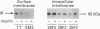Abstract
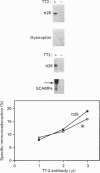
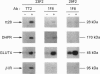
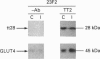
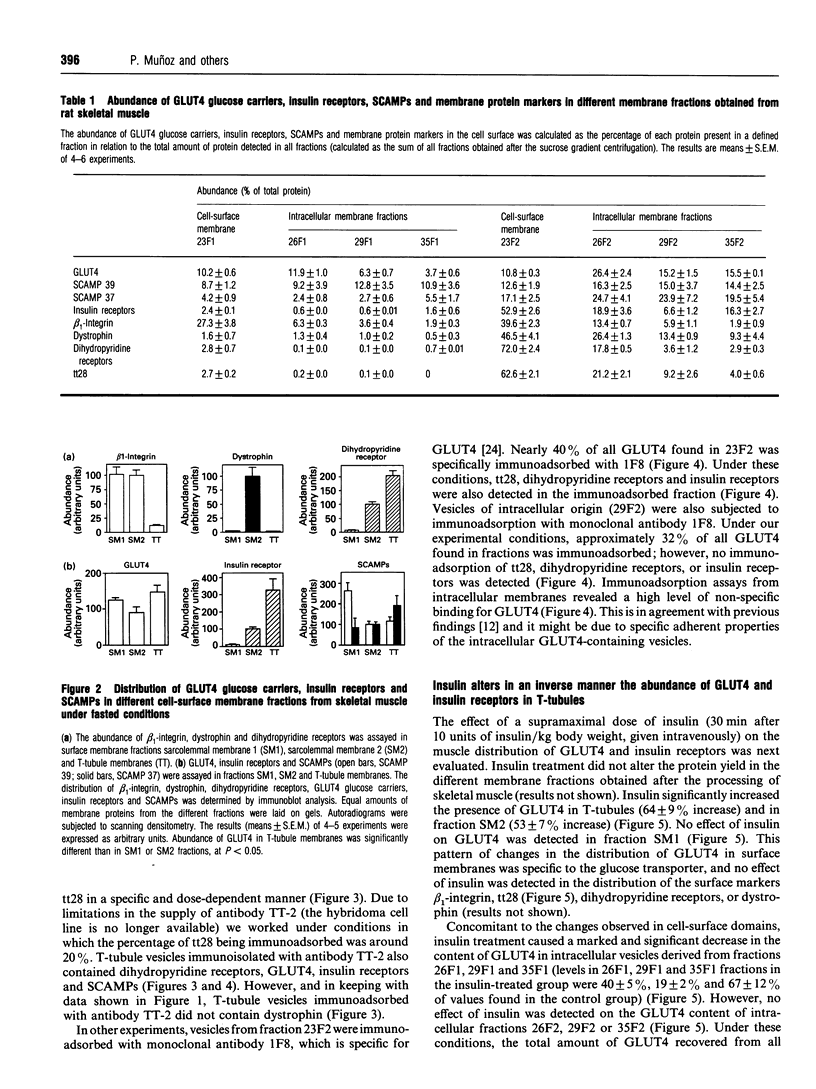
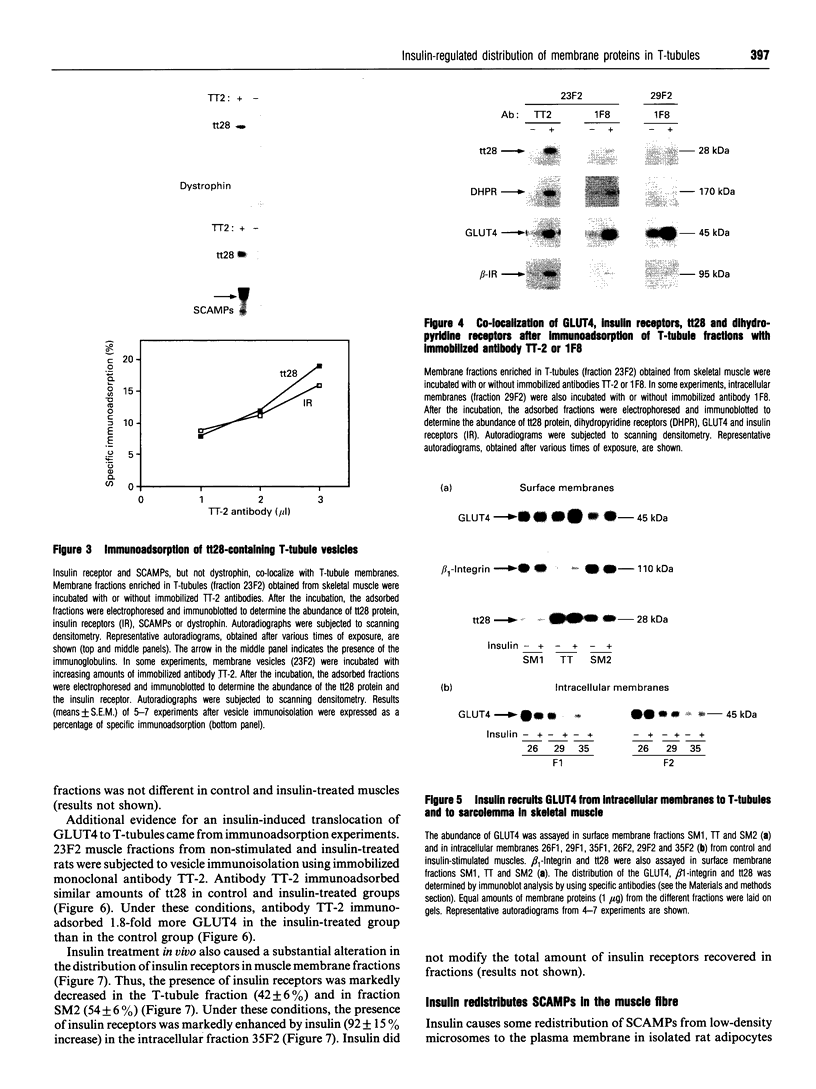
(1) In this study we have determined the distribution of various membrane proteins involved in insulin-activated glucose transport in T-tubules and in sarcolemma from rat skeletal muscle. Two independent experimental approaches were used to determine the presence of membrane proteins in T-tubules: (i) the purification of T-tubules free from sarcolemmal membranes by lectin agglutination, and (ii) T-tubule vesicle immunoadsorption. These methods confirmed that T-tubules from rat skeletal muscle were enriched with dihydropyridine receptors and tt28 protein and did not contain the sarcolemmal markers dystrophin or beta 1-integrin. Both types of experiments revealed an abundant content of GLUT4 glucose carriers, insulin receptors and SCAMPs (secretory carrier membrane proteins) in T-tubule membranes. (2) Acute administration in vivo of insulin caused an increased abundance of GLUT4 in T-tubules and sarcolemma. On the contrary, insulin led to a 50% reduction in insulin receptors present in T-tubules and in sarcolemma, demonstrating that insulin-induced insulin receptor internalization affects T-tubules in the muscle fibre. The alteration in the content of GLUT4 and insulin receptors in T-tubules was a consequence of insulin-induced redistribution of these proteins. SCAMPs also redistributed in muscle membranes in response to insulin. They were recruited by insulin from intracellular high-density fractions to intracellular lighter-density fractions and to the cell surface, showing a pattern of insulin-induced cellular redistribution distinct from those of GLUT4 and the insulin receptor. (3) In conclusion, the T-tubule is a cell-surface target for membrane proteins involved in recycling such as SCAMPs or for membrane proteins that acutely redistribute in response to insulin such as GLUT4 or insulin receptors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angélica Carrasco M., Sierralta J., Hidalgo C. Phospholipase C activity in membranes and a soluble fraction isolated from frog skeletal muscle. Biochim Biophys Acta. 1993 Oct 10;1152(1):44–48. doi: 10.1016/0005-2736(93)90229-s. [DOI] [PubMed] [Google Scholar]
- Arnold H., Nolte J., Pette D. Quantitative and histochemical studies on the desorption and readsorption of aldolase in cross-striated muscle. J Histochem Cytochem. 1969 May;17(5):314–320. doi: 10.1177/17.5.314. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., White M. F. Tyrosine phosphorylation of the insulin receptor during insulin-stimulated internalization in rat hepatoma cells. J Biol Chem. 1989 Jan 25;264(3):1694–1701. [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Compartmentation of hexokinase and creatine phosphokinase, cellular regulation, and insulin action. Curr Top Cell Regul. 1980;16:55–86. doi: 10.1016/b978-0-12-152816-4.50007-8. [DOI] [PubMed] [Google Scholar]
