Abstract
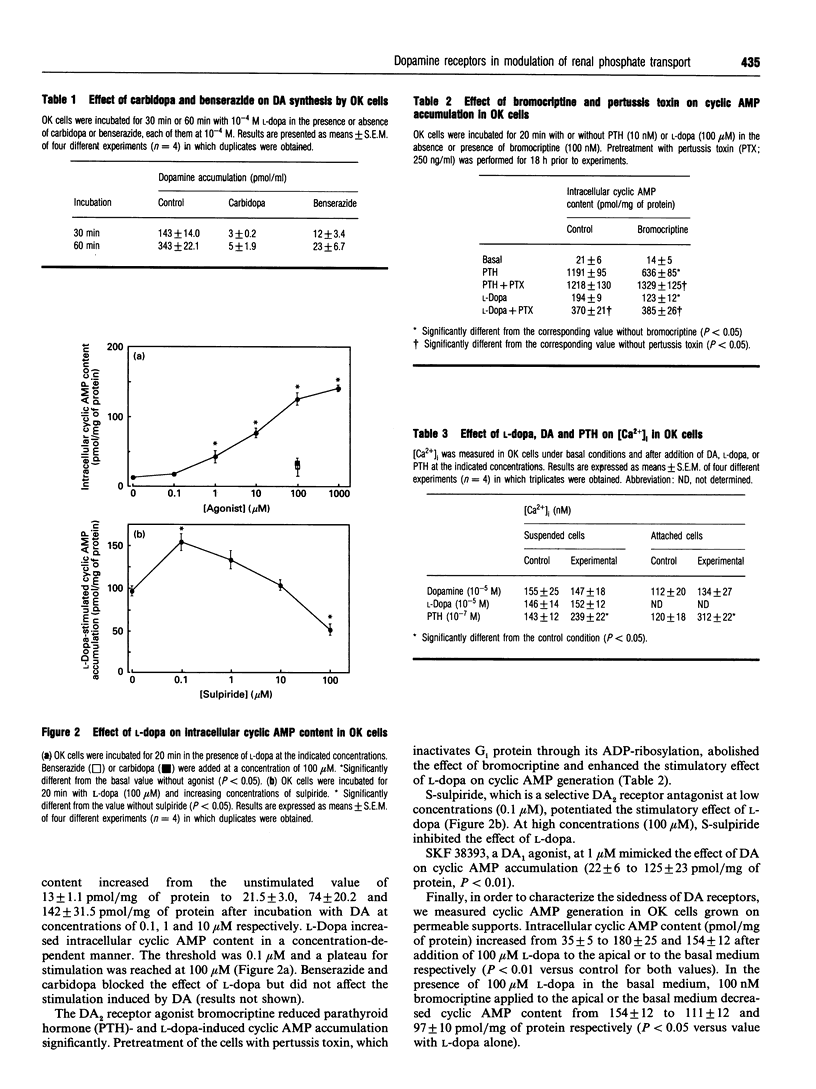
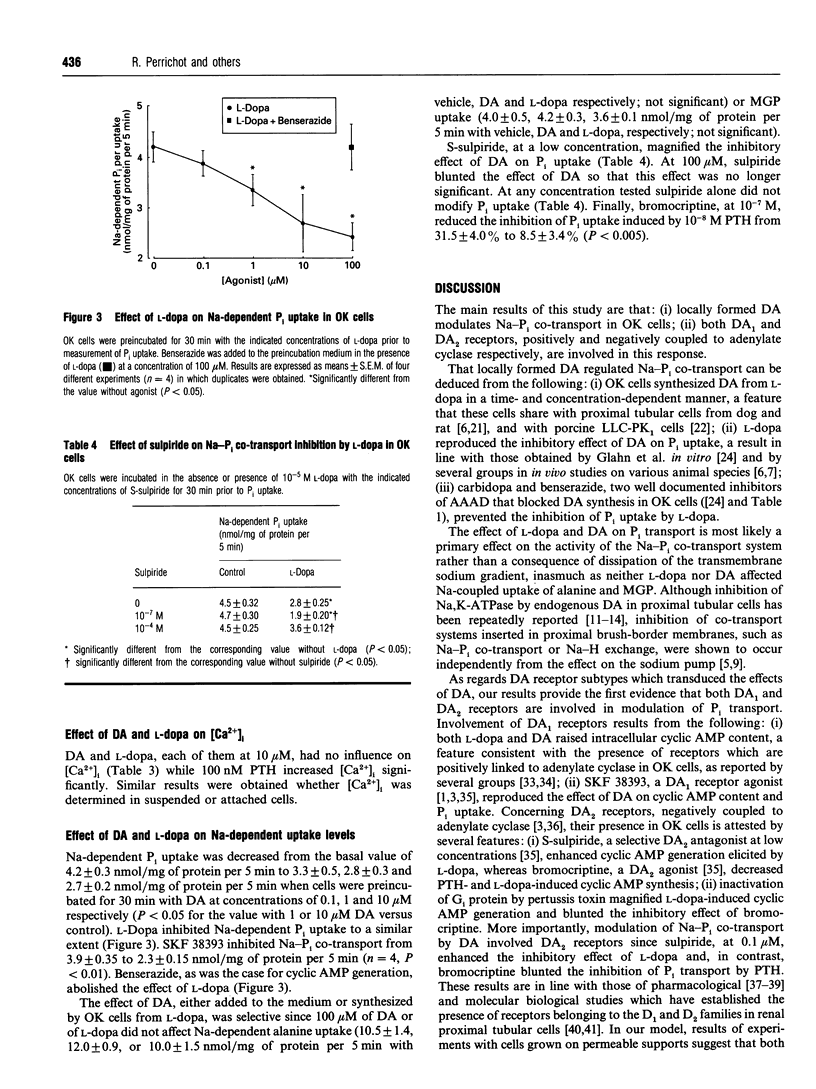
The involvement of dopamine (DA) receptor subtypes in regulation of renal phosphate transport by DA, either exogenous or locally synthesized from L-dihydroxyphenylalanine (L-dopa) was evaluated in opossum kidney (OK) cells with proximal tubular phenotype. DA synthesis from L-dopa by OK cells was abolished by carbidopa and benserazide, two dissimilar inhibitors of aromatic L-amino acid decarboxylase. L-Dopa stimulated cyclic AMP generation and inhibited Na-dependent Pi uptake, and these effects were abolished by carbidopa and benserazide. The effects of L-dopa or DA on cyclic AMP generation and on Na-Pi co-transport were mimicked by SKF 38393, a DA1 receptor agonist, and were potentiated by S-sulpiride, a DA2 receptor antagonist. Bromocriptine, a DA2 receptor agonist, blunted in a pertussis toxin-dependent manner parathyroid hormone (PTH)-induced cyclic AMP generation and inhibition of Pi uptake. In contrast with PTH, neither L-dopa nor DA affected significantly the cytosolic calcium concentration. These results support the involvement of DA1 and DA2 receptors, positively and negatively coupled into adenylate cyclase respectively, in modulation of renal phosphate transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Baines A. D., Drangova R., Hatcher C. Dopamine production by isolated glomeruli and tubules from rat kidneys. Can J Physiol Pharmacol. 1985 Feb;63(2):155–158. doi: 10.1139/y85-027. [DOI] [PubMed] [Google Scholar]
- Baines A. D., Ho P., Drangova R. Proximal tubular dopamine production regulates basolateral Na-K-ATPase. Am J Physiol. 1992 Apr;262(4 Pt 2):F566–F571. doi: 10.1152/ajprenal.1992.262.4.F566. [DOI] [PubMed] [Google Scholar]
- Bates M. D., Caron M. G., Raymond J. R. Desensitization of DA1 dopamine receptors coupled to adenylyl cyclase in opossum kidney cells. Am J Physiol. 1991 Jun;260(6 Pt 2):F937–F945. doi: 10.1152/ajprenal.1991.260.6.F937. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E., Higashi Y., Kaneda Y. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol. 1982 Jun;242(6):F634–F640. doi: 10.1152/ajprenal.1982.242.6.F634. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol. 1990 Dec;259(6 Pt 2):F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng L., Precht P., Frank D., Liang C. T. Dopamine stimulation of cAMP production in cultured opossum kidney cells. Am J Physiol. 1990 Apr;258(4 Pt 2):F877–F882. doi: 10.1152/ajprenal.1990.258.4.F877. [DOI] [PubMed] [Google Scholar]
