Abstract
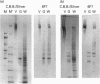
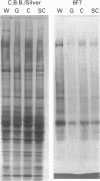
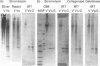
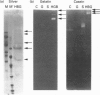
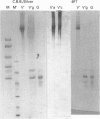
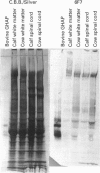
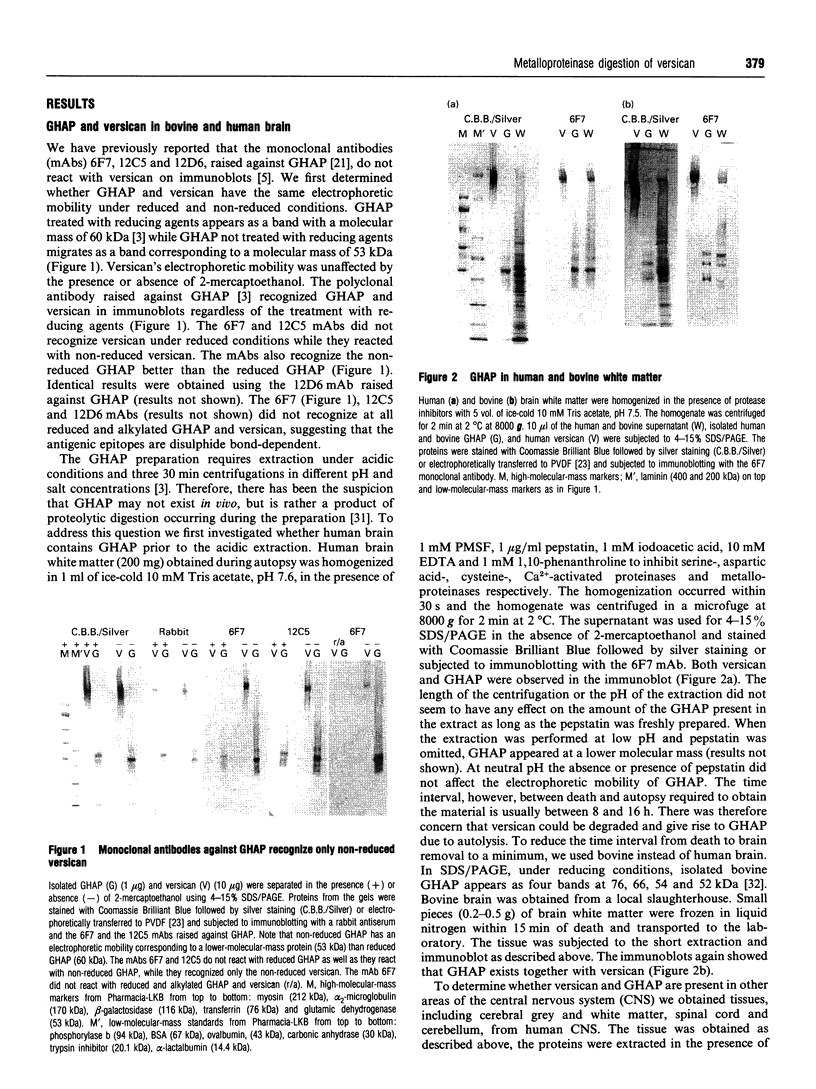
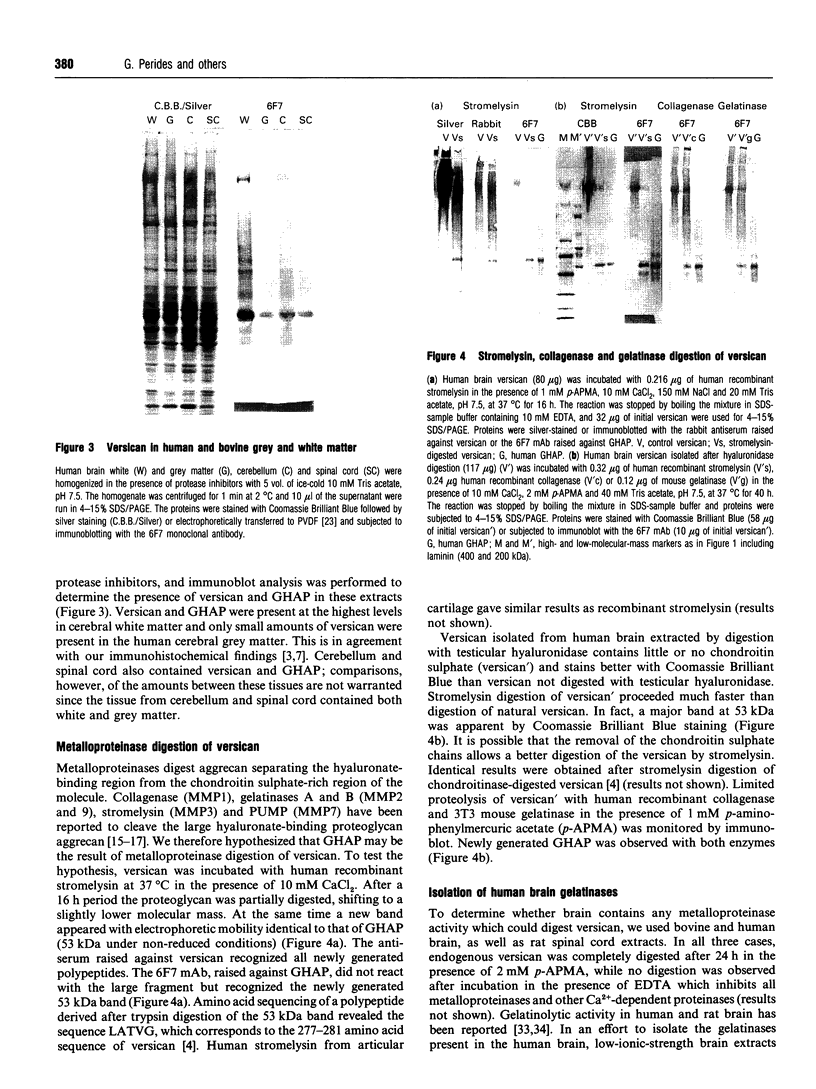
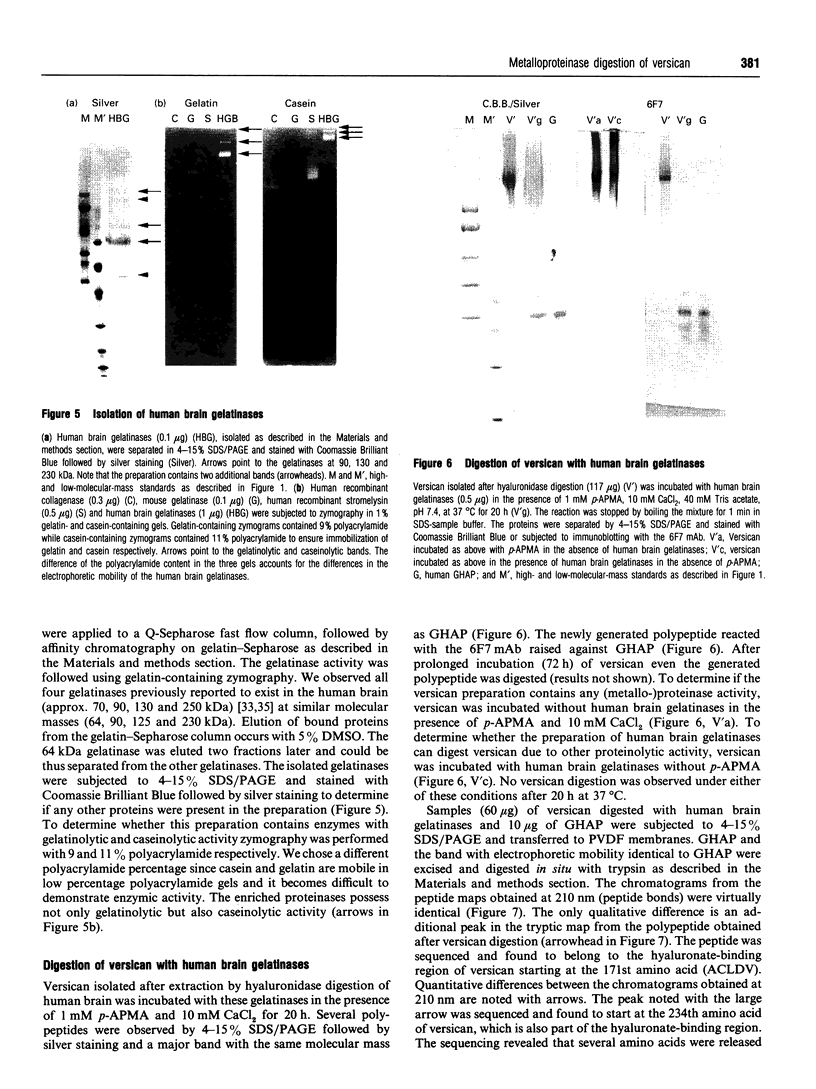
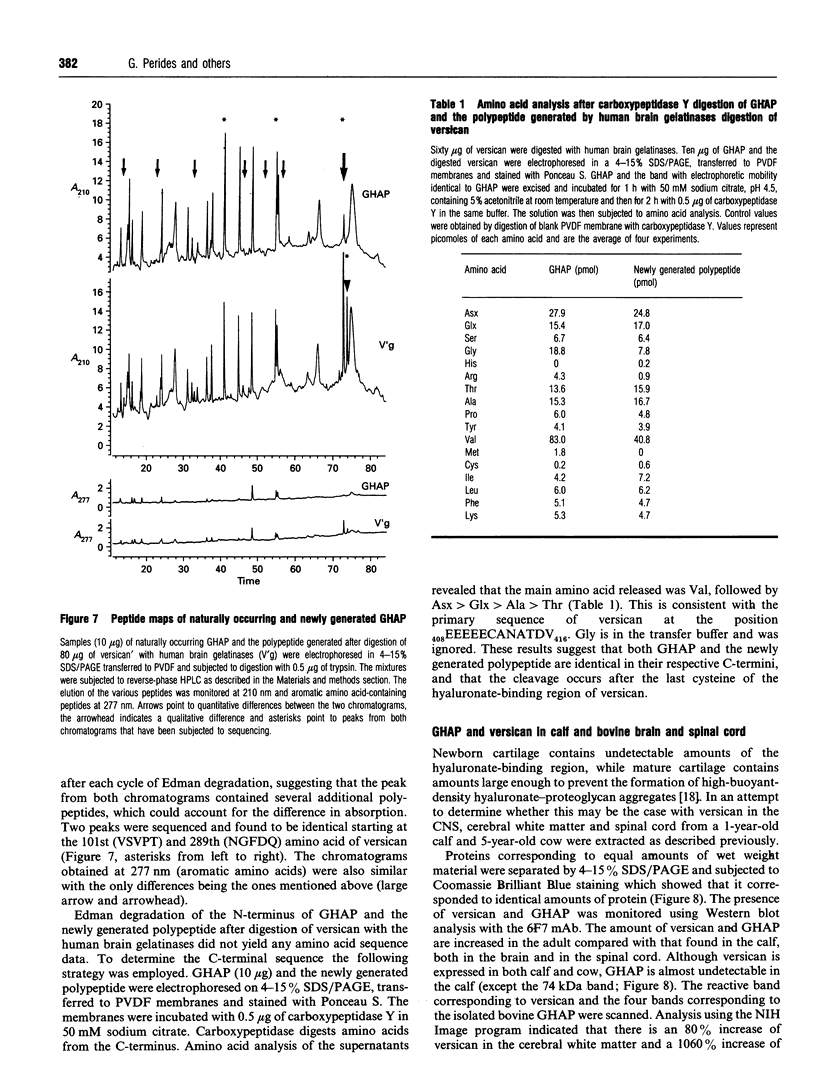
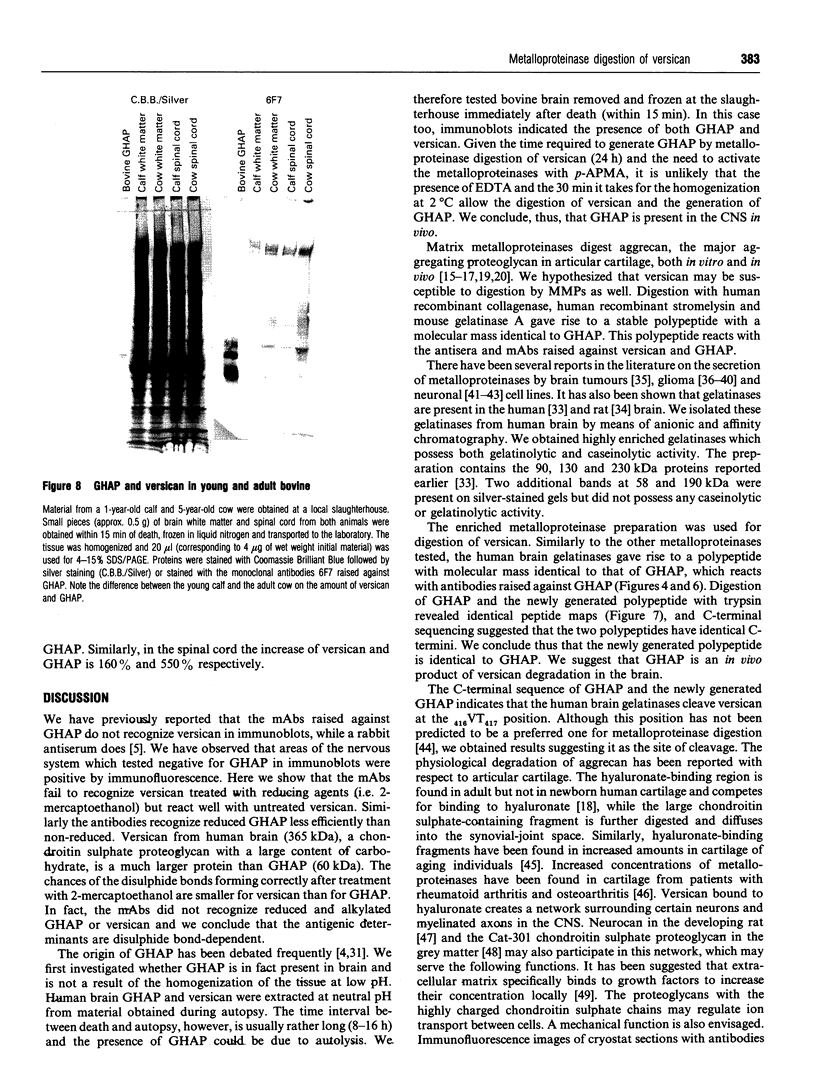
Glial hyaluronate-binding protein (GHAP) is a 60 kDa glycoprotein with an amino acid sequence identical to that of the hyaluronate-binding region of versican, a large fibroblast aggregating proteoglycan found in the brain. Both GHAP and versican were identified by immunoblot in bovine brain extracts prepared only minutes after death. Human recombinant collagenase, stromelysin, mouse gelatinase and gelatinases isolated from human brain by affinity chromatography digest versican and give rise to a polypeptide with electrophoretic mobility identical to GHAP. Immunoblot analysis, peptide mapping and C-terminal amino acid sequencing indicate that the polypeptide generated by digestion with human brain gelatinases is identical to GHAP. We suggest that GHAP is a naturally occurring versican degradation product.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apodaca G., Rutka J. T., Bouhana K., Berens M. E., Giblin J. R., Rosenblum M. L., McKerrow J. H., Banda M. J. Expression of metalloproteinases and metalloproteinase inhibitors by fetal astrocytes and glioma cells. Cancer Res. 1990 Apr 15;50(8):2322–2329. [PubMed] [Google Scholar]