- Bornemann A., Ploug T., Schmalbruch H. Subcellular localization of GLUT4 in nonstimulated and insulin-stimulated soleus muscle of rat. Diabetes. 1992 Feb;41(2):215–221. doi: 10.2337/diab.41.2.215. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brand S. H., Castle J. D. SCAMP 37, a new marker within the general cell surface recycling system. EMBO J. 1993 Oct;12(10):3753–3761. doi: 10.1002/j.1460-2075.1993.tb06053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. H., Laurie S. M., Mixon M. B., Castle J. D. Secretory carrier membrane proteins 31-35 define a common protein composition among secretory carrier membranes. J Biol Chem. 1991 Oct 5;266(28):18949–18957. [PubMed] [Google Scholar]
- Brandau H., Pette D. Topische Muster von Enzymen des energieliefernden Stoffwechsels im quergestreiften Muskel. Enzymol Biol Clin (Basel) 1966;6(2):123–156. [PubMed] [Google Scholar]
- Brozinick J. T., Jr, Etgen G. J., Jr, Yaspelkis B. B., 3rd, Ivy J. L. The effects of muscle contraction and insulin on glucose-transporter translocation in rat skeletal muscle. Biochem J. 1994 Feb 1;297(Pt 3):539–545. doi: 10.1042/bj2970539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett E., Beeler T., Klip A. Distribution of glucose transporters and insulin receptors in the plasma membrane and transverse tubules of skeletal muscle. Arch Biochem Biophys. 1987 Feb 15;253(1):279–286. doi: 10.1016/0003-9861(87)90661-8. [DOI] [PubMed] [Google Scholar]
- Camps M., Castelló A., Muñoz P., Monfar M., Testar X., Palacín M., Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J. 1992 Mar 15;282(Pt 3):765–772. doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Dayer J. M., Lang U., Silverman R., Orci L., Gorden P. Down-regulation and recycling of insulin receptors. Effect of monensin on IM-9 lymphocytes and U-937 monocyte-like cells. J Biol Chem. 1984 Nov 25;259(22):14190–14195. [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charuk J. H., Howlett S., Michalak M. Subfractionation of cardiac sarcolemma with wheat-germ agglutinin. Biochem J. 1989 Dec 15;264(3):885–892. doi: 10.1042/bj2640885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Salviati G., Pozzan T., Volpe P. Is a guanine nucleotide-binding protein involved in excitation-contraction coupling in skeletal muscle? EMBO J. 1986 Feb;5(2):259–262. doi: 10.1002/j.1460-2075.1986.tb04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Rastogi S., Bilan P. J., Cartee G. D., Vranic M., Holloszy J. O., Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990 Aug 15;265(23):13427–13430. [PubMed] [Google Scholar]