- Cote T. E., Felder R., Kebabian J. W., Sekura R. D., Reisine T., Affolter H. U. D-2 dopamine receptor-mediated inhibition of pro-opiomelanocortin synthesis in rat intermediate lobe. Abolition by pertussis toxin or activators of adenylate cyclase. J Biol Chem. 1986 Apr 5;261(10):4555–4561. [PubMed] [Google Scholar]
- Cuche J. L., Marchand G. R., Greger R. F., Lang R. C., Knox F. G. Phosphaturic effect of dopamine in dogs. Possible role of intrarenally produced dopamine in phosphate regulation. J Clin Invest. 1976 Jul;58(1):71–76. doi: 10.1172/JCI108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R., Jr, Phillips M. I. Dopamine synthesis and release in LLC-PK1 cells. Eur J Pharmacol. 1990 Dec 15;189(6):423–426. doi: 10.1016/0922-4106(90)90041-u. [DOI] [PubMed] [Google Scholar]
- Debska-Slizien A., Ho P., Drangova R., Baines A. D. Endogenous renal dopamine production regulates phosphate excretion. Am J Physiol. 1994 Jun;266(6 Pt 2):F858–F867. doi: 10.1152/ajprenal.1994.266.6.F858. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Albrecht F. E., Campbell T., Eisner G. M., Jose P. A. cAMP-independent, G protein-linked inhibition of Na+/H+ exchange in renal brush border by D1 dopamine agonists. Am J Physiol. 1993 Jun;264(6 Pt 2):F1032–F1037. doi: 10.1152/ajprenal.1993.264.6.F1032. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Campbell T., Albrecht F., Jose P. A. Dopamine inhibits Na(+)-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol. 1990 Aug;259(2 Pt 2):F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- Felder C. C., McKelvey A. M., Gitler M. S., Eisner G. M., Jose P. A. Dopamine receptor subtypes in renal brush border and basolateral membranes. Kidney Int. 1989 Aug;36(2):183–193. doi: 10.1038/ki.1989.178. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Blecher M., Calcagno P. L., Jose P. A. Dopamine receptors in the proximal tubule of the rabbit. Am J Physiol. 1984 Sep;247(3 Pt 2):F499–F505. doi: 10.1152/ajprenal.1984.247.3.F499. [DOI] [PubMed] [Google Scholar]
- Friedlander G., Amiel C. Protein kinase C activation has dissimilar effects on sodium-coupled uptakes in renal proximal tubular cells in primary culture. J Biol Chem. 1989 Mar 5;264(7):3935–3941. [PubMed] [Google Scholar]
- Gao D. Q., Canessa L. M., Mouradian M. M., Jose P. A. Expression of the D2 subfamily of dopamine receptor genes in kidney. Am J Physiol. 1994 Apr;266(4 Pt 2):F646–F650. doi: 10.1152/ajprenal.1994.266.4.F646. [DOI] [PubMed] [Google Scholar]
