Version Changes
Revised. Amendments from Version 1
Changes to the text To improve clarity of study design, we have named the discovery GWAS in UK Biobank "Stage 1" and the follow-up of 40 sentinel variants in the 11 independent cohorts "Stage 2" throughout the text. We refer to the meta-analysis of all these data as the "meta-analysis of Stages 1 and 2". We have added some text to clarify the definition of controls. We have added a SNP heritability estimate and a polygenic score analysis to assess evidence of polygenic structure in our hospitalised respiratory infection phenotype. We have added text to the Discussion to encourage caution when interpreting our findings given the lack of statistical replication in the meta-analysis of Stages 1 and 2. We have added text to the Discussion to highlight the limitations of the gene expression data utilised in our study. Further edits to the text were made to resolve typographical errors in the original submission. New or revised/updated figures We have added a new figure (Figure 1) that summarises our overall study design. Changes to the author list Dr Jing Chen has been added as a co-author for her guidance with conducting the polygenic score analysis. Professor Martin Tobin has been replaced by Dr Alexander Williams as the corresponding author.
Abstract
Background: Globally, respiratory infections contribute to significant morbidity and mortality. However, genetic determinants of respiratory infections are understudied and remain poorly understood.
Methods: We conducted a genome-wide association study in 19,459 hospitalised respiratory infection cases and 101,438 controls from UK Biobank (Stage 1). We followed-up well-imputed top signals from our Stage 1 analysis in 50,912 respiratory infection cases and 150,442 controls from 11 cohorts (Stage 2). We aggregated effect estimates across studies using inverse variance-weighted meta-analyses. Additionally, we investigated the function of the top signals in order to gain understanding of the underlying biological mechanisms.
Results: From our Stage 1 analysis, we report 56 signals at P<5×10 -6, one of which was genome-wide significant ( P<5×10 -8). The genome-wide significant signal was in an intron of PBX3, a gene that encodes pre-B-cell leukaemia transcription factor 3, a homeodomain-containing transcription factor. Further, the genome-wide significant signal was found to colocalise with gene-specific expression quantitative trait loci (eQTLs) affecting expression of PBX3 in lung tissue, where the respiratory infection risk alleles were associated with decreased PBX3 expression in lung tissue, highlighting a possible biological mechanism. Of the 56 signals, 40 were well-imputed in UK Biobank and were investigated in Stage 2. None of the 40 signals replicated, with effect estimates attenuated.
Conclusions: Our Stage 1 analysis implicated PBX3 as a candidate causal gene and suggests a possible role of transcription factor binding activity in respiratory infection susceptibility. However, the PBX3 signal, and the other well-imputed signals, did not replicate in the meta-analysis of Stages 1 and 2. Significant phenotypic heterogeneity and differences in study ascertainment may have contributed to this lack of statistical replication. Overall, our study highlighted putative associations and possible biological mechanisms that may provide insight into respiratory infection susceptibility.
Keywords: Respiratory infections, GWAS, UK Biobank, electronic medical records
Introduction
Respiratory infections are a group of diseases characterised by infection and inflammation of the respiratory system. Respiratory infections can be grouped according to their symptomatology, anatomic involvement and causative pathogen 1 . Upper respiratory tract infections are typically benign, self-limiting diseases, and include the common cold, pharyngitis and otitis media. However, upper respiratory tract infections can be particularly burdensome for infants and young children 2 . Lower respiratory tract infections, on the other hand, are often life-threatening diseases that require medical intervention. In 2016, over two million deaths worldwide were caused by lower respiratory tract infections, making this group of infectious diseases the sixth leading cause of death in individuals of all ages and the leading cause of death in very young children 3, 4 . Environmental exposures, such as indoor air pollution and inhalation of tobacco smoke, are important risk factors for upper and lower respiratory tract infections 4 . Genetic factors may also contribute to host susceptibility to infection. Indeed, twin studies have demonstrated a genetic component in susceptibility to otitis media 5, 6 , recurrent tonsillitis 7 and respiratory syncytial virus-related bronchiolitis 8 with heritability estimates as high as 73% 5 . Identifying associations with genes and pathways that influence host susceptibility to infection may reveal novel therapeutic targets and opportunities for drug development.
Further to the environmental and genetic risk factors described above, primary immunodeficiencies (PIDs) are a group of disorders that affect normal immune function, often leading to increased susceptibility to infections 9 . Activated phosphoinositide-3-kinase δ syndrome (APDS) is one such PID that is caused by gain-of-function mutations in genes encoding phosphoinositide-3-kinase δ (PI3Kδ) 9 . In previous studies of APDS 10, 11 , up to 96% of individuals with APDS presented with an upper respiratory tract infection, such as otitis media, and/or a lower respiratory tract infection, such as pneumonia, seemingly distinct respiratory infection diseases. These findings may motivate the need to study a broad respiratory infection phenotype—one that comprises many different kinds of respiratory infection diseases—due to the possibility of shared aetiology between distinct conditions as previously observed in the context of APDS.
In this study, we conducted a genome-wide association study (GWAS) of hospitalised respiratory infections in UK Biobank (Stage 1), utilising the Hospital Episode Statistics (HES) data. We report genetic variants that were putatively associated with hospitalised respiratory infections, of which a subset of well-imputed genetic variants was followed up in 11 independent cohorts (Stage 2). We performed an inverse variance-weighted fixed effects meta-analysis of Stages 1 and 2. Finally, we applied a range of statistical approaches in order to achieve greater insight into the biological mechanisms underlying the putative statistical associations.
Methods
Defining the 14 hospitalised respiratory infection phenotype
The hospitalised respiratory infection (HRI) phenotype was a composition of International Classification of Diseases, 10 th Revision (ICD-10) codes. We initially extracted all ICD-10 codes under Chapter 10: diseases of the respiratory system. Then, by manually exploring the online browser, we extracted further relevant ICD-10 codes that appear under other chapter headings that would have otherwise been missed. Following careful consideration, we restricted the ICD-10 codes to those most likely to be indicative of a respiratory infection (Table S1, Extended data 12 ). An ICD-10 code was deemed relevant by screening its text description, retaining those relating to clinical diagnoses and the detection of common respiratory pathogens.
Stage 1 analysis in UK Biobank
Cases were defined by the presence of one or more of the relevant HRI ICD-10 codes (Table S1, Extended data 12 ) in the linked Hospital Episode Statistics (HES) data over a 20-year period—from the inception of ICD-10 coding in the UK to the end of the period covered by the version of the HES data we analysed. These data reflect all diagnoses recorded while an individual was a patient in hospital, not just the primary discharge diagnosis, and does not include outpatient hospital diagnoses. We restricted the cases to those with (1) genome-wide imputed genetic data; (2) complete information for age (at recruitment), sex and smoking status (at recruitment); (3) no 2 nd degree or closer relative (defined by a kinship estimate >0.0884 from the KING software, provided by UK Biobank) in cases only, and (4) were of European ancestry based on k-means clustering of the first two principal components of ancestry. Among the UK Biobank participants who were not defined as cases, i.e. individuals who had no respiratory infection codes in the secondary care data, we, separately, applied the same quality control measures as described above. Then, controls were randomly selected—to ensure computational feasibility, only a subset of controls was analysed—without replacement from the remaining individuals, using the sample function in R v3.6.1, at a ratio of five controls to every case, such that the distributions of age, sex and smoking status were broadly similar to those of the cases. Following selection of controls, the relatedness was checked between cases and controls. In 2 nd degree or closer related pairings, controls were preferentially excluded in order to maximise the number of cases in the analysis.