- Dudek R. W., Dohm G. L., Holman G. D., Cushman S. W., Wilson C. M. Glucose transporter localization in rat skeletal muscle. Autoradiographic study using ATB-[2-3H]BMPA photolabel. FEBS Lett. 1994 Feb 21;339(3):205–208. doi: 10.1016/0014-5793(94)80416-8. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Dudek R. W., Whitehead D. S., Downes D. L., Frisell W. R., Caro J. F., Dohm G. L. Immunolocalization of glucose transporter GLUT4 within human skeletal muscle. Diabetes. 1991 Jan;40(1):150–154. doi: 10.2337/diab.40.1.150. [DOI] [PubMed] [Google Scholar]
- Gumà A., Mora C., Santalucía T., Viñals F., Testar X., Palacín M., Zorzano A. System A transport activity is stimulated in skeletal muscle in response to diabetes. FEBS Lett. 1992 Sep 21;310(1):51–54. doi: 10.1016/0014-5793(92)81144-b. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Gonzalez M. E., Lagos R. Characterization of the Ca2+- or Mg2+-ATPase of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1983 Nov 25;258(22):13937–13945. [PubMed] [Google Scholar]
- Hidalgo C., Jorquera J., Tapia V., Donoso P. Triads and transverse tubules isolated from skeletal muscle contain high levels of inositol 1,4,5-trisphosphate. J Biol Chem. 1993 Jul 15;268(20):15111–15117. [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Jose M., Biosca J. A., Trujillo R., Itarte E. Characterization of the hepatic insulin receptor undergoing internalization through clathrin-coated vesicles and endosomes. FEBS Lett. 1993 Nov 22;334(3):286–288. doi: 10.1016/0014-5793(93)80696-r. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurie S. M., Cain C. C., Lienhard G. E., Castle J. D. The glucose transporter GluT4 and secretory carrier membrane proteins (SCAMPs) colocalize in rat adipocytes and partially segregate during insulin stimulation. J Biol Chem. 1993 Sep 5;268(25):19110–19117. [PubMed] [Google Scholar]
- Luise M., Presotto C., Senter L., Betto R., Ceoldo S., Furlan S., Salvatori S., Sabbadini R. A., Salviati G. Dystrophin is phosphorylated by endogenous protein kinases. Biochem J. 1993 Jul 1;293(Pt 1):243–247. doi: 10.1042/bj2930243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milting H., Heilmeyer L. M., Jr, Thieleczek R. Phosphoinositides in membranes that build up the triads of rabbit skeletal muscle. FEBS Lett. 1994 May 30;345(2-3):211–218. doi: 10.1016/0014-5793(94)00440-4. [DOI] [PubMed] [Google Scholar]
- Minetti C., Beltrame F., Marcenaro G., Bonilla E. Dystrophin at the plasma membrane of human muscle fibers shows a costameric localization. Neuromuscul Disord. 1992;2(2):99–109. doi: 10.1016/0960-8966(92)90041-4. [DOI] [PubMed] [Google Scholar]
- Muñoz P., Rosemblatt M., Testar X., Palacín M., Zorzano A. Isolation and characterization of distinct domains of sarcolemma and T-tubules from rat skeletal muscle. Biochem J. 1995 Apr 1;307(Pt 1):273–280. doi: 10.1042/bj3070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Snook J. B., Campbell K. P. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991 Jan;112(1):135–148. doi: 10.1083/jcb.112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C., Forsberg E., Enrich C., Johansson S. Changes in cell surface expression of fibronectin and fibronectin receptor during liver regeneration. J Cell Sci. 1992 Aug;102(Pt 4):815–820. doi: 10.1242/jcs.102.4.815. [DOI] [PubMed] [Google Scholar]