- Glahn R. P., Onsgard M. J., Tyce G. M., Chinnow S. L., Knox F. G., Dousa T. P. Autocrine/paracrine regulation of renal Na(+)-phosphate cotransport by dopamine. Am J Physiol. 1993 Apr;264(4 Pt 2):F618–F622. doi: 10.1152/ajprenal.1993.264.4.F618. [DOI] [PubMed] [Google Scholar]
- Grenader A., Healy D. P. Locally formed dopamine stimulates cAMP accumulation in LLC-PK1 cells via a DA1 dopamine receptor. Am J Physiol. 1991 Jun;260(6 Pt 2):F906–F912. doi: 10.1152/ajprenal.1991.260.6.F906. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yamaji Y., Kitajima W., Saruta T. Aromatic L-amino acid decarboxylase activity along the rat nephron. Am J Physiol. 1990 Jan;258(1 Pt 2):F28–F33. doi: 10.1152/ajprenal.1990.258.1.F28. [DOI] [PubMed] [Google Scholar]
- Isaac J., Berndt T. J., Chinnow S. L., Tyce G. M., Dousa T. P., Knox F. G. Dopamine enhances the phosphaturic response to parathyroid hormone in phosphate-deprived rats. J Am Soc Nephrol. 1992 Mar;2(9):1423–1429. doi: 10.1681/ASN.V291423. [DOI] [PubMed] [Google Scholar]
- Isaac J., Glahn R. P., Appel M. M., Onsgard M., Dousa T. P., Knox F. G. Mechanism of dopamine inhibition of renal phosphate transport. J Am Soc Nephrol. 1992 May;2(11):1601–1607. doi: 10.1681/ASN.V2111601. [DOI] [PubMed] [Google Scholar]
- Jose P. A., Raymond J. R., Bates M. D., Aperia A., Felder R. A., Carey R. M. The renal dopamine receptors. J Am Soc Nephrol. 1992 Feb;2(8):1265–1278. doi: 10.1681/ASN.V281265. [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Bello-Reuss E. Effect of dopamine on phosphate reabsorption in isolated perfused rabbit proximal tubules. Miner Electrolyte Metab. 1983;9(3):147–150. [PubMed] [Google Scholar]
- LaBrosse E. H., Comoy E., Bohuon C., Zucker J. M., Schweisguth O. Catecholamine metabolism in neuroblastoma. J Natl Cancer Inst. 1976 Sep;57(3):633–638. doi: 10.1093/jnci/57.3.633. [DOI] [PubMed] [Google Scholar]
- Le Goas F., Amiel C., Friedlander G. Protein kinase C modulates cAMP content in proximal tubular cells: role of phosphodiesterase inhibition. Am J Physiol. 1991 Oct;261(4 Pt 2):F587–F592. doi: 10.1152/ajprenal.1991.261.4.F587. [DOI] [PubMed] [Google Scholar]
- Meister B., Fried G., Holgert H., Aperia A., Hökfelt T. Ontogeny of aromatic L-amino acid decarboxylase-containing tubule cells in rat kidney. Kidney Int. 1992 Sep;42(3):617–623. doi: 10.1038/ki.1992.326. [DOI] [PubMed] [Google Scholar]
