Abstract
Pelvic carcinosarcoma is an aggressive malignancy with significant diagnostic and management hurdles due to its complex vascularity and potential for extensive local invasion. A 59-year-old female presented with severe abdominal pain and significant weight loss, leading to the discovery of a large, complex pelvic mass through CT scans, MRI, and PET CT, suggesting aggressive malignancy. Initial management included a robotic laparoscopic proximal sigmoid loop colostomy to alleviate obstruction. Significant vascularity led to consultations with Vascular Surgery and subsequent preoperative embolization. Definitive surgery involved a supralevator posterior exenteration for en bloc resection of the vagina, mass, and sigmoid colon, combined with a low anterior resection and an omental J flap in anticipation of potential postoperative radiation therapy. This case underscores the importance of integrated imaging and staged surgical interventions in managing pelvic carcinosarcoma, emphasizing a multidisciplinary approach to optimize outcomes and minimize complications.
Keywords: Pelvic carcinosarcoma, Advanced imaging, MRI, PET CT, Biopsy, Posthysterectomy, Oncological diagnosis, Multimodal imaging, J flap
Introduction
Pelvic carcinosarcoma is a rare and intricately challenging malignancy that straddles the categories of sarcoma and carcinoma, originating within the pelvic region [1]. This dual phenotype not only complicates its pathophysiological understanding but also its diagnostic and therapeutic approaches [2]. Typically emerging in postmenopausal women, this aggressive cancer poses significant challenges due to its rapid progression and poor prognosis [3]. The biphasic nature of carcinosarcoma often presents diagnostic challenges, particularly when occurring in anatomically complex regions, such as the pelvis. In patients with a history of significant surgical interventions, like a hysterectomy, the diagnosis becomes even more challenging due to altered anatomical landmarks and potential postsurgical changes that can obscure tumor identification and characterization [4].
The rarity of pelvic carcinosarcoma contributes to the complexities associated with its study. There is a limited but growing body of research focused on its epidemiology, risk factors, and optimal therapeutic strategies. Insights into genetic mutations and the molecular mechanisms driving their development are critical for advancing treatment options, which currently rely heavily on surgery, chemotherapy, and radiation, often applied in a multimodal approach [5].
Enhancing the understanding of pelvic carcinosarcoma also requires a focus on patient-centered care. The impact of this disease extends beyond physical symptoms, affecting emotional and psychosocial well-being. Thus, incorporating supportive care services, such as counseling, pain management, and tailoring treatment modalities to individual patient needs are crucial. This holistic approach aims to treat cancer and improve patients' overall quality of life, providing them with the necessary resources to manage the broader implications of their diagnosis [6].
Imaging plays a pivotal role in the diagnostic pathway of pelvic tumors, offering a noninvasive means to evaluate the extent and nature of the lesions. Advanced imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) are indispensable tools in the diagnosis and staging of pelvic carcinosarcoma [7]. These modalities provide detailed insights into the tumor's location, size, involvement with surrounding structures, and metabolic activity, which are crucial for accurate diagnosis and optimal treatment planning [2].
This case report details the use of sophisticated imaging modalities in a patient presenting with diffuse abdominal pain and a complex medical history, including a total abdominal hysterectomy with bilateral salpingo-oophorectomy. The diagnostic process was compounded by the patient's postsurgical anatomy and the ambiguous nature of the presenting mass. Through a series of imaging studies, the diagnostic team navigated these complexities to arrive at a conclusive diagnosis, demonstrating the critical role of radiology in managing such intricate cases.
Case presentation
A 59-year-old female presented to the emergency department complaining of severe diffuse abdominal pain, which had progressively worsened over several days. Her medical history was notable for hypertension, asthma, obesity, and a total abdominal hysterectomy with bilateral salpingo-oophorectomy performed several years prior. Recent symptoms included nausea, vomiting, constipation, diarrhea, rectal bleeding, dysuria, and a weight loss of 30 pounds over 2 months.
Initial examination revealed vital signs within normal limits and nonspecific abdominal tenderness without any distinct, palpable masses. The patient's recent history of unexplained weight loss and gastrointestinal symptoms prompted further diagnostic workup.
Initial imaging
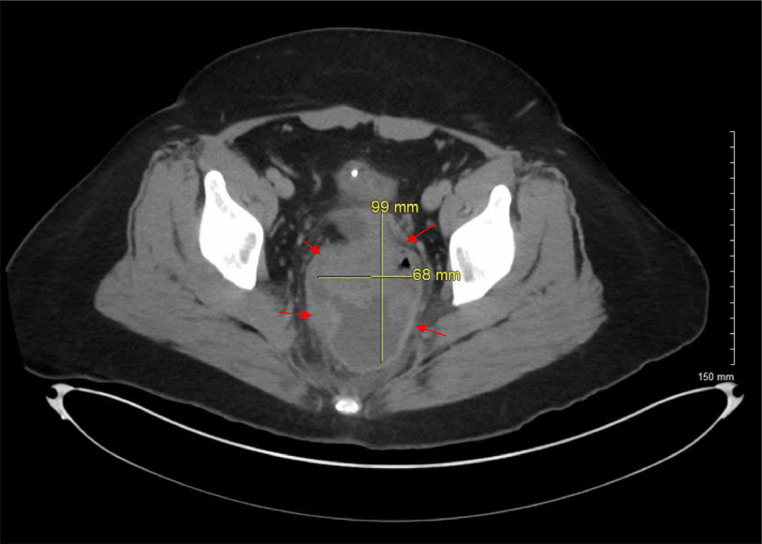
A CT scan of the abdomen and pelvis revealed a complex cystic and solid-appearing mass measuring 9.9 × 6.8 cm located centrally in the pelvis (see Fig. 1, Fig. 2). The mass obscured the distal sigmoid colon and rectum and distorted the vaginal cuff area, raising concerns for a neoplastic process. The ovaries were nonvisualized, and the absence of the uterus was confirmed.