Genotyping was undertaken using the Affymetrix Axiom UK BiLEVE 13 and UK Biobank 14 arrays. Genotype imputation was conducted using the Haplotype Reference Consortium panel and the merged 1000 Genomes phase 3 and UK10K panels 14 . Imputed genotypes with a minor allele count >20 (in all UK Biobank participants with genome-wide imputed genetic data) and an imputation quality score >0.5 were tested for association with the HRI phenotype.
PLINK 2.0 15 was used to perform the genome-wide association study. We assessed autosomal variant associations under an additive genetic model adjusted for age (at recruitment), age 2 , genotyping array, sex, smoking status and the first 10 principal components of ancestry. We analysed variant dosages in order to account for genotype uncertainty.
LD score regression 16 was used to quantify genome-wide inflation in the test statistics due to possible confounding of the genotype-phenotype associations, for example, by population stratification.
Initial signal selection and conditional analyses
We initially defined primary signals of association according to the following criteria: minor allele frequency >0.1% (in cases and controls combined), Hardy-Weinberg exact test P >1×10 -6 (in cases and controls combined), and an association P <5×10 -6.
All genetic variants ±1Mb from the sentinel variant in each association signal were extracted. A conditional analysis was used to identify further, conditionally independent association signals within the 2Mb regions, using GCTA 17, 18 . Conditionally independent signals were defined according to the same criteria as for the primary signals.
Together, the two steps outlined above describe the set of signals to be taken forward for follow-up in the 11 independent cohorts (Stage 2, described below).
Effect of smoking behaviour
The Stage 1 analysis was adjusted for ever-smoking status. However, this may not have fully adjusted for the effect of smoking behaviour. Therefore, we assessed whether any of the association signals for HRIs were driven by smoking behaviour by testing the association between the sentinel variants from the HRI GWAS and smoking initiation (189,159 ever smokers versus 224,349 never smokers), smoking cessation (150,906 current smokers versus 45,075 ex-smokers), the number of cigarettes smoked per day (categorised, 136,391 total individuals), and heaviness of smoking index, a measure of nicotine dependence, (categorised, 31,766 total individuals). We also assessed the association with HRIs in never smokers only (8123 cases and 42,361 controls). These smoking behaviour phenotypes are discussed in more detail in the Supplementary Material ( Extended data 12 ). We used a P-value corrected for the number of sentinel variants tested to define a significant association with a smoking behaviour phenotype.
Stage 2 cohorts
The following cohorts were included in the Stage 2 analysis: The Institute for Personalized Medicine BioMe Biobank (BioMe), Cardiovascular Health Study (CHS) 19 , Electronic Medical Records and Genomics Network (eMERGE) 20, 21 , Estonian Biobank 22 , Generation Scotland: Scottish Family Health Study (GS:SFHS) 23 , Northern Finland 1966 Birth Cohort (NFBC1966) 24 , Orkney Complex Disease Study (ORCADES), Partners Biobank, Penn Medicine Biobank, Trøndelag Health Study (HUNT) 25 and Viking Health Study Shetland (VIKING). A brief summary of each of the cohorts included in the Stage 2 analysis is given in the Supplementary Material ( Extended data 12 ).
The Cardiovascular Health Study and Partners Biobank cohorts defined the HRI phenotype using ICD-9 codes. For this, we mapped the HRI ICD-10 codes to their ICD-9 counterparts, where possible (Table S2, Extended data 12 ).
Meta-analysis of Stages 1 and 2
Of the sentinel variants in each association signal achieving P<5×10 -6 in Stage 1, a subset was followed up in the 11 independent cohorts described above according to the following criteria: all sentinel variants with a minor allele frequency >1%, and any sentinel variant with a minor allele frequency between 0.1% and 1% that additionally had an imputation quality score >0.8. This latter criterion was used to ensure greater confidence in the genotype imputation in lower-frequency sentinel variants and, hence, in the statistical associations.
Where necessary, proxy variants, with a minimum R 2 of 0.6, were substituted based on UK Biobank LD. We used the LDpair tool in the LDlink 26 suite of online applications to match the effect allele of proxy variants to that of the corresponding sentinel variant.
We conducted an inverse variance-weighted (IVW) fixed effects meta-analysis of association results from the Stage 2 cohorts and, separately, combined with the Stage 1 analysis using the meta package in R v3.6.1. We used P<5×10 -8 in the overall meta-analysis (Stages 1 and 2) and a Bonferroni-corrected P-value threshold in the Stage 2 meta-analysis, corrected for the number of variants followed up, to define a replicated signal. An overview of the study design is shown in Figure 1.
Figure 1. Overview of study design.
Identifying putative causal genes
Fine-mapping. In order to restrict the variants in each association signal defined in the Stage 1 analysis to those most likely to be causal, we performed fine-mapping using a Bayesian method 27 . This approach derives approximate Bayes’ factors from GWAS summary statistics, from which the posterior probability of a variant being the true causal variant (under the assumption that the true causal variant was analysed) can be calculated. The variants at each association signal can be sorted by the posterior probability and combined to create a set of variants that is 95% probable to contain the true causal variant, i.e. 95% credible set. Posterior probabilities were calculated for all variants ±1Mb from the sentinel variant in each association signal that had R 2>0.1 with the sentinel variant, using W=0.04 as the prior parameter, representing 95% belief that the relative risk corresponding to departure from the null model lies between 2/3 and 3/2 27, 28 . Association signals in the HLA region were not included in the fine-mapping.
Functional annotation. To identify putative causal genes, we used the Ensembl GRCh37 Variant Effect Predictor (VEP) 29 to annotate all variants in the 95% credible sets. We used the following criteria to annotate variants as deleterious (all criteria implemented in VEP): labelled “deleterious” by SIFT, labelled “probably damaging” or “possibly damaging” by PolyPhen, had a CADD scaled score ≥20, labelled “likely disease causing” by REVEL, labelled “damaging” by MetaLR or “high” by MutationAssessor. The union of the variants defined by each of these methods was taken to be the set of potentially deleterious variants.
Gene expression. We tested whether any variants in the 95% credible sets were associated with gene expression from three expression quantitative trait loci (eQTL) databases: 48 tissues from GTEx v7 30 , three major human immune cell types (CD14 + monocytes, CD16 + neutrophils, and naïve CD4 + T cells) from BLUEPRINT 31 , and cis- and trans-eQTLs in blood from eQTLGen 32 . A false discovery rate (FDR) of 5% was used to define a significant association with gene expression.
Colocalisation with expression quantitative trait loci (eQTLs)
Where a variant (or variants) in the 95% credible set was found to be associated with expression of a particular gene, we assessed whether there was a shared causal variant underlying the corresponding HRI GWAS association signal and expression of the implicated gene in the highlighted tissue or cell type. We performed colocalisation using the coloc 27 package in R v3.6.1 (with default prior probabilities) and all variants within 1Mb of the sentinel variant in the corresponding HRI GWAS signal for which P<0.01 in either the HRI GWAS or the eQTL analysis.
In addition, we also used PICCOLO, which performs colocalisation in the absence of full summary statistics 33 , for example if the association results for a sentinel variant only were available. In addition to eQTL data from the three eQTL databases described above, PICCOLO incorporates quantitative trait loci (QTL) data from additional sources, including protein quantitative trait loci (pQTL) data from four studies 34– 37 . These four studies collected pQTL data for blood plasma 34, 35 , sputum from chronic obstructive pulmonary disease (COPD) patients 36 , and serum from asthma patients 37 .
We used a posterior probability of >80% to identify colocalisation between the GWAS and eQTL traits for both methods described, i.e. >80% probability of a shared causal variant.