- Asher R., Perides G., Vanderhaeghen J. J., Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res. 1991 Mar;28(3):410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- Backstrom J. R., Miller C. A., Tökés Z. A. Characterization of neutral proteinases from Alzheimer-affected and control brain specimens: identification of calcium-dependent metalloproteinases from the hippocampus. J Neurochem. 1992 Mar;58(3):983–992. doi: 10.1111/j.1471-4159.1992.tb09352.x. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Expression of brain-specific hyaluronectin (BHN), a hyaluronate-binding protein, in dog postnatal development. Exp Neurol. 1988 Jan;99(1):107–117. doi: 10.1016/0014-4886(88)90131-8. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D., Gilad V. H., Gilad G. M. Hyaluronate-binding protein produced by white matter astrocytes: an axonal growth repellent? Exp Neurol. 1988 Apr;100(1):253–256. doi: 10.1016/0014-4886(88)90218-x. [DOI] [PubMed] [Google Scholar]
- Bignami A., LeBlanc A., Perides G. A role for extracellular matrix degradation and matrix metalloproteinases in senile dementia? Acta Neuropathol. 1994;87(3):308–312. doi: 10.1007/BF00296747. [DOI] [PubMed] [Google Scholar]
- Bignami A., Perides G., Rahemtulla F. Versican, a hyaluronate-binding proteoglycan of embryonal precartilaginous mesenchyma, is mainly expressed postnatally in rat brain. J Neurosci Res. 1993 Jan;34(1):97–106. doi: 10.1002/jnr.490340110. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Colton C. A., Keri J. E., Chen W. T., Monsky W. L. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993 Jun 15;35(3):297–304. doi: 10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- Crawford T. J., Melhado I. G., Jirik F. R. Expression of versican mRNA is developmentally regulated in the brain of the embryonic chick and the developing rat. Brain Res Dev Brain Res. 1993 Dec 17;76(2):264–267. doi: 10.1016/0165-3806(93)90216-w. [DOI] [PubMed] [Google Scholar]
- Dours-Zimmermann M. T., Zimmermann D. R. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem. 1994 Dec 30;269(52):32992–32998. [PubMed] [Google Scholar]
- Fillmore H. L., Mainardi C. L., Hasty K. A. Differentiation of PC12 cells with nerve growth factor is associated with induction of transin synthesis and release. J Neurosci Res. 1992 Apr;31(4):662–669. doi: 10.1002/jnr.490310410. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Girard M. T. Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Invest Ophthalmol Vis Sci. 1990 Sep;31(9):1779–1788. [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fosang A. J., Last K., Knäuper V., Neame P. J., Murphy G., Hardingham T. E., Tschesche H., Hamilton J. A. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993 Oct 1;295(Pt 1):273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Last K., Hardingham T. E., Murphy G., Hamilton J. A. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992 Sep 25;267(27):19470–19474. [PubMed] [Google Scholar]
- Fryer H. J., Kelly G. M., Molinaro L., Hockfield S. The high molecular weight Cat-301 chondroitin sulfate proteoglycan from brain is related to the large aggregating proteoglycan from cartilage, aggrecan. J Biol Chem. 1992 May 15;267(14):9874–9883. [PubMed] [Google Scholar]