Fig. 1.
Axial view CT of the abdomen and pelvis with IV contrast reveals a complex, centrally located mass in the pelvis that displays both cystic and solid characteristics. Marked by red arrows, the mass's ambiguous nature and central pelvic position raise concerns about a possible neoplastic process.
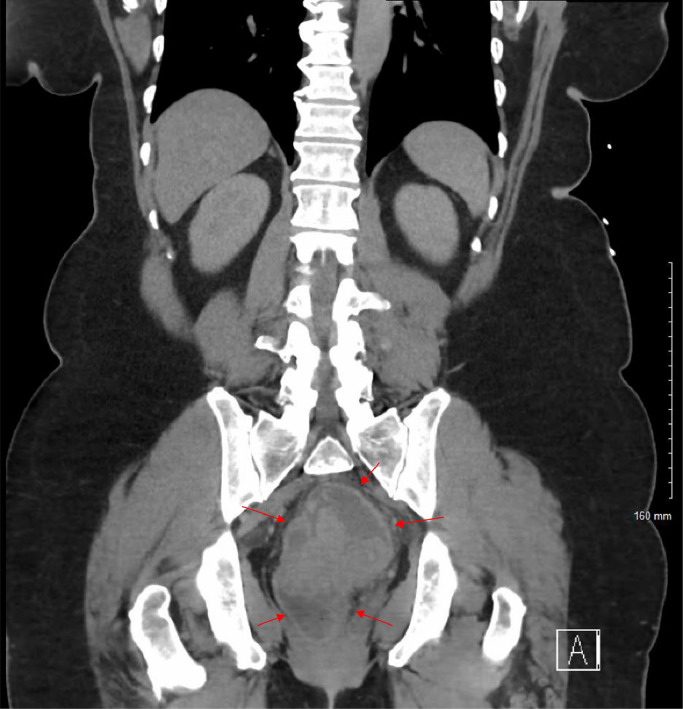
Fig. 2.
Coronal view CT of the abdomen and PELVIS with and without IV contrast showcases a complex mass centrally located in the pelvis, exhibiting both cystic and solid components. Highlighted by red arrows, this mass presents an ambiguous origin but prompts concerns for a neoplastic process.
Follow-up imaging
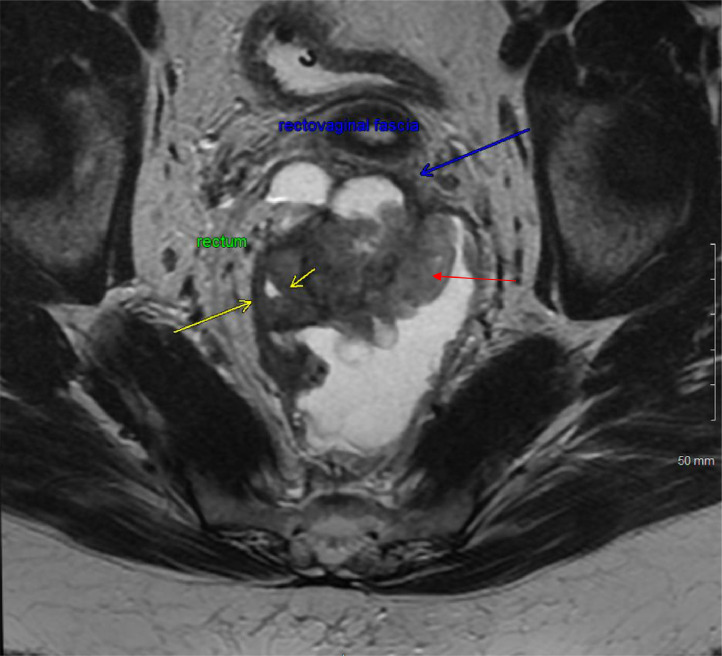
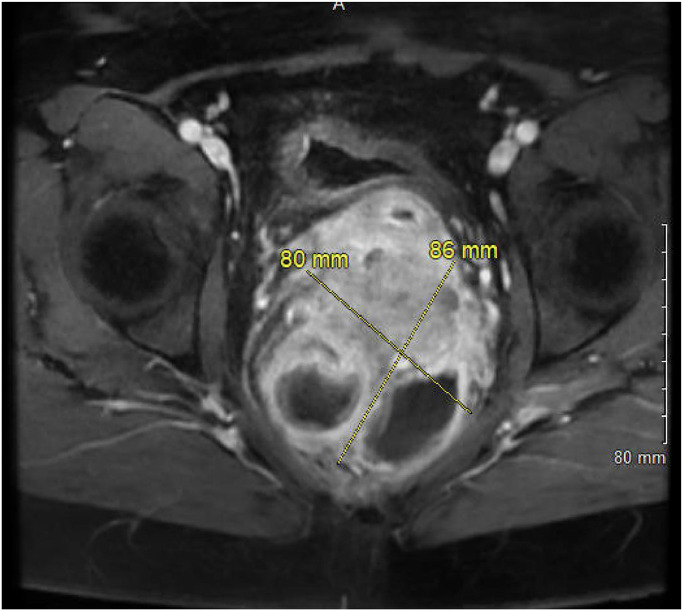
An MRI of the rectum with and without contrast was obtained to characterize the mass further. The MRI images showed an 8.0 × 8.6 cm heterogeneously enhancing mass centered in the retro-rectal space, causing mass effect, luminal narrowing of the mid-rectum, and anterior displacement of the rectovaginal fascia (see Fig. 3, Fig. 4). This raised the suspicion for malignancy with possible extension into the lower posterior wall of the vagina.