Pathway analysis
We tested for enrichment of genes harbouring association signals in pathways defined in the MetaBase 38 and Gene Ontology: Biological Processes 39, 40 (GOBP) databases using Pascal 41 . With Pascal, variants are mapped to genes by genomic position. To ensure computational feasibility, only GOBP pathways with >10 and <1000 genes were tested. A false discovery rate (FDR) <5% was used to define a significantly enriched pathway.
Assessment of sentinel variants in published GWAS
We assessed whether any of the sentinel variants in the association signals were associated with other traits and diseases from existing GWAS. The traits studied included, but were not limited to, UK Biobank baseline measures (from questionnaires and physical measures), curated health outcomes from primary and/or secondary care data, and self-reported diseases and medications. P<5×10 -8 was used to define a significant association between the sentinel variants and existing GWAS traits. Further, likely relevant, traits were also highlighted at P<5×10 -6.
In addition, we investigated the association between the sentinel variants and four COVID-19 phenotypes from the COVID-19 Host Genetics Initiative 42 meta-analyses (release 6) ranging from 8779 cases (very severe COVID-19) to 112,612 cases (any COVID-19) from up to 165 cohorts worldwide. A significant association between a sentinel variant and a COVID-19 phenotype was defined using P<5×10 -8.
Polygenic score (PGS)
In order to assess evidence of polygenic structure in our hospitalised respiratory infection phenotype, we applied PRS-CS-auto 43 to create a polygenic score (PGS) using the summary statistics from a GWAS of a randomly selected half of the original Stage 1 population as the training dataset. PRS-CS-auto applies a fully Bayesian approach that automatically learns the global scaling parameter from the training dataset, and no validation dataset is needed. We tested the association of this PGS with our hospitalised respiratory infection phenotype in the half of the original Stage 1 population that was not used to generate the PGS. This association was tested using a logistic regression model adjusted for age, age2, genotyping array, sex, smoking status and the first 10 principal components of ancestry. We report the effect estimate of the PGS as a measure of polygenic structure.
Ethics statement
UK Biobank: The human samples were sourced ethically, and their research use was in accord with the terms of the informed consents under an IRB/EC approved protocol (16/NW/0274).
Estonian Biobank: This study and the use of data acquired from biobank participants was approved by the Research Ethics Committee of the University of Tartu (Approval number 288/M-18).
Ethical approval for the GS:SFHS study was obtained from the Tayside Committee on Medical Research Ethics (on behalf of the National Health Service).
The HUNT study was approved by the Regional Committee for Medical and Health Research Ethics and written informed consent was given by all participants.
The research protocols of NFBC1966 have been approved by the Ethics Committee of the Northern Finland Ostrobothnia Hospital District and all participants have given their written informed consent.
No further ethics approvals were required for the analyses of these data.
Results
Defining the hospitalised respiratory infection phenotype
Our hospitalised respiratory infection phenotype was a composition of 114 ICD-10 codes (Table S1, Extended data 12 ). Due to the specificity of certain codes (for example, “pneumonia due to Klebsiella pneumoniae” versus the more generic “pneumonia, unspecified”), 59 (51.8%) of these 114 ICD-10 codes occurred in fewer than 10 individuals, and 28 (24.6%) codes did not occur at all. Furthermore, 95% of cases were captured by the 16 most frequently recorded codes – the most common code, "J22 unspecified acute lower respiratory infection", accounted for more than one third (37.8%) of all cases ( Figure 2).
Figure 2. Frequency of individual ICD-10 codes used to define the 19hospitalised respiratory infection phenotype.
Frequency (log 10 scale) of individual ICD-10 codes used to define the hospitalised respiratory infection phenotype. To improve visualisation, only codes that occurred in 10 or more individuals are shown. Individuals may contribute to the overall count of more than one ICD-10 code. A description of each ICD-10 code, as well as the ICD-10 code itself, is shown.
Stage 1 analysis in UK Biobank
Following quality control, 19,459 cases and 101,438 controls were included in the association testing of 52,488,101 genetic variants. The intercept of LD score regression 16 was found to be 1.013, hence we did not correct the GWAS results for inflation ( Methods). The SNP heritability for the Stage 1 analysis was 9.48% (95% CI: 5.80-13.16%, liability scale). We defined 56 signals showing association at P<5×10 -6 with hospitalised respiratory infections (HRIs), including one signal on chromosome 9 that was genome-wide significant ( P<5×10 -8; Table S3, Extended data 12 ) for which the sentinel variant, rs10564495 (risk allele: A, risk allele frequency: 65.0%, risk allele count (cases): 25,806, risk allele count (controls): 131,232) was located in an intron of PBX3, a gene that encodes pre-B-cell leukaemia transcription factor 3, a homeodomain-containing transcription factor. The conditional analysis 18 did not identify further conditionally independent signals in any of the 2Mb regions.
Effect of smoking behaviour
We assessed the association between the sentinel variants in the 56 signals and smoking behaviour traits ( Methods and Supplementary Material, Extended data 12 ). The rs10564495 variant was found to be significantly associated with smoking cessation ( P=1.53×10 -4; Table S3, Extended data 12 ). The A allele for this variant was associated with 3.1% (odds ratio (OR): 0.969; 95% CI: 0.954-0.985) lower odds of quitting smoking and 7.6% (OR: 1.076; 95% CI: 1.051-1.101) greater odds of HRIs. In a stratified analysis, the association between this variant and HRIs was stronger in never-smokers than in both ever-smokers and in the overall GWAS: 8.9% (OR: 1.089; 95% CI: 1.051-1.129) greater odds of HRIs in never-smokers versus 6.6% (OR: 1.066; 95% CI: 1.034-1.099) greater odds of HRIs in ever-smokers (effect size for overall GWAS as above). These latter findings may suggest that the effect of the rs10564495 variant was not mediated by smoking behaviour.
Meta-analysis of Stages 1 and 2
Across the 11 Stage 2 cohorts ( Methods), there were 50,912 additional cases and 150,442 additional controls, bringing the total number of cases to 70,371 and controls to 251,880, effectively more than tripling the number of cases included in the Stage 1 analysis ( Table 1).
Table 1. Summary demographics of the case-control populations in Stage 1 and each of the Stage 2 cohorts.
Demographics of the case-control populations in Stage 1 and in each of the Stage 2 cohorts. *The HUNT cohort provided average year of birth rather than average age. For age, the mean and standard deviation are reported in cases and controls separately. For sex and smoking status, the number and proportion of females and never-smokers are reported in cases and controls separately.
