., Shin D. Melanocortin 1 receptor (MC1R): pharmacological and therapeutic aspects. Int J Mol Sci. 2023;24(15) doi: 10.3390/ijms241512152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D., Taylor A.W. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J Leukoc Biol. 2008;84(1):191–198. doi: 10.1189/jlb.0707463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gómez-SanMiguel A.B., Villanúa M.Á., Martín A.I., López-Calderón A. D-TRP(8)-γMSH prevents the effects of endotoxin in rat skeletal muscle cells through TNFα/NF-KB signalling pathway. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam C.W., Getting S.J., Perretti M. In vitro and in vivo induction of heme oxygenase 1 in mouse macrophages following melanocortin receptor activation. J Immunol. 2005;174(4):2297–2304. doi: 10.4049/jimmunol.174.4.2297. [DOI] [PubMed] [Google Scholar]
- 92.Lam C.W., Perretti M., Getting S.J. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides. 2006:404–412. doi: 10.1016/j.peptides.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 93.Lee D.J., Preble J., Lee S., Stephen Foster C., Taylor A.W. MC5r and A2Ar deficiencies during experimental autoimmune uveitis identifies distinct T cell polarization programs and a biphasic regulatory response. Sci Rep. 2016 doi: 10.1038/srep37790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang Q.H., Hruby V.J., Tatro J.B. Systemic alpha-MSH suppresses LPS fever via central melanocortin receptors independently of its suppression of corticosterone and IL-6 release. Am J Physiol. 1998;275(2):R524–R530. doi: 10.1152/ajpregu.1998.275.2.R524. [DOI] [PubMed] [Google Scholar]
- 95.Lipton J.M., Ceriani G., Macaluso A., McCoy D., Carnes K., Biltz J., et al. Antiinflammatory effects of the neuropeptide alpha-MSH in acute, chronic, and systemic inflammation. Ann N Y Acad Sci. 1994;741:137–148. doi: 10.1111/j.1749-6632.1994.tb39654.x. [DOI] [PubMed] [Google Scholar]
- 96.Caruso C., Mohn C., Karara A.L., Rettori V., Watanobe H., Schiöth H.B., et al. Alpha-melanocyte-stimulating hormone through melanocortin-4 receptor inhibits nitric oxide synthase and cyclooxygenase expression in the hypothalamus of male rats. Neuroendocrinology. 2004;79(5):278–286. doi: 10.1159/000079321. [DOI] [PubMed] [Google Scholar]
- 97.Caruso C., Durand D., Schiöth H.B., Rey R., Seilicovich A., Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. 2007;148(10):4918–4926. doi: 10.1210/en.2007-0366. [DOI] [PubMed] [Google Scholar]
- 98.Colombo G., Gatti S., Sordi A., Turcatti F., Carlin A., Rossi C., et al. Production and effects of alpha-melanocyte-stimulating hormone during acute lung injury. Shock. 2007;27(3):326–333. doi: 10.1097/01.shk.0000239764.80033.7e. [DOI] [PubMed] [Google Scholar]
- 99.Chiao H., Foster S., Thomas R., Lipton J., Star R.A. Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Investig. 1996;97(9):2038–2044. doi: 10.1172/JCI118639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen S., Zhao L., Sherchan P., Ding Y., Yu J., Nowrangi D., et al. Activation of melanocortin receptor 4 with RO27-3225 attenuates neuroinflammation through AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2018 doi: 10.1186/s12974-018-1140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carniglia L., Ramírez D., Durand D., Saba J., Caruso C., Lasaga M. [Nle4, D-phe7]-α-MSH inhibits toll-like receptor (TLR)2- and TLR4-induced microglial activation and promotes a M2-like phenotype. PLoS One. 2016 doi: 10.1371/journal.pone.0158564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamermans A., Verhoeven T., van het Hof B., Koning J.J., Borghuis L., Witte M., et al. Setmelanotide, a novel, selective melanocortin receptor-4 agonist exerts anti-inflammatory actions in astrocytes and promotes an anti-inflammatory macrophage phenotype. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sergeyev V., Broberger C., Hökfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Research. Mol Brain Res. 2001;90(2):93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 104.Ericsson A., Liu C., Hart R.P., Sawchenko P.E. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361(4):681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 105.Reyes T.M., Sawchenko P.E. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J Neurosci: Off J Soc Neurosci. 2002;22(12):5091–5099. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howard M., Muchamuel T., Andrade S., Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.da Silva A.A., do Carmo J.M., Wang Z., Hall J.E. Melanocortin-4 receptors and sympathetic nervous system activation in hypertension. Curr Hypertens Rep. 2019;21(6):46. doi: 10.1007/s11906-019-0951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brandfon S., Eylon A., Khanna D., Parmar M.S. Advances in anti-obesity pharmacotherapy: current treatments, emerging therapies, and challenges. Cureus. 2023;15(10) doi: 10.7759/cureus.46623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lonati C., Gatti S., Catania A. Activation of melanocortin receptors as a potential strategy to reduce local and systemic reactions induced by respiratory viruses. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.569241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.