Fig. 3.
MRI of the rectum with and without contrast in a coronal view displays a heterogeneously enhancing mixed solid and cystic lesion in the extraperitoneal pelvic region, marked by red arrow. This lesion, predominantly centered in the retro-rectal space, exerts pressure on the rectum, pushing it anterolaterally as indicated by yellow arrows, causing mass effect and probable luminal narrowing of the mid rectum. Additionally, as shown by blue arrow, the lesion is pushing the rectovaginal fascia anteriorly with possible extension into the lower posterior wall of the vagina.
Fig. 4.
Axial LAVA Flex MRI reveals a heterogeneously enhancing lesion in the extraperitoneal pelvic region, centered predominantly in the retro-rectal space. This mixed solid and cystic lesion is captured in detail, illustrating its complex structure and positioning relative to critical pelvic anatomy.
Advanced imaging
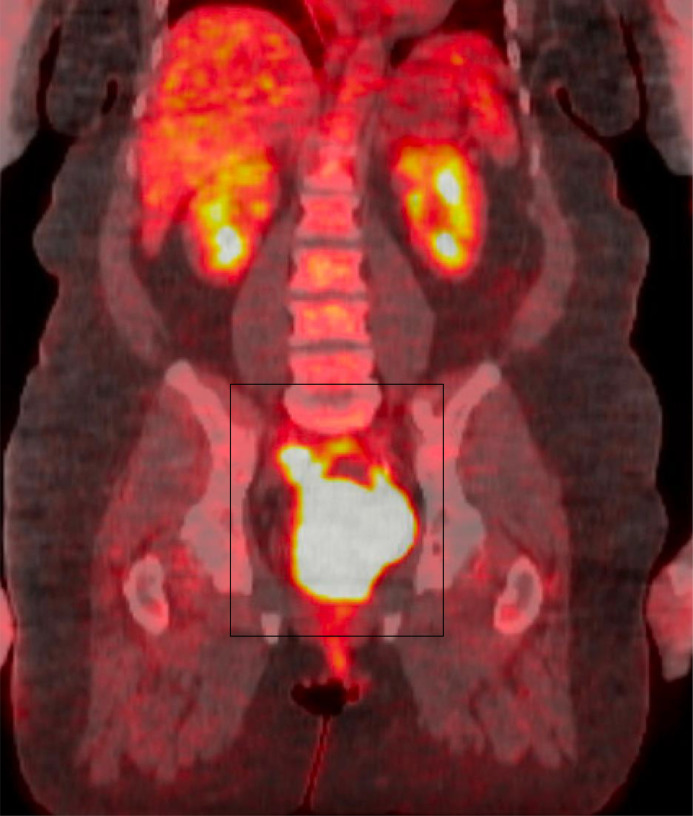
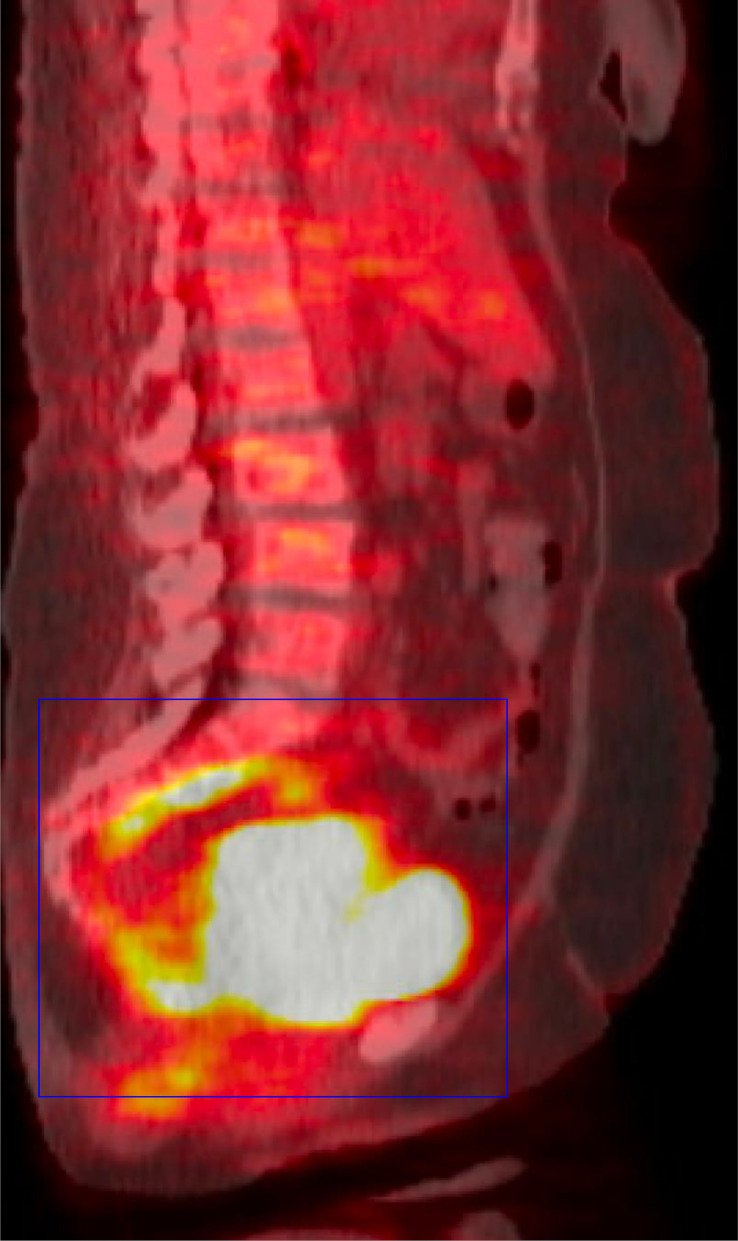
A PET CT scan was undertaken, which showed the mass in the posterior pelvis with a markedly elevated FDG uptake (Max SUV 43.6). This indicated high metabolic activity typical of malignant lesions. Additionally, a smaller mass with FDG accumulation (Max SUV 10.1) was observed along the right pelvic sidewall, suggestive of possible nodal involvement (see Fig. 5, Fig. 6, Fig. 7).