| Cohort | Sample size | Age, mean (SD) | Sex, n (%) – female | Smoking status,
n (%)
– never-smoker |
||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
|
UK Biobank
(discovery) |
19,459 | 101,438 | 59.1 (7.7) | 59.0 (7.7) | 9280 (47.7) | 48,312 (47.6) | 8123 (41.7) | 42,361 (41.8) |
| BioMe | 585 | 2182 | 60.0 (17.2) | 56.9 (17.4) | 335 (57.3) | 1058 (48.5) | 328 (56.1) | 1205 (55.2) |
| CHS | 1098 | 2169 | 72.5 (5.1) | 72.3 (5.5) | 647 (58.9) | 1342 (61.9) | 476 (43.4) | 1088 (50.2) |
| eMERGE | 26,353 | 33,252 | 67.3 (24.0) | 58.6 (24.8) | 11,644 (44.2) | 16,964 (51.0) | 18,265 (69.3) | 26,938 (81.0) |
| Estonian Biobank | 4420 | 19,937 | 59.2 (17.9) | 58.9 (17.6) | 2961 (67.0) | 13,230 (66.4) | 2446 (55.3) | 10,916 (54.8) |
| GS:SFHS | 1133 | 15,729 | 41.8 (17.3) | 47.4 (14.4) | 651 (57.5) | 9223 (58.6) | 535 (47.2) | 8271 (52.6) |
| HUNT * | 9887 | 58,243 | 1940 (16.8) | 1950 (17.7) | 4794 (48.5) | 31,203 (53.6) | 3106 (31.4) | 25,153 (43.2) |
| NFBC1966 | 1340 | 2899 | 31.1 (0.4) | 31.1 (0.4) | 534 (39.9) | 1795 (61.9) | 820 (61.2) | 1628 (56.2) |
| ORCADES | 141 | 1886 | 55.6 (19.5) | 53.6 (15.0) | 93 (66.0) | 1131 (60.0) | 86 (61.0) | 1159 (61.5) |
| Partners Biobank | 3342 | 4386 | 62.5 (15.5) | 59.0 (16.6) | 1959 (58.6) | 2387 (54.4) | 2023 (60.5) | 2797 (63.8) |
|
Penn Medicine
Biobank |
2488 | 7755 | 69.7 (13.6) | 70.5 (13.6) | 916 (36.8) | 2569 (33.1) | 953 (38.3) | 3398 (43.8) |
| VIKING | 125 | 2004 | 45.4 (16.8) | 50.1 (15.1) | 71 (56.8) | 1208 (60.3) | 72 (57.6) | 1100 (54.9) |
| Total | 70,371 | 251,880 | 33,885 (48.2) | 130,422 (51.8) | 37,233 (52.9) | 126,014 (50.0) | ||
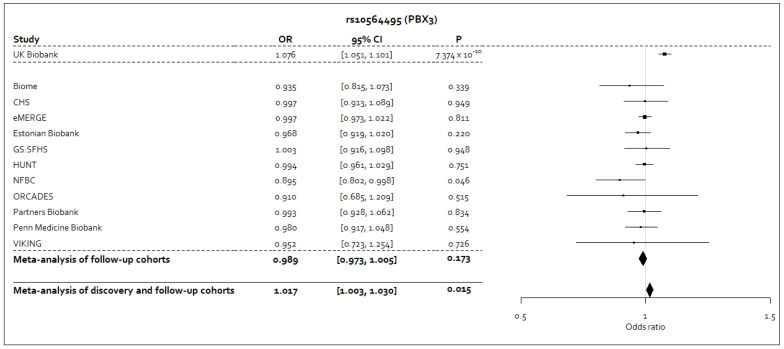
In Stage 2, we followed up a total of 40 variants. The availability of each variant across the 11 Stage 2 cohorts is shown in Table S4, Extended data 12 . In the meta-analysis of Stages 1 and 2, no variants achieved P<5×10 -8 (Table S5, Extended data 12 ). Furthermore, in the Stage 2 meta-analysis, no variants met a Bonferroni-corrected P-value threshold for 40 tests ( P<0.05/40=1.25×10 -3). The effect estimates in the Stage 2 cohorts for rs10564495-A, or its proxy rs10819083-T, were consistently in the opposite direction, or were close to the null value, to the effect estimate from the Stage 1 analysis ( Figure 3). In the meta-analysis of Stages 1 and 2 for the rs10564495 variant, we observed an I 2 statistic of 70.8% (95% CI: 45.9%-84.2%; P=0.0002), representing significant heterogeneity in the meta-analysis for this variant.
Figure 3. Forest plot for the sentinel variant in the genome-wide significant signal from the Stage 1 analysis following meta-analysis of Stages 1 and 2.
Forest plot for the sentinel variant, rs10564495, in the genome-wide significant signal identified in the Stage 1 following inverse variance-weighted fixed effects meta-analysis of results from Stages 1 and 2. The A allele for this variant was taken to be the coded allele. Where a proxy variant was used, which was consistently the rs10819083 variant, the T allele was taken to be the allele that corresponds to the A allele of the rs10564495 variant, as reported by the LDpair tool in the LDlink 26 suite of online applications.
Identifying putative causal genes
Fine-mapping. There were 107 variants in the 95% credible set at the genome-wide significant locus from the Stage 1 analysis. The sentinel variant, rs10564495, at this locus was assigned 16.2% probability of being causal, the highest probability in the corresponding 95% credible set (Table S6, Extended data 12 ).
Functional annotation. According to the criteria defined in Methods, there were six variants in five unique genes across four signals that were annotated as deleterious (Table S7, Extended data 12 ): DNAH6 (rs72832548 and rs72836490), ZNF608 (rs10040793), PBX3 (rs7849076 and rs1411352), RNU6-457P (rs2172310) and RBFOX1 (rs2172310). The two missense variants in DNAH6 (rs72832548 and rs72836490) were low frequency (minor allele frequencies of 0.55% and 0.56%, respectively) and result in amino acid changes from serine to glycine and alanine to threonine, respectively. The consequence(s) of these base changes has not been reported. DNAH6 encodes a protein that is involved in regulating motile ciliary beating 44, 45 and has been implicated in primary ciliary dyskinesia 46 , a disorder characterised by chronic respiratory tract infections. PBX3 houses the genome-wide significant signal from the Stage 1 analysis. However, the two variants in PBX3 annotated as deleterious were non-coding (Table S7, Extended data 12 ).
Gene expression and colocalisation with expression quantitative trait loci (eQTLs)
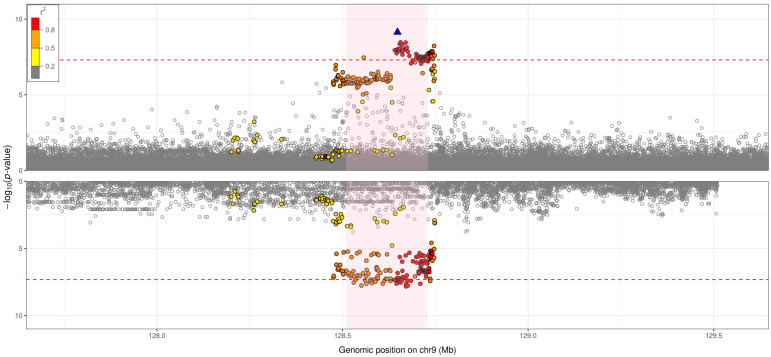
Using GTEx v7 30 data, the genome-wide significant signal from the Stage 1 analysis was found to colocalise (PP>80%) with PBX3-specific eQTLs in heart atrial appendage tissue, tibial artery tissue, not-sun-exposed suprapubic skin tissue, stomach tissue, lung tissue, aortic artery tissue, and sigmoid colon tissue ( Figure 4 and Supplementary Figures, Extended data 12 ). The HRI risk alleles were consistently associated with decreased PBX3-specific gene expression in all of the aforementioned tissues (Table S8, Extended data 12 ). We also found colocalisation between the genome-wide significant signal and expression of the proximal GOLGA1 gene in sun-exposed lower leg skin tissue (PP=81%). We did not identify additional colocalisation using BLUEPRINT 31 or eQTLGen 32 data.
Figure 4. Hospitalised respiratory infection GWAS versus eQTL for PBX3 in lung tissue (GTEx v7): probability of colocalisation = 87%.
Each point corresponds to a genetic variant, with genomic position (GRCh37) on the x-axis and –log 10( p-value) on the y-axis. The top plot shows regional association results for the genome-wide significant signal (sentinel variant: rs10564495) from the hospitalised respiratory infection GWAS. The bottom plot shows regional association results for the genome-wide significant signal from the eQTL analysis. The plotting window extends 1Mb either side of the sentinel variant in the region. The sentinel variant is represented by a blue triangle, with all other genetic variants in the region coloured according to the extent of pairwise linkage disequilibrium with the sentinel variant: red points reflect genetic variants that have r 2 >0.8 with the sentinel variant, orange points reflect genetic variants that have 0.5< r 2 ≤0.8 with the sentinel variant, yellow points reflect genetic variants that have 0.2< r 2 ≤0.5 with the sentinel variant, and grey points reflect genetic variants that have r 2 ≤0.2 with the sentinel variant. The area shaded in light pink represents the gene implicated by the eQTL analysis. The red dashed line represents a p-value threshold of 5×10 -8.
