Abstract
The decomposition of benzenesulphohydroxamic acid (Piloty's acid; PA) and some of its derivatives has been reported to yield nitroxyl ions (NO-), a species with potent vasodilator properties. In a previous study we demonstrated that the oxidative breakdown of PA results in the formation of nitric oxide (NO) and suggested that NO rather than NO- may account for its vasorelaxant properties. Using isolated aortic rings in organ baths, we now show that high concentrations of cysteine potentiate the vasorelaxant response to PA, whereas responses to Angeli's salt (AS), a known generator of NO-, were almost completely inhibited. These different behaviours of PA and AS are mirrored by their distinct chemistries. By using HPLC it was shown that, at physiological pH and in the absence of oxidizing conditions, PA is a relatively stable compound. Direct chemical determination of NO, stimulation of soluble guanylyl cyclase, and measurement of platelet aggregation under various experimental conditions confirmed the requirement for oxidation to release NO from PA, and quite weak oxidants were found to be sufficient to promote this reaction. In contrast, at pH 7.4 AS decomposed rapidly to yield nitrite (NO2-) and NO-, bu did not produce NO on reaction with dioxygen (O2) or hydrogen peroxide (H2O2). Thus sulphohydroxamic acids are a new class of thiol-independent NO-donors that generate NO rather than NO- under physiological conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
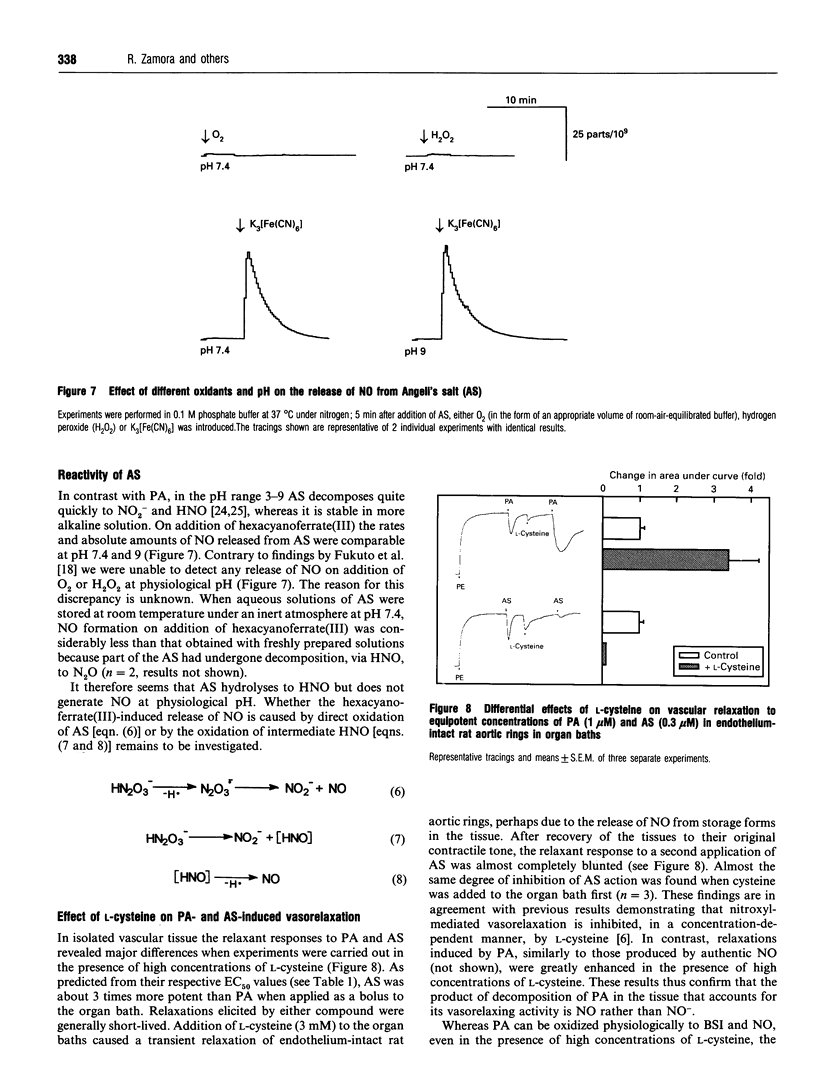
- Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993 Jan 26;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Feelisch M. Biotransformation to nitric oxide of organic nitrates in comparison to other nitrovasodilators. Eur Heart J. 1993 Nov;14 (Suppl 1):123–132. [PubMed] [Google Scholar]
- Fukuto J. M., Chiang K., Hszieh R., Wong P., Chaudhuri G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1992 Nov;263(2):546–551. [PubMed] [Google Scholar]
- Fukuto J. M., Hobbs A. J., Ignarro L. J. Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems: the role of physiological oxidants and relevance to the biological activity of HNO. Biochem Biophys Res Commun. 1993 Oct 29;196(2):707–713. doi: 10.1006/bbrc.1993.2307. [DOI] [PubMed] [Google Scholar]
- Fukuto J. M., Hszieh R., Gulati P., Chiang K. T., Nagasawa H. T. N,O-diacylated-N-hydroxyarylsulfonamides: nitroxyl precursors with potent smooth muscle relaxant properties. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1367–1373. doi: 10.1016/0006-291x(92)90453-r. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J., Baricos W. H. Evidence that regulation of hepatic guanylate cyclase activity involves interactions between catalytic site -SH groups and both substrate and activator. Arch Biochem Biophys. 1981 Apr 15;208(1):75–86. doi: 10.1016/0003-9861(81)90125-9. [DOI] [PubMed] [Google Scholar]
- Kimura H., Mittal C. K., Murad F. Activation of guanylate cyclase from rat liver and other tissues by sodium azide. J Biol Chem. 1975 Oct 25;250(20):8016–8022. [PubMed] [Google Scholar]
- Pino R. Z., Feelisch M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO-) using L-cysteine. Biochem Biophys Res Commun. 1994 May 30;201(1):54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]