Fig. 5.
FDG PET CT Whole Body, axial view, shows a posterior pelvic mass exhibiting marked FDG uptake, indicated by blue arrows. This significant metabolic activity suggests high cellular activity, often associated with aggressive neoplastic processes.
Fig. 6.
FDG PET CT Whole Body, coronal view, displays a posterior pelvic mass highlighted by a black box, showing marked FDG uptake. This significant uptake indicates intense metabolic activity within the mass, which is characteristic of aggressive neoplastic processes.
Fig. 7.
FDG PET CT Whole Body in sagittal view showcases a posterior pelvic mass, highlighted by a blue box, which exhibits marked FDG uptake. This significant uptake suggests active metabolic processes typically associated with malignant tissue, providing essential information on the mass's behavior and aggressiveness.
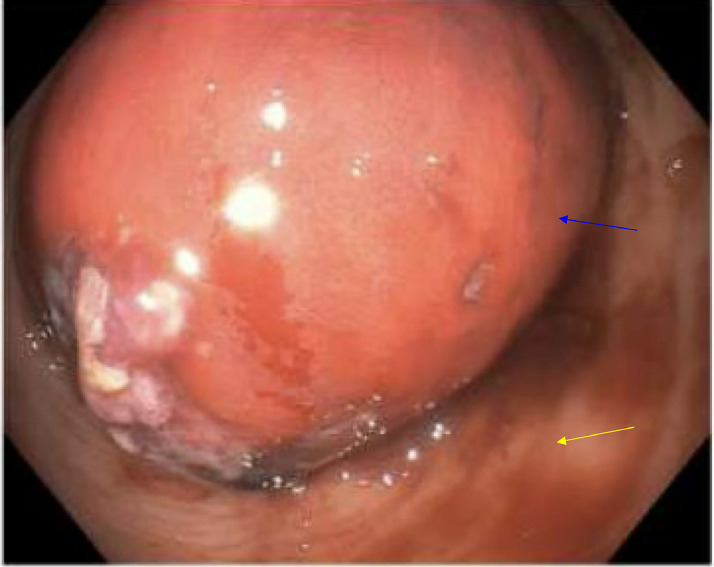
A sigmoidoscopy was attempted, and a mass was appreciated just inside the rectal vault (see Fig. 8). The tissue appearance was suspicious for GYN origin, although a history of total abdominal hysterectomy with bilateral salpingo-oophorectomy was reported. Multiple cold biopsies were obtained for pathology.
Fig. 8.
Colonoscopy image showing an obstructing rectal mass, indicated by a blue arrow, surrounded by normal mucosa, highlighted with a yellow arrow. This visualization contrasts the abnormal mass with the surrounding healthy tissue, providing a clear view of the extent and nature of the obstruction within the rectal lumen.
Challenges in diagnosis
The patient's surgical history complicated the interpretation of imaging findings. The ambiguity regarding the origin of the mass, whether ovarian, colonic, or cervical/vaginal, necessitated a multidisciplinary review and further biopsies to establish a definitive diagnosis.
The patient's multidisciplinary management involved consultations from gynecologic oncology, gastroenterology, urology, and colorectal surgery. Interventions included biopsies during sigmoidoscopy, which initially did not confirm a malignancy, and subsequent CT-guided biopsies that identified the lesion as carcinosarcoma with aggressive characteristics.
Therapeutic intervention
Given the size, location, suspicious nature of the lesion, and the potential risks associated with direct biopsy due to the proximity of major vascular structures, a percutaneous CT-guided biopsy was elected. This approach aimed to minimize the risk of contamination and obtain a sufficient sample for histopathological evaluation.
Pathology
The CT-guided biopsy revealed a carcinosarcoma with chondroid differentiation. Tumor cells were positive for ER, PR, P16, CK7, and PAX8, confirming the diagnosis of a malignancy likely of gynecologic origin. This was supported by the absence of CK20 and CDX-2, which are typically found in colorectal carcinomas. Tumor markers were also obtained with CEA 0.7, CA 19-9 <1.2, and CA 125 7.00. See Fig. A, Fig. B, Fig. C, Fig. D, Fig. E, Fig. F.
Fig. A.