Using PICCOLO 33 , the genome-wide significant signal from the Stage 1 analysis was found to additionally colocalise (PP>80%) with PBX3-specific eQTLs in CD4/8 + naïve T cells, coronary artery tissue and whole blood (Table S11, Extended data 12 ). PICCOLO did not highlight colocalisation between eQTLs and any proximal genes to PBX3. At the time of analysis, PICCOLO did not provide effect estimates for the eQTL traits. Therefore, we queried the Open Targets Genetics 47 portal in order to assess directionality for these additional eQTL traits. The HRI risk alleles were associated with decreased PBX3-specific expression in coronary artery tissue. Summary statistics for the T cell and whole blood traits were not available, however.
For the remaining signals, the chromosome 5 signal (sentinel variant: rs7730012) was found to colocalise with ZNF608-specific eQTLs in tibial artery tissue (GTEx v7 30 ) using the coloc 27 method (Supplementary Figure 1, Extended data 12 ). Additional results from PICCOLO 33 can be seen in Table S11 ( Extended data 12 ).
Pathway analysis
We tested for significant enrichment of genes from the HRI GWAS in known pathways: 1383 pathways from the MetaBase 38 resource and 6405 pathways from the Gene Ontology: Biological Processes 39, 40 resource ( Methods). We did not identify any significantly enriched pathways at a false discovery rate of 5%.
Assessment of sentinel variants in published GWAS
The A allele of the rs10564495 variant was associated with increased overall health rating, increased odds of requiring the use of dentures and decreased standing height at P <5×10 -8 (Table S12, Extended data 12 ), and decreased lung function and increased odds of various respiratory disease phenotypes, including respiratory infections, at P <5×10 -6 (Table S13, Extended data 12 ). Significant associations for the other sentinel variants are presented in Tables S12&S13 and include various respiratory infection phenotypes such as acute pharyngitis and pneumonia.
Finally, there were no significant associations found between any of the sentinel variants and the four COVID-19 phenotypes from the COVID-19 Host Genetics Initiative 42 (Table S14, Extended data 12 ).
Polygenic score (PGS)
There were 9730 cases and 50,719 controls randomly selected for the GWAS from which the PGS was constructed. When we tested the association between the PGS and our hospitalised respiratory infection phenotype in the other half of the Stage 1 population (9729 hospitalised respiratory infection cases and 50,719 controls), we found an 8.1% (95% CI: 5.8%-10.5%; P=2.50×10 -12) increase in disease odds per standard deviation unit increase in the PGS.
Discussion
We conducted one of the largest GWAS of respiratory infections to date, combining data from UK Biobank and 11 international cohorts.
In our Stage 1 analysis, the strongest association signal was in an intron of the PBX3 gene, which encodes the pre-B-cell transcription factor 3 protein. PBX3 contributes to DNA-binding transcription factor activity and sequence-specific DNA binding. The hospitalised respiratory infection risk alleles at this locus were associated with decreased expression of PBX3 in lung tissue (Table S8, Extended data 12 ). In a recent preprint, PBX3 was found to be associated with pneumonia in almost 25,000 cases from UK Biobank and FinnGen 48 . The authors also found that genetic variants in PBX3 were associated with PBX3 expression in lung tissue (effect direction not reported). In a study of a range of infectious diseases using 23andMe data, including some respiratory infections such as pneumonia and childhood ear infections, neither PBX3, nor any neighbouring genes, were found to be associated with the diseases studied 49 . However, it should be noted that the respiratory infection phenotypes in the 23andMe study were defined from self-reported questionnaire data which may have been subject to recall bias, particularly for diseases that occurred during childhood.
Evidence that PBX3 is a functionally significant transcription factor in a range of cancers, in addition to its expression being linked to more aggressive disease and shorter overall survival, has been reported 50 . Cancer patients are more susceptible to infections for a number of reasons. One such reason may be due to the receipt of immunosuppressants compromising the individual’s immune system, resulting in greater risk of opportunistic infection, as has been observed in lung cancer patients 51 .
We followed up 40 signals from our Stage 1 analysis in 11 independent cohorts (Stage 2). None of the 40 signals surpassed P<5×10 -8 in the meta-analysis of Stages 1 and 2, highlighting the importance of statistical replication and the potential influence of winner’s curse bias in our Stage 1 analysis. However, it is possible that there was significant phenotypic heterogeneity, reflected in a significant I 2 statistic for many of the 40 signals (Table S5, Extended data 12 ), between cohorts owing to differences in exposure to circulating pathogens, health care systems and coding practices which may have influenced the representation of particular infections in the medical record data. For example, the respiratory infection phenotype in UK Biobank was defined using ICD-10 codes (Table S1, Extended data 12 ). In two of the Stage 2 cohorts, the corresponding phenotype was defined entirely or partly by ICD-9 codes (Table S2, Extended data 12 ). As there is no exact mapping between ICD-9 and ICD-10 codes, the resulting phenotypes may differ and give rise to greater heterogeneity between cohorts. In addition, controls were not selected for similar distributions of age, sex and smoking status in the Stage 2 cohorts which, in some cases, led to large differences in the distributions of the aforementioned factors between cases and controls for some cohorts ( Table 1).
In addition to considerations around phenotypic differences, it is important to consider that the association test statistics for specific variants may be subject to ascertainment biases given that not all variants were available to study in all cohorts and participant ascertainment strategies – such as hospital-based versus population-based recruitment - varied between studies.
We applied a range of statistical techniques to further understand the biological mechanisms underlying the statistical associations identified in Stage 1. Following fine-mapping 28 , the sentinel variant, rs10564495, in PBX3 was attributed 16.2% probability of being causal for the GWAS trait among a set of 107 genetic variants that was attributed 95% probability of containing the true causal variant. These 107 genetic variants were located in, or slightly upstream of, PBX3. We found two variants in PBX3 that were annotated as deleterious. However, these variants were non-coding/regulatory region variants (Table S7, Extended data 12 ), which may suggest that these variants are involved in gene expression. Further work is needed to understand the role of these particular variants in influencing susceptibility to hospitalised respiratory infections. In a colocalisation analysis, the PBX3 signal was found to colocalise with PBX3-specific eQTLs in a range of tissues and cell types including, but not limited to, lung tissue, CD4/8 + T cells and whole blood. At the PBX3 locus, the alleles that were associated with increased risk of hospitalised respiratory infections were also associated with decreased expression of PBX3 in the tissues and cell types highlighted and may implicate PBX3 as a candidate causal gene. Furthermore, we found that the PBX3 sentinel variant was associated with overall health rating, denture use and standing height at P<5×10 -8, and a broader respiratory system disease phenotype and FEV 1 at P<5×10 -6 when looking across a large number of published GWAS. These associations, particularly those with FEV 1 and standing height, may implicate lung function as a driver of the PBX3 association signal. In the absence of independent replication, however, any interpretation of functional evidence relating to the PBX3 signal, or the remaining signals, must be viewed with caution. We presented findings for additional signals from our Stage 1 analysis. However, since these signals were not genome-wide significant in Stage 1, or in the meta-analysis of Stages 1 and 2, our interpretation of the functional evidence relating to these signals should be viewed with caution.