- Gunja-Smith Z., Nagase H., Woessner J. F., Jr Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989 Feb 15;258(1):115–119. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Shinomura T., Zako M., Ujita M., Kimata K. Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J Biol Chem. 1995 Jan 13;270(2):958–965. doi: 10.1074/jbc.270.2.958. [DOI] [PubMed] [Google Scholar]
- Koklitis P. A., Murphy G., Sutton C., Angal S. Purification of recombinant human prostromelysin. Studies on heat activation to give high-Mr and low-Mr active forms, and a comparison of recombinant with natural stromelysin activities. Biochem J. 1991 May 15;276(Pt 1):217–221. doi: 10.1042/bj2760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Hoerrner L. A., Lark M. W. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993 Feb;36(2):181–189. doi: 10.1002/art.1780360207. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Neame P. J., Sandy J. D. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993 Sep;36(9):1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Lund R. D., Zhou H. F., Yee K. T. The migration of astrocytes after implantation to immature brains. Int J Dev Neurosci. 1993 Oct;11(5):595–601. doi: 10.1016/0736-5748(93)90048-i. [DOI] [PubMed] [Google Scholar]
- Machida C. M., Rodland K. D., Matrisian L., Magun B. E., Ciment G. NGF induction of the gene encoding the protease transin accompanies neuronal differentiation in PC12 cells. Neuron. 1989 Jun;2(6):1587–1596. doi: 10.1016/0896-6273(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Ballin M., Pelina M. D., Farina A. R., Nason A. M., Hartzler J. L., Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. 1992;12(3-4):168–184. [PubMed] [Google Scholar]