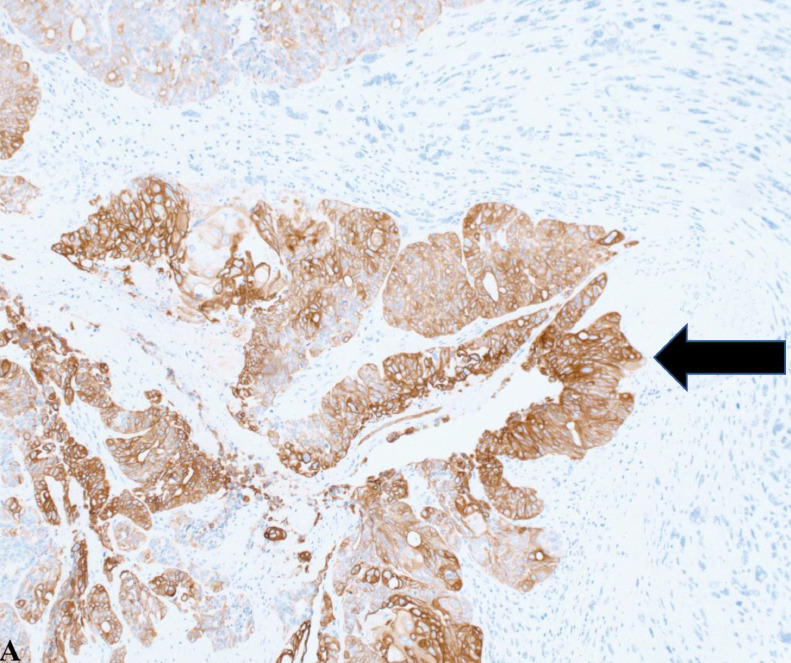
This image highlights tumor cells positively stained brown for cytokeratin 7 (CK7), indicated by a black arrow. The presence of CK7 is critical for distinguishing between gynecologic and nongynecologic tumors, as it is typically expressed in tissues like the breast, endometrium, lung, and ovary but absent in lower gastrointestinal cancers. This image, taken at 20x magnification, highlights the diagnostic utility of CK7 in identifying the tissue origin of tumors.
Fig. B.
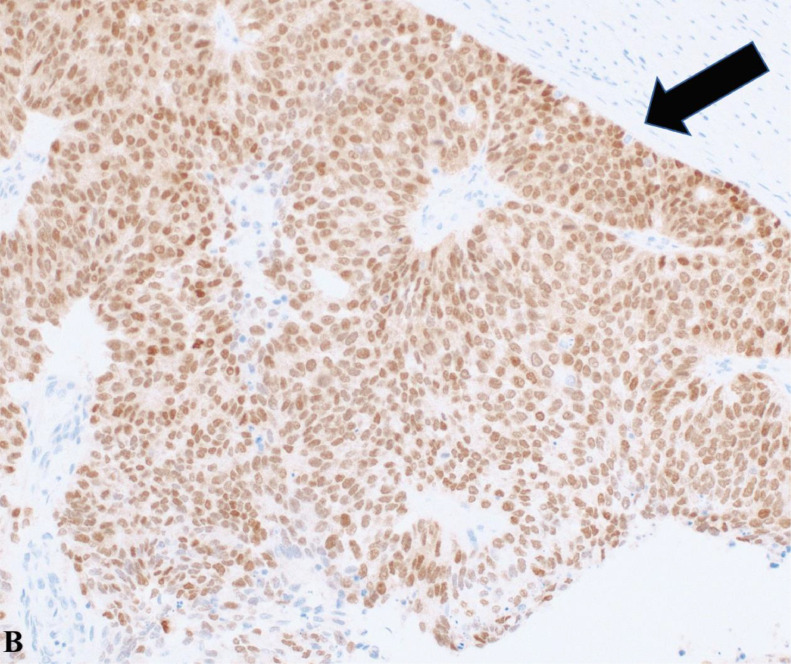
Displayed at 20x magnification, the tumor cells show positive staining for paired box gene 8 (PAX8), marked by a black arrow, suggesting a Mullerian origin. Pax 8 is expressed in gynecologic malignancies, thyroid cancer, and renal cell carcinoma.
Fig. C.
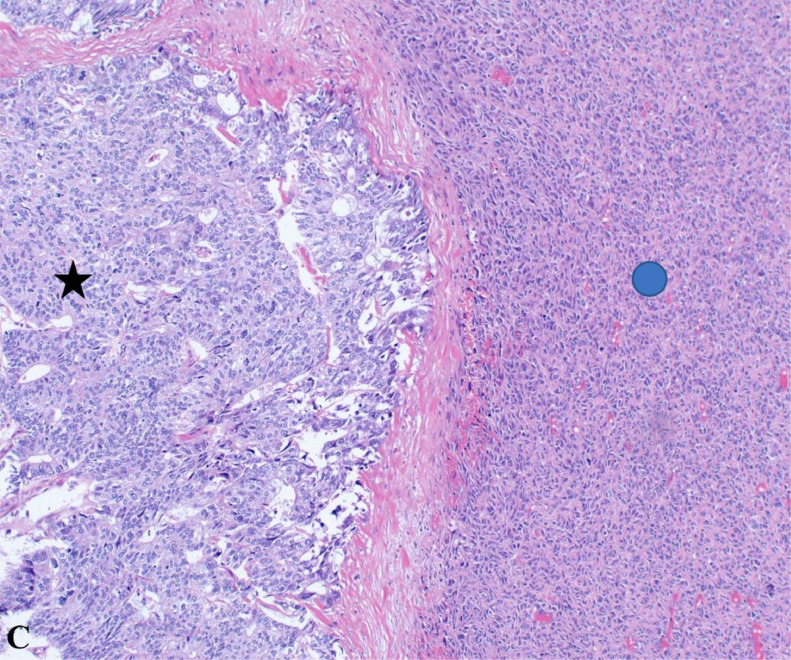
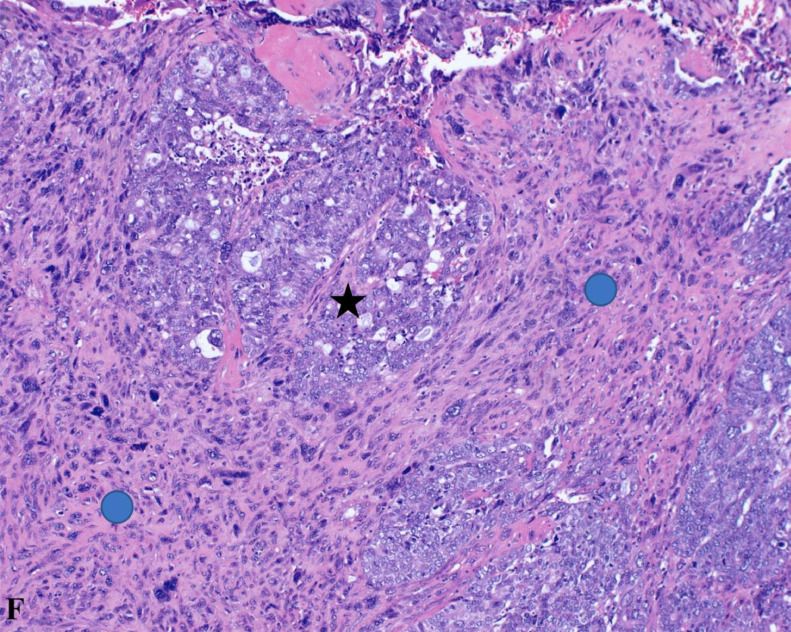
This H&E-stained section at 20x magnification reveals the tumor's dual nature, comprising both carcinomatous (black star) and sarcomatous (blue circle) components. The carcinomatous areas depict poorly differentiated malignant epithelial cells, while the sarcomatous regions contain mesenchymal elements, illustrating the complex histopathological landscape of carcinosarcomas.