As with all research based on medical records, misclassification of diagnoses may have occurred, and we did not have the benefit of microbiological or virological data to confirm the infective agent. Nevertheless, the use of medical records enabled us to study much larger sample sizes than have been attained in studies that do not use such data—historically, GWAS that define cases of respiratory infection by other means included fewer than 1000 cases 52– 57 . We combined multiple respiratory infection codes to define our overall phenotype, motivated by previous findings in the context of APDS, which resulted in a larger sample size but likely increased heterogeneity. Controls were individuals with no evidence of having had a respiratory infection in hospital, but we did not consider other data sources, such as primary care data, where there may be records of respiratory infection among the controls, reflecting misclassification and a possible loss of statistical power. Gene expression data generated from healthy tissues and cells, as is the case for the three eQTL datasets we used, may not accurately represent the biological landscape during disease. Furthermore, the gene expression data provides insight into gene expression at the tissue level, but some effects may be mediated via specific cell types within a tissue that may have been missed in our analysis. Finally, we restricted our analysis to unrelated individuals of European ancestry in order to limit the potential impact of population stratification and cryptic relatedness. However, this may limit the generalisability of the results we report.
Using genome-wide SNPs, we observed a highly-significant SNP heritability, with a point estimate of 9.48% (liability scale), which is higher than estimates provided in previous large GWAS of respiratory infections 49, 50 . Given this SNP heritability, the lack of signals reaching genome-wide significance in the overall (Stage 1+2) meta-analysis is consistent with a polygenic trait – that is, many variants of individually small effect. In our polygenic score analysis, we observed an 8.1% (95% CI: 5.8%-10.5%) increase in hospitalised respiratory infection odds per standard deviation unit increase in the polygenic score, which is also consistent with polygenic architecture of hospitalised respiratory infections. Despite this being the largest such study undertaken to date, these findings would suggest that discovery of additional genetic associations with hospitalised respiratory infections would require even larger sample sizes, and ideally discovery and follow-up populations subject to relatively homogeneous approaches to coding respiratory infections.
To conclude, genetic variants in PBX3 were found to be associated with hospitalised respiratory infection susceptibility in UK Biobank, which may implicate transcription factor binding activity in susceptibility to a general respiratory infection phenotype. However, this finding did not replicate in independent cohorts. Future genome-wide association studies of hospitalised respiratory infection susceptibility would benefit from larger sample sizes and reduced phenotypic heterogeneity that may arise when utilising linked electronic healthcare records across different healthcare systems.
Acknowledgements
Generation Scotland is grateful to all the families who took part, the general practitioners and the Scottish School of Primary Care for their help in recruiting them, and the whole Generation Scotland team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, healthcare assistants and nurses.
The Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. The genotype quality control and imputation in HUNT has been conducted by the K.G. Jebsen Center for Genetic Epidemiology, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology.
We gratefully acknowledge the contributions of all cohort members and researchers who participated in the 46 years study. We would also like to acknowledge the work of the NFBC project center.
DNA extractions for ORCADES were performed at the Edinburgh Clinical Research Facility, University of Edinburgh. We would like to acknowledge the invaluable contributions of the research nurses in Orkney, the administrative team in Edinburgh and the people of Orkney. DNA extractions and genotyping for VIKING were performed at the Edinburgh Clinical Research Facility, University of Edinburgh. We would like to acknowledge the invaluable contributions of the research nurses in Shetland, the administrative team in Edinburgh and the people of Shetland. The authors acknowledge the support of the eDRIS Team (Public Health Scotland) for their involvement in obtaining approvals, provisioning and linking Electronic Health Record data for the ORCADES and VIKING cohorts.
The authors would like to thank Giovanni M Dall’Olio, Jorge Esparza Gordillo, Cong Guo, Aidan MacNamara, David Mayhew, Nikolina Nakic and Karsten B Sieber of GSK for their advice, guidance and support in running the downstream GWAS analyses. We would also like to acknowledge the work of all those contributing to the eMERGE Network which contributed valuable data to our analysis.
This research used the ALICE and SPECTRE High Performance Computing Facilities at the University of Leicester. Analysis by Estonian Biobank was carried out in the High Performance Computing Center of the University of Tartu, Estonia.
Funding Statement
This research was partially supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre; the views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health. ATW was supported by a BBSRC industrial CASE studentship between the University of Leicester and GlaxoSmithKline. AY, DM, IPH, JB, LVW and MDT lead a research collaboration between the Universities of Leicester and Nottingham, and GlaxoSmithKline. IPH has been partially supported by the NIHR Nottingham Biomedical Research Centre. LVW holds a GSK/British Lung Foundation Chair in Respiratory Research. MDT was supported by a Wellcome Trust Investigator Award [202849, <a href=https://doi.org/10.35802/202849>https://doi.org/10.35802/202849</a>] and an NIHR Senior Investigator Award (NIHR201371). LVW and MDT have been supported by the Medical Research Council (MRC) (MR/N011317/1). CJ holds a Medical Research Council Clinical Research Training Fellowship (MR/P00167X/1). This work was supported by BREATHE - The Health Data Research Hub for Respiratory Health [MC_PC_19004] in partnership with the SAIL Databank. BREATHE is funded through the UK Research and Innovation Industrial Strategy Challenge Fund and delivered through Health Data Research UK. This work used data provided by patients and collected by the NHS as part of their care and support. CH was supported by an MRC Human Genetics Unit programme grant ‘Quantitative traits in health and disease’ (U. MC_UU_00007/10). MHC was supported by NHLBI R01HL135142, R01HL137927, R01HL089856, R01HL147148. SC was supported by NHLBI K01HL153941. This CHS research was supported by NHLBI contracts HHSN268201200036C, HHSN268200960009C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006; and NHLBI grants U01HL080295, R01HL087652, R01HL105756, R01HL103612, R01HL120393, and U01HL130114 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This phase of the eMERGE Network was initiated and funded by the NHGRI through the following grants: U01HG008657 (Kaiser Permanente Washington/University of Washington); U01HG008685 (Brigham and Women’s Hospital); U01HG008672 (Vanderbilt University Medical Center); U01HG008666 (Cincinnati Children’s Hospital Medical Center); U01HG006379 (Mayo Clinic); U01HG008679 (Geisinger Clinic); U01HG008680 (Columbia University Health Sciences); U01HG008684 (Children’s Hospital of Philadelphia); U01HG008673 (Northwestern University); U01HG008701 (Vanderbilt University Medical Center serving as the Coordinating Center); U01HG008676 (Partners Healthcare/Broad Institute); U01HG008664 (Baylor College of Medicine); and U54MD007593 (Meharry Medical College). The work of Estonian Genome Center, University of Tartu has been supported by the European Regional Development Fund and grants no. GENTRANSMED (2014-2020.4.01.15-0012), MOBERA5 (Norface Network project no 462.16.107) and 2014-2020.4.01.16-0125. This study was also funded by the European Union through Horizon 2020 research and innovation programme under grant no. 810645 and through the European Regional Development Fund project no. MOBEC008 and Estonian Research Council Grants PRG1291 and PUT1660. Generation Scotland received core support from the Chief Scientist Office of the Scottish Government Health Directorates [CZD/16/6] and the Scottish Funding Council [HR03006] and is currently supported by the Wellcome Trust [216767, <a href=https://doi.org/10.35802/216767>https://doi.org/10.35802/216767</a>]. Genotyping of the GS:SFHS samples was carried out by the Genetics Core Laboratory at the Edinburgh Clinical Research Facility, University of Edinburgh, Scotland and was funded by the Medical Research Council UK and the Wellcome Trust (Wellcome Trust Strategic Award “STratifying Resilience and Depression Longitudinally” (STRADL) [104036, <a href=https://doi.org/10.35802/104036>https://doi.org/10.35802/104036</a>]). The NFBC1966 follow-up studies were supported by the University of Oulu (Grants no. 65354, 24000692), Oulu University Hospital (Grants no. 2/97, 8/97, 24301140), National research funding via City of Oulu, Ministry of Health and Social Affairs (Grants no. 23/251/97, 160/97, 190/97), National Institute for Health and Welfare, Helsinki (Grant no. 54121), Regional Institute of Occupational Health, Oulu, Finland (Grants no. 50621, 54231), and ERDF European Regional Development Fund (Grant no. 539/2010 A31592). The research on NFBC1966 data has been supported in part by H2020-633595 DynaHealth, H2020-733206 LifeCycle, H2020-824989 EUCANCONNECT, H2020-873749 LongITools, H2020-848158 EarlyCause, the JPI HDHL, PREcisE project, and ZonMw the Netherlands no. P75416. The Orkney Complex Disease Study (ORCADES) was supported by the Chief Scientist Office of the Scottish Government (CZB/4/276, CZB/4/710), a Royal Society URF to J.F.W., the MRC Human Genetics Unit quinquennial programme “QTL in Health and Disease”, Arthritis Research UK and the European Union framework program 6 EUROSPAN project (contract no. LSHG-CT-2006-018947). The Viking Health Study – Shetland (VIKING) was supported by the MRC Human Genetics Unit quinquennial programme grant “QTL in Health and Disease”. J.F.W. acknowledges support from the MRC Human Genetics Unit programme grant, “Quantitative traits in health and disease” (U. MC_UU_00007/10).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 1 approved, 2 approved with reservations]
Data availability
Underlying data
This research has been conducted using the UK Biobank resource under applications 648 and 4892. The genetic and phenotypic UK Biobank data can be requested upon application to the UK Biobank resource for all bona fide researchers (see https://www.ukbiobank.ac.uk/researchers/ for more details).