- Margolis R. K., Margolis R. U. Nervous tissue proteoglycans. Experientia. 1993 May 15;49(5):429–446. doi: 10.1007/BF01923587. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Fujimoto N., Obata K., Cloutier J. M., Pelletier J. P. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994 Jun;70(6):807–815. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Kubota T., Kabuto M., Sato K., Kawano H., Hayakawa T., Okada Y. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg. 1994 Jul;81(1):69–77. doi: 10.3171/jns.1994.81.1.0069. [DOI] [PubMed] [Google Scholar]
- Nakano A., Tani E., Miyazaki K., Furuyama J., Matsumoto T. Expressions of matrilysin and stromelysin in human glioma cells. Biochem Biophys Res Commun. 1993 May 14;192(3):999–1003. doi: 10.1006/bbrc.1993.1515. [DOI] [PubMed] [Google Scholar]
- Naso M. F., Zimmermann D. R., Iozzo R. V. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994 Dec 30;269(52):32999–33008. [PubMed] [Google Scholar]
- Nelson R. B., Siman R. Identification and characterization of calcium-dependent metalloproteases in rat brain. J Neurochem. 1989 Aug;53(2):641–647. doi: 10.1111/j.1471-4159.1989.tb07381.x. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S., Sang Q. X., Moore W. G., Navre M., Birkedal-Hansen H., Van Wart H. E. Comparative sequence specificities of human 72- and 92-kDa gelatinases (type IV collagenases) and PUMP (matrilysin). Biochemistry. 1993 Jun 29;32(25):6427–6432. doi: 10.1021/bi00076a016. [DOI] [PubMed] [Google Scholar]