Fig. D.
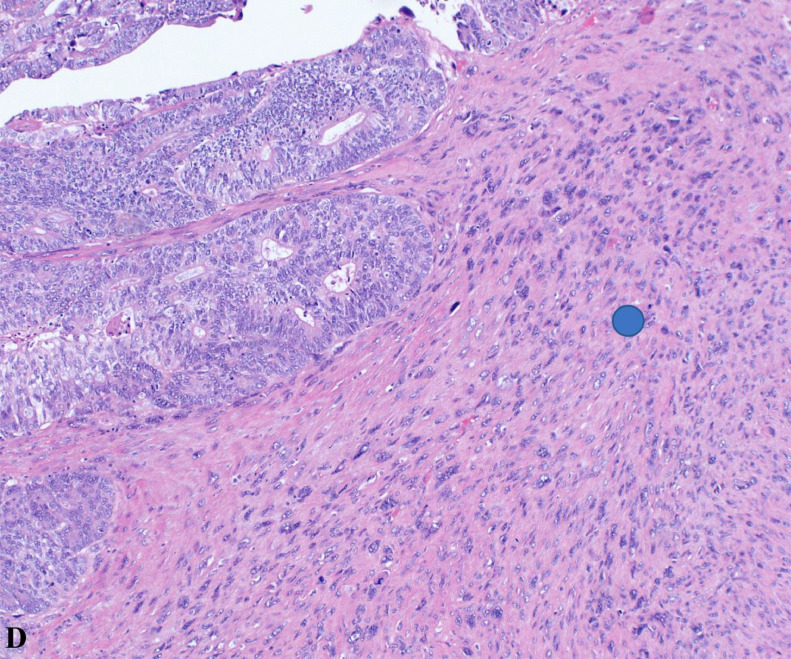
Highlighting sarcomatous features (blue circle) at 20x magnification, this H&E-stained image provides a close-up view of the tumor's mesenchymal elements, contributing to understanding its aggressive behavior.
Fig. E.
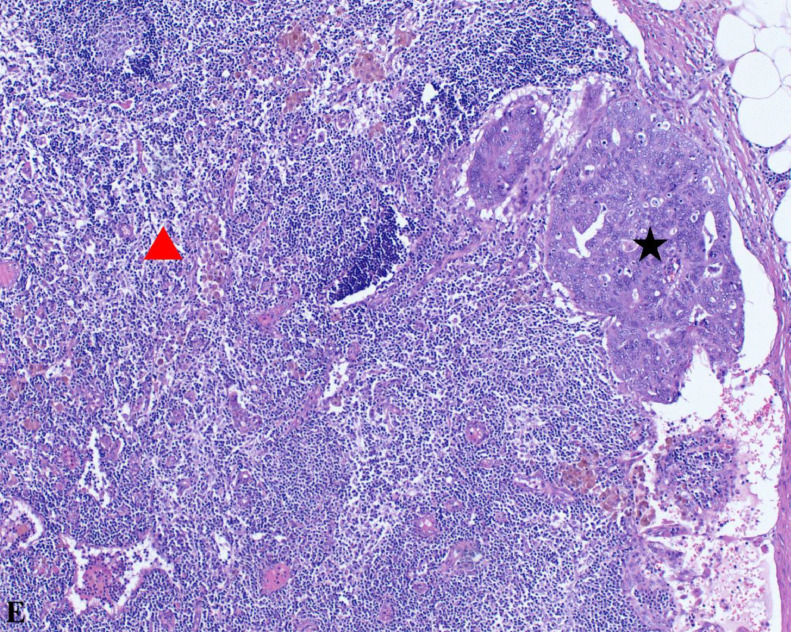
At 10x magnification, this image captures carcinoma metastasis into a lymph node. It contrasts carcinomatous components (black star) with normal lymph node tissue (red triangle), emphasizing the tumor's invasive potential.
Fig. F.
Displayed at 20x magnification, this H&E stained section shows the interplay between carcinomatous (black star) and sarcomatous (blue circle) features, providing a detailed view of the tumor's complex cellular composition.