Figshare: WilliamsAT_prePMID_HRI.tsv.gz. https://doi.org/10.6084/m9.figshare.16622062 58 .
Extended data
Figshare: Williams_et_al_extended_data. https://doi.org/10.6084/m9.figshare.16622191 12 .
This project contains the following extended data:
supplementary_material.docx (Supplementary material and methods)
supplementary_figures.docx (Supplementary figures)
tableS1_icd10_codes.csv (Table S1, ICD-10 codes used to define the hospitalised respiratory infection phenotype).
tableS2_icd9_codes.txt (Table S2, ICD-9 codes used to define the hospitalised respiratory infection phenotype in CHS and Partners Biobank).
tableS3_discovery_summstats_sentinels.csv (Table S3, Summary statistics for the 56 sentinel variants from the discovery GWAS in UK Biobank).
tableS4_followup_availability_sentinels.csv (Table S4, Availability of the 40 sentinel variants in the 11 follow-up cohorts).
tableS5_metaanalysis_sentinels.csv (Table S5, Results of the inverse variance-weighted fixed effects meta-analysis).
tableS6_finemapping_results.csv (Table S6, Fine-mapping of the 56 association signals).
tableS7_annotation_results.csv (Table S7, Functional annotation of variants in the 95% credible sets).
tableS8_geneexpression_results_gtex.csv (Table S8, Association between variants in the 95% credible sets and gene expression across 48 tissues from GTEx v7).
tableS9_geneexpression_results_blueprint.csv (Table S9, Association between variants in the 95% credible sets and gene expression across three major human immune cell types from BLUEPRINT).
tableS10_geneexpression_results_eqtlgen.csv (Table S10, Association between variants in the 95% credible sets and gene expression (cis-eQTLs) from eQTLGen).
tableS11_coloc_piccolo_results.csv (Table S11, Colocalisation results from PICCOLO).
tableS12_gwsig_lookup_results.csv (Table S12, Look-up of the sentinel variants in the 56 association signals in existing GWAS).
tableS13_suggestive_lookup_results.csv (Table S13, Look-up of the sentinel variants in the 56 association signals in existing GWAS).
tableS14_covid19hgi_lookup_results.csv (Table S14, Look-up of the sentinel variants in the 56 association signals in the COVID-19 Host Genetics Initiative meta-analysis results).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
References
- 1. Dasaraju PV, Liu C: Infections of the Respiratory System.In: Baron S, editor. Medical Microbiology. 4th edition, Galveston (TX): University of Texas Medical Branch at Galveston;1996; Chapter 93. Reference Source [PubMed] [Google Scholar]
- 2. Monasta L, Ronfani L, Marchetti F, et al. : Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7(4):e36226. 10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2016 Causes of Death Collaborators: Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016.[published correction appears in Lancet. 2017 Oct 28; 390(10106):e38]. Lancet. 2017;390(10100):1151–1210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2016 Lower Respiratory Infections Collaborators: Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. 10.1016/S1473-3099(18)30310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casselbrant ML, Mandel EM, Fall PA, et al. : The heritability of otitis media: a twin and triplet study. JAMA. 1999;282(22):2125–2130. 10.1001/jama.282.22.2125 [DOI] [PubMed] [Google Scholar]
- 6. Rovers M, Haggard M, Gannon M, et al. : Heritability of Symptom Domains in Otitis Media: A Longitudinal Study of 1,373 Twin Pairs. Am J Epidemiol. 2002;155(10):958–964. 10.1093/aje/155.10.958 [DOI] [PubMed] [Google Scholar]
- 7. Kvestad E, Kvaerner KJ, Røysamb E, et al. : Heritability of recurrent tonsillitis. Arch Otolaryngol Head Neck Surg. 2005;131(5):383–387. 10.1001/archotol.131.5.383 [DOI] [PubMed] [Google Scholar]
- 8. Thomsen SF, Stensballe LG, Skytthe A, et al. : Increased concordance of severe respiratory syncytial virus infection in identical twins. Pediatrics. 2008;121(3):493–496. 10.1542/peds.2007-1889 [DOI] [PubMed] [Google Scholar]
- 9. Michalovich D, Nejentsev S: Activated PI3 Kinase Delta Syndrome: From Genetics to Therapy. Front Immunol. 2018;9:369. 10.3389/fimmu.2018.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coulter TI, Chandra A, Bacon CM, et al. : Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: A large patient cohort study. J Allergy Clin Immunol. 2017;139(2):597–606.e4. 10.1016/j.jaci.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elkaim E, Neven B, Bruneau J, et al. : Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: A cohort study. J Allergy Clin Immunol. 2016;138(1):210–218.e9. 10.1016/j.jaci.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 12. Williams AT, Shrine N, Naghra-van Gijzel H, et al. : Williams_et_al_extended_data. figshare. Dataset.2021. 10.6084/m9.figshare.16622191.v2 [DOI]
- 13. Wain LV, Shrine N, Miller S, et al. : Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. [published correction appears in Lancet Respir Med. 2016 Jan; 4(1): e4]. Lancet Respir Med. 2015;3(10):769–781. 10.1016/S2213-2600(15)00283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bycroft C, Freeman C, Petkova D, et al. : The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang CC, Chow CC, Tellier LC, et al. : Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bulik-Sullivan BK, Loh PR, Finucane HK, et al. : LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Lee SH, Goddard ME, et al. : GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J, Ferreira T, Morris AP, et al. : Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369–375, S1–3. 10.1038/ng.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried LP, Borhani NO, Enright P, et al. : The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 20. McCarty CA, Chisholm RL, Chute CG, et al. : The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13. 10.1186/1755-8794-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gottesman O, Kuivaniemi H, Tromp G, et al. : The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet Med. 2013;15(10):761–71. 10.1038/gim.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leitsalu L, Haller T, Esko T, et al. : Cohort Profile: Estonian Biobank of the Estonian Genome Center, University of Tartu. Int J Epidemiol. 2015;44(4):1137–47. 10.1093/ije/dyt268 [DOI] [PubMed] [Google Scholar]
- 23. Smith BH, Campbell A, Linksted P, et al. : Cohort Profile: Generation Scotland: Scottish Family Health Study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J Epidemiol. 2013;42(3):689–700. 10.1093/ije/dys084 [DOI] [PubMed] [Google Scholar]
- 24. University of Oulu: Northern Finland Birth Cohort 1966. University of Oulu. Reference Source [Google Scholar]
- 25. Krokstad S, Langhammer A, Hveem K, et al. : Cohort Profile: the HUNT Study, Norway. Int J Epidemiol. 2013;42(4):968–77. 10.1093/ije/dys095 [DOI] [PubMed] [Google Scholar]