- Rodnick K. J., Slot J. W., Studelska D. R., Hanpeter D. E., Robinson L. J., Geuze H. J., James D. E. Immunocytochemical and biochemical studies of GLUT4 in rat skeletal muscle. J Biol Chem. 1992 Mar 25;267(9):6278–6285. [PubMed] [Google Scholar]
- Rosemblatt M. S., Scales D. J. Morphological, immunological and biochemical characterization of purified transverse tubule membranes isolated from rabbit skeletal muscle. Mol Cell Biochem. 1989 May 4;87(1):57–69. doi: 10.1007/BF00421083. [DOI] [PubMed] [Google Scholar]
- Russell R. R., 3rd, Mrus J. M., Mommessin J. I., Taegtmeyer H. Compartmentation of hexokinase in rat heart. A critical factor for tracer kinetic analysis of myocardial glucose metabolism. J Clin Invest. 1992 Nov;90(5):1972–1977. doi: 10.1172/JCI116076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori S., Furlan S., Millikin B., Sabbadini R., Betto R., Margreth A., Salviati G. Localization of protein kinase C in skeletal muscle T-tubule membranes. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1073–1080. doi: 10.1006/bbrc.1993.2360. [DOI] [PubMed] [Google Scholar]
- Schmid A., Barhanin J., Coppola T., Borsotto M., Lazdunski M. Immunochemical analysis of subunit structures of 1,4-dihydropyridine receptors associated with voltage-dependent Ca2+ channels in skeletal, cardiac, and smooth muscles. Biochemistry. 1986 Jun 17;25(12):3492–3495. doi: 10.1021/bi00360a002. [DOI] [PubMed] [Google Scholar]
- Sigel P., Pette D. Intracellular localization of glycogenolytic and glycolytic enzymes in white and red rabbit skeletal muscle: a gel film method for coupled enzyme reactions in histochemistry. J Histochem Cytochem. 1969 Apr;17(4):225–237. doi: 10.1177/17.4.225. [DOI] [PubMed] [Google Scholar]
- Thoidis G., Kotliar N., Pilch P. F. Immunological analysis of GLUT4-enriched vesicles. Identification of novel proteins regulated by insulin and diabetes. J Biol Chem. 1993 Jun 5;268(16):11691–11696. [PubMed] [Google Scholar]
- Toutant M., Gabrion J., Vandaele S., Peraldi-Roux S., Barhanin J., Bockaert J., Rouot B. Cellular distribution and biochemical characterization of G proteins in skeletal muscle: comparative location with voltage-dependent calcium channels. EMBO J. 1990 Feb;9(2):363–369. doi: 10.1002/j.1460-2075.1990.tb08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E., MacDonald G., Phillips L., Jorgensen A. O., MacLennan D. H. Monoclonal antibodies to the Ca2+ + Mg2+-dependent ATPase of sarcoplasmic reticulum identify polymorphic forms of the enzyme and indicate the presence in the enzyme of a classical high-affinity Ca2+ binding site. J Bioenerg Biomembr. 1984 Dec;16(5-6):441–464. doi: 10.1007/BF00743238. [DOI] [PubMed] [Google Scholar]