Patient perspective
Despite the considerable physical discomfort and psychological distress caused by her symptoms and the prolonged diagnostic process, the patient exhibited remarkable fortitude and a positive demeanor throughout her interactions with the healthcare team. Her journey, marked by severe pain, unexplained weight loss, and the uncertainty of a complex diagnosis, would understandably lead to frustration and anxiety. Yet she approached each step with patience and optimism, rarely voicing complaints. Her pleasant nature and strong faith seemed to provide her with a reservoir of strength, allowing her to face each new diagnostic test and treatment decision with hope and resilience. This attitude eased the inherent challenges of her medical journey and inspired the medical staff working with her. Her case proves the profound impact of a patient's outlook on the therapeutic relationship and the importance of maintaining hope and positivity in the face of medical adversity.
Initial surgical interventions
The first stage involved a robotic laparoscopic loop proximal sigmoid colostomy and flexible sigmoidoscopy with biopsies. These procedures were critical for managing symptoms and obtaining diagnostic tissue without extensive surgical trauma. The complexity of the mass, indicated by third spacing edema and the cystic nature, required careful handling to prevent spillage and aggravation of the condition.
The discovery of a single drop of mucin in the cul-de-sac, although seemingly minor, was critical as it suggested the presence of a complex, potentially ruptured cystic lesion, guiding the need for careful handling of the tumor.
Vascular management
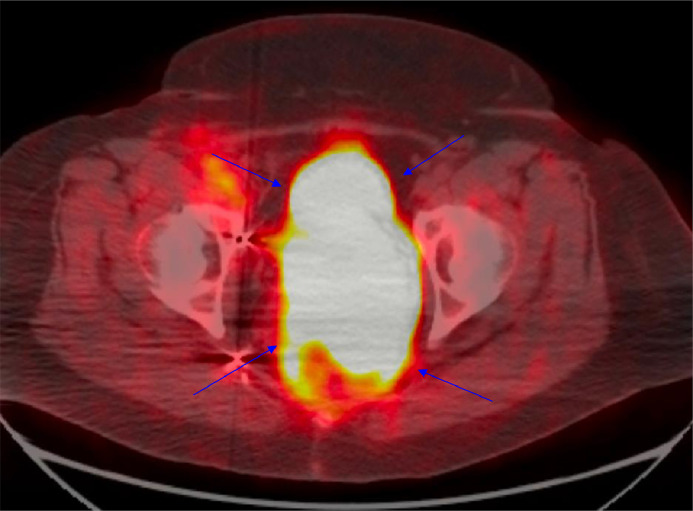
Due to the increased vascularity of the mass, there was a significant risk of bleeding, so targeted vascular intervention was crucial. A pelvic angiogram and coil embolization of the right and left uterine arteries and right vaginal artery were performed (see Fig. 9). These procedures reduced the risk of hemorrhage during subsequent surgical excision.
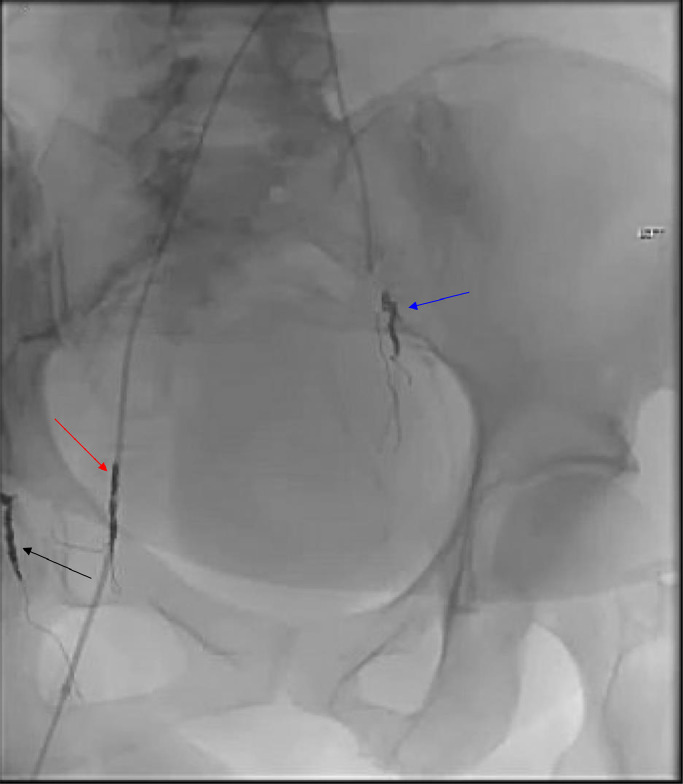
Fig. 9.
The pelvic angiogram postembolization depicts successful targeting of the right uterine artery (red arrow), right vaginal artery (black arrow), and left uterine artery (blue arrow). This strategic intervention is crucial for reducing the blood supply to the pelvic mass, potentially minimizing bleeding during subsequent surgical procedures, and reducing tumor viability.
Definitive surgical excision
The definitive surgical treatment involved a supralevator posterior exenteration with an omental J flap to achieve clear margins. The operation involved meticulous dissection in the retroperitoneal space, careful isolation and protection of vital structures such as the ureters, and strategic navigation around the pelvic anatomy altered by the patient's previous surgical history and current disease state. The surgery aimed to remove the mass entirely while preserving some vaginal tissue, given that the disease was above the levator plate and the posterior aspect of the mass was well-circumscribed.
To further prepare for possible postoperative radiation therapy, an intra-abdominal omental pedicle flap was created and positioned to exclude the small bowel from the pelvis. This step was crucial in reducing the risk of radiation-induced complications should additional therapy be required. A low anterior resection, in-depth revision of the previous loop colostomy to an end colostomy, and careful hemostasis accompanied this.
The surgical approach taken in this case shows the importance of a meticulously planned, multi-stage strategy when dealing with advanced pelvic malignancies. Each step, from initial symptom management and diagnostic biopsies to major oncologic resection and postoperative preventive measures, was designed to maximize disease control while minimizing patient morbidity. This case is a detailed example of the critical interplay between surgical foresight, detailed anatomical understanding, and interventional radiology in managing complex oncological conditions.