- 26. Machiela MJ, Chanock SJ: LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31(21):3555–7. 10.1093/bioinformatics/btv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giambartolomei C, Vukcevic D, Schadt EE, et al. : Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383. 10.1371/journal.pgen.1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wakefield J: Reporting and interpretation in genome-wide association studies. Int J Epidemiol. 2008;37(3):641–653. 10.1093/ije/dym257 [DOI] [PubMed] [Google Scholar]
- 29. McLaren W, Gil L, Hunt SE, et al. : The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(1):122. 10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. GTEx Consortium, Laboratory, Data Analysis & Coordinating Center (LDACC)—Analysis Working Group, Statistical Methods groups—Analysis Working Group: Genetic effects on gene expression across human tissues. [published correction appears in Nature. 2017 Dec 20;:]. Nature. 2017;550(7675):204–213. 10.1038/nature24277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen L, Ge B, Casale FP, et al. : Genetic Drivers of Epigenetic and Transcriptional Variation in Human Immune Cells. Cell. 2016;167(5):1398–1414.e24. 10.1016/j.cell.2016.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Võsa U, Claringbould A, Westra HJ, et al. : Unraveling the polygenic architecture of complex traits using blood eQTL metaanalysis. bioRχiiv. 2018;447367. 10.1101/447367 [DOI] [Google Scholar]
- 33. Guo C, Sieber KB, Esparza-Gordillo J, et al. : Identification of putative effector genes across the GWAS Catalog using molecular quantitative trait loci from 68 tissues and cell types. bioRχiv. 2019;808444. 10.1101/808444 [DOI] [Google Scholar]
- 34. Suhre K, Arnold M, Bhagwat AM, et al. : Connecting genetic risk to disease end points through the human blood plasma proteome. [published correction appears in Nat Commun. 2017 Apr 11; 8: 15345]. Nat Commun. 2017;8:14357. 10.1038/ncomms14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun BB, Maranville JC, Peters JE, et al. : Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–79. 10.1038/s41586-018-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qiu W, Cho MH, Riley JH, et al. : Genetics of sputum gene expression in chronic obstructive pulmonary disease. PLoS One. 2011;6(9):e24395. 10.1371/journal.pone.0024395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. U-BIOPRED (Unbiased BIOmarkers in PREDiction of respiratory disease outcomes). Reference Source [Google Scholar]
- 38. Bolser DM, Chibon PY, Palopoli N, et al. : MetaBase--the wiki-database of biological databases. Nucleic Acids Res. 2012;40(Database issue):D1250–D1254. 10.1093/nar/gkr1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashburner M, Ball CA, Blake JA, et al. : Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. The Gene Ontology Consortium: The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47(D1):D330–D338. 10.1093/nar/gky1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamparter D, Marbach D, Rueedi R, et al. : Fast and Rigorous Computation of Gene and Pathway Scores from SNP-Based Summary Statistics. PLoS Comput Biol. 2016;12(1):e1004714. 10.1371/journal.pcbi.1004714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. COVID-19 Host Genetics Initiative: Mapping the human genetic architecture of COVID-19. Nature. 2021;600(7889):472–477. 10.1038/s41586-021-03767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ge T, Chen CY, Ni Y, et al. : Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10(1):1776. 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vaughan KT, Mikami A, Paschal BM, et al. : Multiple mouse chromosomal loci for dynein-based motility. Genomics. 1996;36(1):29–38. 10.1006/geno.1996.0422 [DOI] [PubMed] [Google Scholar]
- 45. Maiti AK, Mattéi MG, Jorissen M, et al. : Identification, tissue specific expression, and chromosomal localisation of several human dynein heavy chain genes. Eur J Hum Genet. 2000;8(12):923–32. 10.1038/sj.ejhg.5200555 [DOI] [PubMed] [Google Scholar]
- 46. Li Y, Yagi H, Onuoha EO, et al. : DNAH6 and Its Interactions with PCD Genes in Heterotaxy and Primary Ciliary Dyskinesia. PLoS Genet. 2016;12(2):e1005821. 10.1371/journal.pgen.1005821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghoussaini M, Mountjoy E, Carmona M, et al. : Open Targets Genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 2021;49(D1):D1311–D1320. 10.1093/nar/gkaa840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campos AI, Kho P, Vazquez-Prada KX, et al. : Genetic susceptibility to pneumonia: A GWAS meta-analysis between UK Biobank and FinnGen. medRχiv. 2020. 10.1101/2020.06.22.20103556 [DOI] [PubMed] [Google Scholar]
- 49. Tian C, Hromatka BS, Kiefer AK, et al. : Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat Commun. 2017;8(1):599. 10.1038/s41467-017-00257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morgan R, Pandha HS: PBX3 in Cancer. Cancers (Basel). 2020;12(2):431. 10.3390/cancers12020431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akinosoglou KS, Karkoulias K, Marangos M: Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2013;17(1):8–18. [PubMed] [Google Scholar]
- 52. Allen EK, Chen WM, Weeks DE, et al. : A genome-wide association study of chronic otitis media with effusion and recurrent otitis media identifies a novel susceptibility locus on chromosome 2. J Assoc Res Otolaryngol. 2013;14(6):791–800. 10.1007/s10162-013-0411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allen EK, Manichaikul A, Chen WM, et al. : Evaluation of replication of variants associated with genetic risk of otitis media. PLoS One. 2014;9(8):e104212. 10.1371/journal.pone.0104212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Garcia-Etxebarria K, Bracho MA, Galán JC, et al. : No Major Host Genetic Risk Factor Contributed to A(H1N1)2009 Influenza Severity. PLoS One. Erratum in: PLoS One. 2015; 10(10):e0141661.2015;10(9):e0135983. 10.1371/journal.pone.0135983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McMahon G, Ring SM, Davey-Smith G, et al. : Genome-wide association study identifies SNPs in the MHC class II loci that are associated with self-reported history of whooping cough. Hum Mol Genet. 2015;24(20):5930–9. 10.1093/hmg/ddv293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Einarsdottir E, Hafrén L, Leinonen E, et al. : Genome-wide association analysis reveals variants on chromosome 19 that contribute to childhood risk of chronic otitis media with effusion. Sci Rep. 2016;6:33240. 10.1038/srep33240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pasanen A, Karjalainen MK, Bont L, et al. : Genome-Wide Association Study of Polymorphisms Predisposing to Bronchiolitis. Sci Rep. 2017;7:41653. 10.1038/srep41653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams AT, Shrine N, Naghra-van Gijzel H, et al. : WilliamsAT_prePMID_HRI.tsv.gz. figshare. Dataset,2021. 10.6084/m9.figshare.16622062.v1 [DOI]