- Murer H., Werner A., Reshkin S., Wuarin F., Biber J. Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol. 1991 May;260(5 Pt 1):C885–C899. doi: 10.1152/ajpcell.1991.260.5.C885. [DOI] [PubMed] [Google Scholar]
- Pages N., Orosco M., Rouch C., Fournier G., Comoy E., Bohuon C. Brain and adrenal monoamines and neuropeptide Y in codeine tolerant rats. Gen Pharmacol. 1992 Mar;23(2):159–163. doi: 10.1016/0306-3623(92)90003-3. [DOI] [PubMed] [Google Scholar]
- Puybasset L., Lacolley P., Laurent S., Mignon F., Billaud E., Cuche J. L., Comoy E., Safar M. Effects of clonidine on plasma catecholamines and neuropeptide Y in hypertensive patients at rest and during stress. J Cardiovasc Pharmacol. 1993 Jun;21(6):912–919. doi: 10.1097/00005344-199306000-00010. [DOI] [PubMed] [Google Scholar]
- Seeman P., Van Tol H. H. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994 Jul;15(7):264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Seri I., Kone B. C., Gullans S. R., Aperia A., Brenner B. M., Ballermann B. J. Locally formed dopamine inhibits Na+-K+-ATPase activity in rat renal cortical tubule cells. Am J Physiol. 1988 Oct;255(4 Pt 2):F666–F673. doi: 10.1152/ajprenal.1988.255.4.F666. [DOI] [PubMed] [Google Scholar]
- Siegfried G., Vrtovsnik F., Prié D., Amiel C., Friedlander G. Parathyroid hormone stimulates ecto-5'-nucleotidase activity in renal epithelial cells: role of protein kinase-C. Endocrinology. 1995 Mar;136(3):1267–1275. doi: 10.1210/endo.136.3.7867581. [DOI] [PubMed] [Google Scholar]
- Stephenson R. K., Sole M. J., Baines A. D. Neural and extraneural catecholamine production by rat kidneys. Am J Physiol. 1982 Mar;242(3):F261–F266. doi: 10.1152/ajprenal.1982.242.3.F261. [DOI] [PubMed] [Google Scholar]
- Strange P. G. Dopamine receptors: structure and function. Prog Brain Res. 1993;99:167–179. doi: 10.1016/s0079-6123(08)61345-x. [DOI] [PubMed] [Google Scholar]
- Wahbe F., Hagege J., Loreau N., Ardaillou R. Endogenous dopamine synthesis and dopa-decarboxylase activity in rat renal cortex. Mol Cell Endocrinol. 1982 Jun;27(1):45–54. doi: 10.1016/0303-7207(82)90061-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I., Jose P. A., Mouradian M. M., Canessa L. M., Monsma F. J., Jr, Sibley D. R., Takeyasu K., Felder R. A. Expression of dopamine D1A receptor gene in proximal tubule of rat kidneys. Am J Physiol. 1993 Feb;264(2 Pt 2):F280–F285. doi: 10.1152/ajprenal.1993.264.2.F280. [DOI] [PubMed] [Google Scholar]