Discussion
The management of pelvic carcinosarcoma, particularly when compounded by previous surgical alterations and complex vascular involvement, necessitates a meticulous multi-staged approach to optimize outcomes and minimize patient morbidity [8,9]. This case report highlights the sophisticated integration of advanced imaging, interventional radiology, and strategic surgical interventions to manage such challenging cases effectively.
The patient's management began with a robotic laparoscopic loop proximal sigmoid colostomy. This initial procedure aimed to relieve the obstructive symptoms caused by the mass, allowing for further diagnostic and therapeutic steps to be planned. Using minimally invasive techniques early in management shows the importance of promptly addressing acute symptoms to improve overall treatment tolerance and outcome.
Recognizing the extensive vascular nature of the mass, characterized by hypertrophied feeding vessels, the team proceeded with a pelvic angiogram and coil embolization of the right and left uterine arteries and right vaginal artery. This preemptive vascular management was critical in reducing the risk of significant hemorrhage during the subsequent surgical resection. The decision to utilize coil embolization reflects an understanding of the importance of controlling the vascular supply to highly vascular tumors, which can lead to major intraoperative and postoperative complications.
The centerpiece of the therapeutic strategy was the supralevator posterior exenteration, involving en bloc resection of the vagina, mass, and sigmoid colon, accompanied by a low anterior resection and the placement of an omental J flap. This extensive surgical endeavor was not only aimed at removing the malignant mass but also preparing the site for potential adjuvant radiation therapy. The omental J flap is particularly noteworthy as it protects the resection site, enhances postoperative healing, and decreases the side effects of subsequent radiation therapy. The omental J flap provides a physical barrier to decrease small bowel adhesions in the field, thus limiting GI side effects.
This case exemplifies the critical need for an integrated approach with multiple specialties. The interplay between gastroenterology, gynecologic oncology, interventional radiology, and surgical oncology was pivotal in formulating a coherent and effective management plan. Each specialty provided essential insights that influenced the overall treatment strategy, demonstrating the importance of collaborative practice in managing complex oncological conditions.
Conclusion
Pelvic carcinosarcoma is a rare and aggressive cancer that manifests within the pelvic region, straddling the properties of both carcinoma and sarcoma. This type of tumor is known for its rapid progression and poor prognosis, often presenting significant challenges in management. Effective treatment necessitates a multimodal individualized approach, combining surgery with chemotherapy and radiation. The importance of early detection through vigilant monitoring and the use of advanced imaging techniques cannot be overstated, as they significantly impact treatment outcomes. The role of a multidisciplinary team is crucial in managing such intricate cases, ensuring comprehensive care that addresses the patient's oncological and supportive needs.
Diagnosing and managing pelvic carcinosarcoma, particularly in patients with complex postsurgical anatomy, exemplify the challenges and advancements in diagnostic radiology. This case reveals the essential role of comprehensive imaging strategies in characterizing malignancies that may not present with typical features due to altered anatomical landscapes. Advanced imaging modalities such as CT, MRI, and PET CT are invaluable in these scenarios, providing critical insights that guide clinical decision-making and treatment planning.
Patient consent
We confirm that we have obtained written, informed consent from the patient for the publication of this case report. The patient has been thoroughly informed about the details that will be published and understands the implications of the publication. The written consent is stored securely and is available for review by the editorial team upon request.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kitazono I, Akahane T, Kobayashi Y, Yanazume S, Tabata K, Tasaki T, et al. Pelvic carcinosarcoma showing a diverse histology and hierarchical gene mutation with a common pole mutation to endometrial endometroid carcinoma: a case report. Int J Surg Pathol. 2022;30(8):891–899. doi: 10.1177/10668969221088880. [DOI] [PubMed] [Google Scholar]
- 2.Elshaikh MA, Modh A, Jhingran A, Biagioli MC, Coleman RL, Gaffney DK, et al. Executive summary of the American Radium Society® appropriate use criteria for management of uterine carcinosarcoma. Gynecol Oncol. 2020;158(2):460–466. doi: 10.1016/j.ygyno.2020.04.683. [DOI] [PubMed] [Google Scholar]
- 3.Singh R. Review literature on uterine carcinosarcoma. J Cancer Res Ther. 2014;10(3):461–468. doi: 10.4103/0973-1482.138197. [DOI] [PubMed] [Google Scholar]
- 4.Arend R, Doneza JA, Wright JD. Uterine carcinosarcoma. Curr Opin Oncol. 2011;23(5):531–536. doi: 10.1097/CCO.0b013e328349a45b. [DOI] [PubMed] [Google Scholar]
- 5.Cowdell I, Smyth SL, Eltawab S, Soleymani Majd H. Radical abdomino-pelvic surgery in the management of uterine carcinosarcoma with concomitant para-aortic lymphadenopathy metastasising from anal carcinoma. BMJ Case Rep. 2022;15(11):e252233. doi: 10.1136/bcr-2022-252233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain Z. The Holistic Approach to Cancer Pain Management. Ulster Med J. 2022;91(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- 7.Brocker KA, Alt CD, Eichbaum M, Sohn C, Kauczor HU, Hallscheidt P. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT : part 1. Strahlenther Onkol. 2011;187(10):611–618. doi: 10.1007/s00066-011-4001-0. [DOI] [PubMed] [Google Scholar]
- 8.Terblanche L, Botha MH. Uterine carcinosarcoma: a 10-year single institution experience. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0271526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol. 2012;125(1):271–277. doi: 10.1016/j.ygyno.2011.12.418. [DOI] [PubMed] [Google Scholar]