- Paganetti P. A., Caroni P., Schwab M. E. Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease. J Cell Biol. 1988 Dec;107(6 Pt 1):2281–2291. doi: 10.1083/jcb.107.6.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perides G., Biviano F., Bignami A. Interaction of a brain extracellular matrix protein with hyaluronic acid. Biochim Biophys Acta. 1991 Oct 31;1075(3):248–258. doi: 10.1016/0304-4165(91)90273-j. [DOI] [PubMed] [Google Scholar]
- Perides G., Lane W. S., Andrews D., Dahl D., Bignami A. Isolation and partial characterization of a glial hyaluronate-binding protein. J Biol Chem. 1989 Apr 5;264(10):5981–5987. [PubMed] [Google Scholar]
- Perides G., Rahemtulla F., Lane W. S., Asher R. A., Bignami A. Isolation of a large aggregating proteoglycan from human brain. J Biol Chem. 1992 Nov 25;267(33):23883–23887. [PubMed] [Google Scholar]
- Rauch U., Gao P., Janetzko A., Flaccus A., Hilgenberg L., Tekotte H., Margolis R. K., Margolis R. U. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified with monoclonal antibodies. J Biol Chem. 1991 Aug 5;266(22):14785–14801. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Nishida Y., Ito K., Kimata K. cDNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationships to versican. J Biol Chem. 1993 Jul 5;268(19):14461–14469. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venstrom K. A., Reichardt L. F. Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 1993 Aug;7(11):996–1003. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- Vilim V., Fosang A. J. Proteoglycans isolated from dissociative extracts of differently aged human articular cartilage: characterization of naturally occurring hyaluronan-binding fragments of aggrecan. Biochem J. 1994 Dec 15;304(Pt 3):887–894. doi: 10.1042/bj3040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]